FDA Overhauls Nutrition Facts Label
Major changes to the label include a declaration of added sugar, updated serving sizes, and requirements for listing vitamin D and potassium.
The new rules for the Nutrition Facts label on food and beverage packaging announced last month by the U.S. Food and Drug Administration (FDA) will require manufacturers of roughly 800,000 food and beverage products to overhaul labeling in order to help consumers make more healthy food choices. Specifically, the new labeling requirements call for changes in the way a number of nutrients and food components, most notably added sugars, are listed on the label as well as changes designed to more accurately represent serving sizes. These are the first major changes proposed for the Nutrition Facts label since it was introduced in 1993
The new regulations reflect current scientific information, including research on the link between diet and chronic diseases such as obesity, heart disease, and diabetes. These changes are intended to increase consumers’ awareness of portion sizes and the amount of calories and components such as added sugar that are contained in the foods they eat and to help them make more informed food choices. As First Lady Michelle Obama said when making the announcement about the changes, “This is going to make a real difference in providing families across the country the information they need to make healthy choices” (White House 2016; FDA 2016a).
So What’s New About the Nutrition Facts Label?
The changes can be divided into two parts—nutrients and food components and serving sizes—as indicated in the final rules released by the FDA.
Nutrients and Food Components
The changes in nutritional information required on the label are based on updated scientific evidence, nutrition intake recommendations from the Institute of Medicine, and recommendations from the 2015-2020 Dietary Guidelines for Americans (FDA 2016b). Here’s a look at the highlights.
-
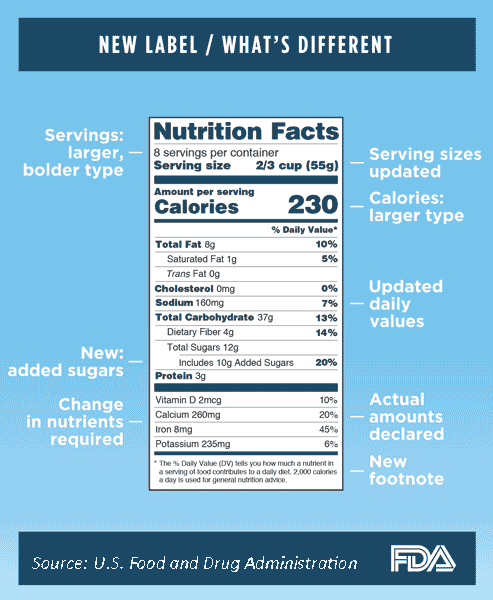
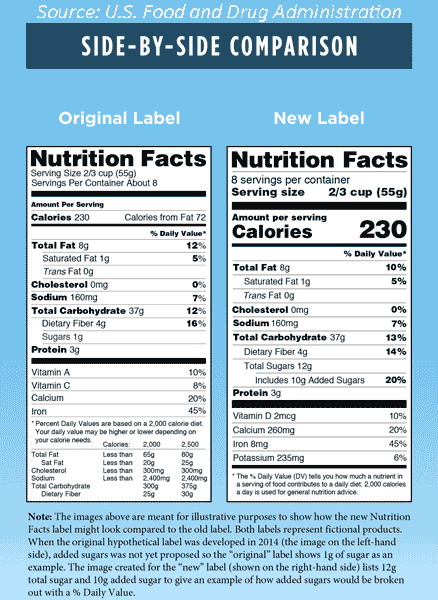
Added Sugars. Sugars added to foods will be required on the label in grams and as percent Daily Value (% DV) to allow the distinction between added and inherent sugar. This requirement is consistent with the 2015–2020 Dietary Guidelines for Americans, which recommends to “consume less than 10% of calories per day from added sugar” (HHS/USDA 2016). Added sugars will be declared as “Includes ‘X’ g Added Sugars” directly under total sugars (FDA 2016b).
-
The FDA defines added sugars in this way: “Added sugars are either added during the processing of foods, or are packaged as such, and include sugars (free, mono- and disaccharides), sugars from syrups and honey, and sugars from concentrated fruit or vegetable juices that are in excess of what would be expected from the same volume of 100 percent fruit or vegetable juice of the same type, except that fruit or vegetable juice concentrated from 100 percent juices sold to consumers, fruit or vegetable juice concentrates used towards the total juice percentage label declaration under § 101.30 or for Brix standardization under § 102.33(g)(2) of this chapter, fruit juice concentrates which are used to formulate the fruit component of jellies, jams, or preserves in accordance with the standard of identities set forth in §§ 150.140 and 150.160 of this chapter, or the fruit component of fruit spreads shall not be labeled as added sugars” (FDA 2016b).
