Functional Foods: Opportunities & Challenges
New IFT Expert Panel report provides a comprehensive review of functional foods and a seven-step process for designing, developing, and marketing them. Here’s a summary.
While food has long been used to improve health, our knowledge of health is now being used to improve food. Strictly speaking, all food is functional, in that it provides energy and nutrients necessary for survival. But the term “functional food” in use today conveys health benefits that extend far beyond mere survival. Food and nutrition science has moved from identifying and correcting nutritional deficiencies to designing foods that promote optimal health and reduce the risk of disease.
The costly and complex process of translating scientific advances and nutritional innovations into consumer products is not without pitfalls. Sound science must underlie the development, marketing, and regulation of these new functional foods to protect and inform consumers. Regulatory oversight must ensure the safety and efficacy of products and the accuracy of their marketing claims.
To advance the scientific perspective on these issues, the Institute of Food Technologists convened a panel of internationally renowned experts to review the science related to functional foods and the regulatory environment for developing and marketing such products. The panel members worked tirelessly through an initial meeting, scores of conference calls, and innumerable e-mail exchanges.
The resulting IFT Expert Report, “The Promise of Functional Foods: Opportunities and Challenges,” contains insight from the extensive deliberations of this multidisciplinary panel. As such, it joins two previous IFT Expert Reports—”Emerging Microbiological Food Safety Issues: Implications for Control in the 21st Century” (Arthur, 2002) and “Biotechnology and Foods” (IFT, 2000)—and an IFT Authoritative Report, “Managing Food Safety: Use of Performance Standards and Other Criteria in Food Inspection Systems” (IFT, 2004).
As our nonprofit scientific society, IFT is committed to becoming the indispensable resource for information on food science and technology, and these authoritative IFT documents are central to achieving this goal. The in-depth analysis provided on timely issues such as functional foods provides valuable information for IFT members in their professional activities. The IFT Office of Science, Communications, and Government Relations coordinates the development of these publications as part of its mission to promote regulatory policies that are based on sound science. This new Expert Report will be the subject of numerous outreach efforts to legislators, regulators, and the news media.
The Expert Report provides a comprehensive review of functional foods that emphasizes their importance, summarizes the applicable United States regulations, and presents scientifically based guidance for demonstrating both safety and efficacy. The report recommends approaches for improving the regulatory framework to better address evolving science and food composition. It also identifies potential incentives to expand the availability of new products and facilitate consumer understanding of the benefits of functional foods.
--- PAGE BREAK ---
Defining Functional Foods
The first step in a comprehensive review of functional foods is to define what exactly is included. The term “functional food,” although arbitrary, is nonetheless useful to convey to consumers the unique characteristics of the food and its associated health benefits. The Expert Report defines functional foods as foods and food components that provide a health benefit beyond basic nutrition (for the intended population). Examples may include conventional foods; fortified, enriched, or enhanced foods; and dietary supplements. Functional foods provide essential nutrients beyond quantities necessary for normal maintenance, growth, and development, and/or provide other biologically active components that impart health benefits or desirable physiological effects.
When defining functional foods, a word about dietary supplements is necessary. Some current legal standards classify dietary supplements separately from whole foods and apply different requirements for benefit claims and supporting scientific documentation. The panel considered this legal distinction and decided that, from a scientific perspective, dietary supplements should be included in the definition of functional foods. Supplements merely constitute a different delivery vehicle for a bioactive component, and therefore the scientific demonstration of efficacy and safety remains the same.
Research is beginning to provide clear evidence of relationships between dietary components and health benefits, and consumers have enthusiastically embraced the evolving role of food in health. Appropriately defining functional foods is the first step in providing consumers with accurate, non-misleading information about the health benefits of these foods.