-
-
Total Sugars. Sugars will now be declared as total sugars. Total sugars includes both added and inherent sugars (FDA 2016b).
-
Dietary Fiber. In the past, there was no definition of dietary fiber. The FDA now defines dietary fiber as “non-digestible soluble and insoluble carbohydrates (with 3 or more monomeric units), and lignin that are intrinsic and intact in plants; isolated or synthetic non-digestible carbohydrates (with 3 or more monomeric units) determined by FDA to have physiological effects that are beneficial to human health” (FDA 2016b).
-
The Daily Reference Value (DRV) is increased to 28 g from 25 g (FDA 2016b).
-
-
Vitamin D and Potassium. Both nutrients will now be required on the label as they are considered “nutrients of public health concern” since many Americans do not consume these in sufficient amounts, thereby putting themselves at a higher risk for chronic diseases. In addition to the % DV, the actual quantities of vitamin D and potassium will be required on the label (FDA 2016b; HHS/USDA 2016).
-
The % DV for vitamin D and potassium is based on the Reference Daily Intake (RDI) of 20 mcg and 4,700 mg, respectively (FDA 2016b).
-
-
Calcium and Iron. In addition to the % DV, declaration of the actual quantities of calcium and iron will be required (FDA 2016b).
-
The % DV for calcium and iron is based on the RDI of 1,300 mg and 18 mg, respectively (FDA 2016b).
-
-
Sodium. The DRV for sodium is decreased to 2,300 mg from 2,400 mg, consistent with the new scientific data and consensus reports on sodium (FDA 2016b).
-
Vitamins A and C. Neither is now required on the label but may be included on a voluntary basis. These vitamins are not considered to be nutrients of public health concern in the 2010 Dietary Guidelines for Americans and the 2015 Dietary Guidelines for Americans. If a specific claim is made with regard to either nutrient, then it would need to be listed on the label (FDA 2016b; HHS/USDA 2016).
-
The RDI for vitamin A is 900 mcg RAE (retinol activity equivalents). The RDI for vitamin C increased to 90 mg from 60 mg (FDA 2016b).
-
-
Other Vitamins and Minerals. The quantity, in grams, of other vitamins and minerals will be permitted on a voluntary basis (FDA 2016b).
-
Calories From Fat. Current scientific evidence indicates that the type of fat is more relevant than total fat intake with respect to the risk of chronic diseases; hence, the information on calories from fat will no longer be required (FDA 2016b).
-
Total Fat, Saturated Fat, and Trans Fat. All will continue to be required on the label (FDA 2016b).
-
Daily Values. The % DV helps consumers understand the nutrition information in the context of a total daily diet. For sodium, dietary fiber, and vitamin D, updated daily values consistent with the Institute of Medicine’s recommendations and the 2015-2020 Dietary Guidelines for Americans will be required on the label (FDA 2016b). The % DV for nutrients will change for foods and beverages with updated serving sizes as well as updated DRVs and RDIs for specific nutrients.
Serving Size
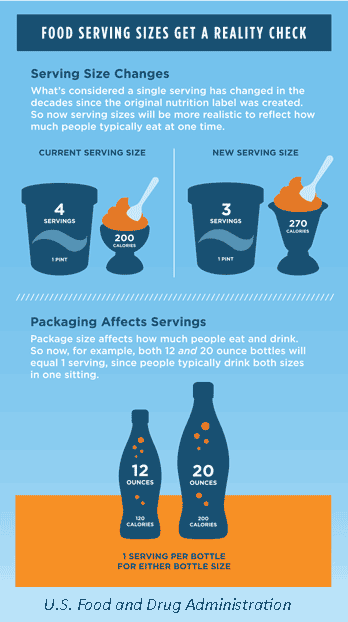
The updates on the serving sizes are based on newer consumption data, which show that the amounts of food consumed have changed since 1993. Thus, the updated serving size more accurately reflects the amount that is typically consumed in one sitting, giving more direct and realistic information to encourage people to maintain and/or improve their dietary practices.