Applying Scientific Advances
Creating a scientifically valid distinction between food and medicine has never been easy. Centuries ago, Hippocrates advised, “Let food be thy medicine and medicine be thy food.” Early nutrition research focused on establishing the necessary intake levels for vitamins and minerals, resulting in cures for numerous widespread deficiency-based diseases. Recent scientific advances have further blurred the line between food and medicine, as scientists identify bioactive food components that can reduce the risk of chronic disease, improve quality of life, and promote proper growth and development.
Traditional definitions of and divisions between food and medicine should not restrict consumer access to knowledge about the benefits of functional foods. Likewise, the framework for strong regulatory oversight should not present unnecessary barriers to the development and marketing of functional foods. Where existing terminology and regulatory frameworks are inadequate to address the full scope of benefits and opportunities for promoting health through functional foods, the terminology and frameworks must be modified.
Research currently underway at academic, industry, and government facilities will reveal how a myriad of substances can be used as functional food components. In some cases, advances are as simple as better understanding the role and optimal levels of traditional nutrients, especially for specific subpopulations. Research on bioactive components known to have health benefits (e.g., omega-3 fatty acids) may demonstrate additional benefits beyond those currently identified. And the vast range of potentially bioactive substances remain to be catalogued and linked to health outcomes.
Our understanding of human dietary requirements results from developments in many scientific disciplines, including food science, nutrition, chemistry, biochemistry, physiology, and genetics. New research in proteomics, nutrigenomics, metabolomics, and other disciplines is helping to identify the biological basis by which food components promote health and wellness. Continuing and accelerating this research will uncover the effects of nutrients on the molecular-level processes in the body and document the variable effects of nutrients under different conditions.
--- PAGE BREAK ---
Shifting the Health Care Paradigm
“An apple a day keeps the doctor away” could perhaps be considered the first functional food advertisement. Functional foods offer opportunities to reduce disease risk and promote wellness with minimal health professional involvement.
A growing number of consumers perceive the ability to control their health by improving their present health and/or hedging against aging and future disease. These consumers create a demand for food products with enhanced characteristics and associated health benefits. In one study (IFIC, 2002), 93% of consumers believed that certain foods have health benefits that may reduce the risk of disease or other health concerns. In addition, 85% expressed interest in learning more about the health benefits offered by functional foods. The combination of consumer interest, advances in food technology, and new evidence-based science linking diet to disease and disease prevention provides an unprecedented opportunity to improve public health.
A new self-care paradigm, adapted from Clydesdale (1998), recognizes that foods can provide health benefits that can coexist with traditional medical approaches to disease treatment. Science has clearly demonstrated additional dietary roles in reducing disease risk, and consumers have learned that food has a greater impact on health than previously known. At the same time, consumers recognize problems with the current health care system, perceiving that it is often expensive, time-constrained, and impersonal.
Functional foods fit into a continuum that ranges from health maintenance/promotion to disease treatment (Fig. 1). On one end of the continuum are public health programs aimed at reducing disease risk in a large segment of the population through self-directed lifestyle changes. On the other end of the continuum is individualized treatment of disease by health care professionals, using drugs and other medical interventions.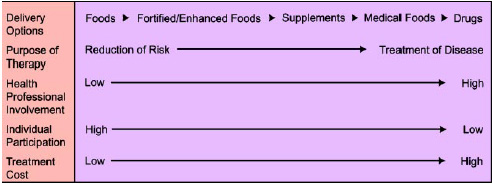
Our health care system has a role for all these treatment options. Functional foods should be integral components of established health programs to reduce the risk of specific diseases while enhancing consumer control and minimizing cost.
Understanding the Interaction of Food and Genes
The insight gained from genetic research provides opportunities to prevent disease and improve quality of life through functional foods and tailored diets. Discoveries in genetics make it possible to understand the effects of nutrients in processes at the molecular level in the body and also the variable effects of dietary components on each individual.
Nutrigenomics, proteomics, and metabolomics are three new disciplines that will contribute to the rapid development of functional foods.