-
Serving Size. The new rules will require listing the serving size that is typically consumed in one sitting. As stated in the Code of Federal Regulations, the term serving or serving size means an amount of food customarily consumed per eating occasion by persons 4 years of age or older (CFR 2010). The amount of food consumed typically in one sitting has changed over the years and no longer matches the serving sizes published in 1993. For example, the serving size of ice cream increased to 2/3 cup from 1/3 cup, and the serving size for yogurt decreased to 6 oz from 8 oz (FDA 2016c).
-
Dual Column Labels. Food packages with multiple servings that could be consumed in one or multiple sittings will require both “per serving” and “per package” calorie and nutrient information. The dual column label is intended to help consumers easily understand how many calories and nutrients they are consuming if they eat or drink the entire package/unit in one sitting. Examples include a pint of ice cream and a 3 oz bag of chips (FDA 2016c).
-
For food packages that contain between one and two servings, the calories and other nutrients will be required to be labeled as one serving because it is typically consumed in one sitting. An example of such a product is a 20 oz bottle of soda (FDA 2016c).
Refined Label Design
In addition to the changes described thus far, the label design has been refined. The iconic look of the label will remain, with some refinements in the design to increase the visibility of essential information that consumers need to make informed decisions about the foods they eat. These include the following:
-
Increasing the type size for “Calories,” “Servings per container,” and “Serving size” to make them more prominent (FDA 2016b).
-
Bolding the “number of calories” and the “Serving size” (FDA 2016b).
-
An abbreviated footnote to better explain the % DV: “The % Daily Value tells you how much a nutrient in a serving of food contributes to a daily diet. 2,000 calories a day is used for general nutrition advice” (FDA 2016b).
-
The footnote table listing the reference values for certain nutrients for 2,000 and 2,500 calorie diets will not be required (FDA 2016b).
Opportunities and Challenges for Product Reformulation and Innovation
The final rules become effective on July 26, 2016. The compliance date is July 26, 2018 for manufacturers with $10 million or more in annual food sales and July 26, 2019 for manufacturers with less than $10 million in annual food sales (FDA 2016b; FDA 2016c). Between now and then and beyond, the new rules present opportunities and challenges for product reformulation and innovation. The extent of the impact of the new rules will vary across food products and will depend on the products’ nutrient contributions (type and amount of nutrient), recommended portion and frequency of consumption, and consumer demands. For example, manufacturers may consider the use of low- or non-caloric sweeteners in food products that currently contribute significant amounts of added sugars in the diet. However, addition of sweeteners or other components may be objectionable to consumers who may be more inclined to consume foods containing fewer ingredients. The replacement of added sugars with low- or non-caloric sweeteners is unpopular, so there is an opportunity for food companies to reformulate products with other alternatives. The mandatory declaration of vitamin D, which is naturally available in few foods (NIH 2016), offers opportunities for the development of fortified/enriched foods. However, the amount and type of foods to which it can be added is regulated by the FDA (CFR 2016). The voluntary declaration of vitamin C may be an advantage as many manufacturers add more than needed (due to its instability) to comply with the regulations at the point of sale. Reformulation of products containing vitamin C to reflect the increased RDI are not essential unless the product bears a nutrient content claim for vitamin C. Similarly, the changes in the serving sizes offer potential challenges and opportunities. For example, foods that have a smaller serving size and bear a low- or no- fat claim may require reformulation. This may pose formulation challenges in terms of taste and texture as some fat is required for palatability. In addition, these changes will impact nutrient content and health claims, front-of-pack labels, standards of identity, and other regulatory requirements, which the FDA intends to address in the future. The new rules will also impact graphic design, brand identity, packaging, and marketing decisions.
The new rule also imposes written record-keeping requirements to support the declarations of certain nutrients under specified circumstances. For example, current analytical methods cannot distinguish between dietary fiber and nondigestible carbohydrates that do not meet the definition of dietary fiber, added and naturally occurring sugars, various forms of vitamin E, or folate and folic acid. As a result, manufacturers are required to keep written records to verify the declarations of these food components in the labeling of the food. These records are required to be maintained for at least two years after introduction or delivery for introduction of the food into interstate commerce. Additionally, due to the lack of analytical methods that can distinguish between added and naturally occurring sugars in food products, manufacturers are required to keep records to verify the declared amount of added sugars in specific foods, alone or in combination with naturally occurring sugars, where the added sugars are subject to nonenzymatic browning and/or fermentation. These record-keeping requirements will help ensure that the nutrient declarations are accurate, truthful, and not misleading and will assist with enforcing the requirements when necessary (FDA 2016b).
Is the ‘E’ in NLEA (Nutritional Labeling and Education Act) Important?
“For more than 20 years, Americans have relied on the Nutrition Facts label as a leading source of information regarding calories, fat and other nutrients to help them understand more about the foods they eat in a day. The updated label makes improvements to this valuable resource so consumers can make more informed food choices—one of the most important steps a person can take to reduce the risk of heart disease and obesity,” said FDA Commissioner Robert Califf (White House 2016; FDA 2016a).
In 2013, about 48% of consumers said that they used the Nutrition Facts label to help with food decisions; this was down from 64% in 1995 after the labels were added to food packaging (NPD 2014). However, there has been concern that the labels are misleading due in part to small serving sizes. The required changes and redesign of the label may encourage consumers to review the nutritional content of foods before making purchasing and consumption choices.
Hence, consumers’ understanding of “how to read the labels” is crucial for effective use of the labels to help select and consume the foods that meet healthy dietary patterns. Consumer education efforts by government agencies, the food industry, and food and nutrition professionals are needed to enhance consumers’ understanding of the label and to reduce confusion. For example, a larger serving size for some foods (those that are recommended to be consumed in limited amounts and/or occasionally) should not be viewed as a recommendation to eat more. After all, the “E” in the NLEA stands for “education,” which is essential if the new Nutrition Facts label is to serve as a valuable resource for consumers in order to help them make informed choices about the foods and beverages they consume.
Concluding Thoughts
The release of the new Nutrition Facts label is a great opportunity for both food reformulation and innovation and nutrition education. The new Nutrition Facts label is based on scientific research conducted in the past 20 years, particularly on new evidence on the link between nutrition and diet-related chronic diseases. The label changes, especially the declaration of added sugar and the changes in serving sizes, provide many opportunities for the food industry to be innovative and creative with new and existing food products. Thus, despite the challenges for manufacturers, the new rules could act as an incentive to provide more healthy food choices to consumers who are concerned about diet and long-term health, to help manufacturers differentiate their products from competitors’ products, and to communicate and educate consumers on the nutritional and health benefits of their products.
Farida Mohamedshah is Director, Food, Health and Nutrition, IFT, Washington, D.C. ([email protected]). Cathy Davies, PhD, Food Science and Nutrition, Education and Advocacy, Co-chair, IFT Nutrition Division ([email protected])
References
CFR. 2010. Nutrition labeling of food. Code of Federal Regulations. 21CFR101.9.
CFR. 2016. Vitamin D. Code of Federal Regulations. 21CFR184.1950.
FDA. 2016a. “FDA modernizes Nutrition Facts label for packaged foods.” Press release, May 20. U.S. Food and Drug Administration, Washington, D.C. fda.gov.
FDA. 2016b. Food labeling: Revision of the Nutrition and Supplement Facts labels. U.S. Food and Drug Administration, Fed. Reg. 81: 33742–33999.
FDA. 2016c. Food Labeling: Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments. U.S. Food and Drug Administration, Fed. Reg. 81: 34000–34047.
HHS/USDA. 2016. Dietary Guidelines for Americans 2015-2020. U.S. Dept. of Health and Human Services/U.S. Dept. of Agriculture, Washington, D.C. health.gov.
NIH. 2016. Vitamin D. National Institutes of Health, Bethesda, Md. nih.gov. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/.
NPD. 2014. “U.S. Consumers’ Interest in Reading Nutrition Facts Labels Wanes as Time Goes On, Reports NPD.” Press release, Feb. 27. The NPD Group, Port Washington, N.Y.
White House. 2016. “The White House and FDA Announce Modernized Nutrition Facts Label.” Press release, May 20. The White House, Office of the First Lady, Washington, D.C. whitehouse.gov.