Nutrigenomics is defined as the interaction of dietary components with genes. The dietary components of interest can be essential nutrients (e.g., vitamins, minerals, fatty acids), other bioactive substances (e.g., phytochemicals), or metabolites of food components (e.g., retinoic acid, eicosanoids).
--- PAGE BREAK ---
Proteomics is the study of the full set of proteins encoded and expressed by a genome. Proteomics identifies the large number of proteins in the organism, maps their interactions, and analyzes the proteins’ biologic activities.
Metabolomics (or metabonomics) is metabolite profiling, measuring the real outcome of the potential changes suggested by genomics and proteomics. Metabolomics investigates regulation and metabolic fluxes in individual cells or cell types.
At a simplistic level, nutrigenomics describes how dietary components affect the protein profile of an individual; proteomics describes how that altered protein profile affects the biological systems of the individual; and metabolomics describes the cellular response to the changes.
Bioinformatics is a new tool that uses computer database technology to integrate data from multiple, and sometimes disparate, disciplines.
Already these disciplines and tools have improved our understanding of food science and human nutrition.
Now that the human genome has been catalogued, the race is on to determine the functional significance of each gene, understand the complex functional networks and control mechanisms, and figure out the role that genotype and environment play in determining the physical characteristics of an individual (i.e., his or her phenotype). Functional studies to date have largely evaluated one gene at a time. However, to truly understand the biology of processes directed by genes, researchers need to simultaneously study functional interactions, networks, and pathways. With enough data and proper bioinformatics, scientists will be able to model the genetic circuitry to identify interventions that can optimize biological outcomes through health and wellness lifestyle choices such as diet.
Research has shown that nutrients affect gene expression and formation of various proteins at discrete points in the processes that lead to enzymes, structural proteins, and other chemicals on which life depends. Thus, the amount, and even the form, of nutrients present during gene expression can affect the manufacture of protein, resulting in less of a protein being produced, production of a less than optimally functional form, or no protein at all. Each of those possibilities exists as a result of the hereditary form of genes present and whether the genes are normal or contain polymorphisms that affect gene expression.
The challenges facing nutrigenomics are similar to those encountered in drug development. Many common diseases are not caused by a genetic variation within a single gene. Instead, diseases are caused by complex interactions among multiple genes, in conjunction with environmental and lifestyle factors. Although both environmental and lifestyle factors contribute tremendously to disease risk, their effect is currently difficult to measure and evaluate.
Genetic factors may confer susceptibility or resistance to a disease and may determine the severity or progression of disease. Since we do not yet know all of the factors involved in these intricate pathways, researchers have found it difficult to develop screening tests for most diseases and disorders. By studying stretches of DNA that have been found to harbor a genetic variation associated with a disease trait, researchers may begin to find relevant genes associated with a disease and variable response to dietary components. Defining and understanding the role of genetic factors in disease also will allow researchers to better evaluate the role that non-genetic factors—such as behavior, diet, lifestyle, and physical activity—have on disease.
--- PAGE BREAK ---
Regulating Health Claims
In the U.S., statutes and regulations have not been implemented specifically for functional foods, although as noted earlier, dietary supplements are considered distinct from functional foods in some portions of the regulatory structure. Functional foods are addressed through regulation of the claims about their health benefits.
A full review of the legal and scientific standards for functional food health claims is beyond the scope of this article. Nonetheless, a few key concepts are discussed below. For more-detailed information, interested parties should rely on the extensive discussion in the Expert Report.
Regardless of the type of claim, all food labeling statements must be truthful and not misleading in any particular, as required by the Federal Food, Drug and Cosmetic Act.
In general, any claim that a food cures, mitigates, treats, or prevents a disease would subject the food to regulation as a drug. However, the Nutrition Labeling and Education Act of 1990 authorized the Food and Drug Administration to allow certain disease-risk-reduction claims, known as health claims, to appear in food labeling. Health claims approved to date have generally been statements to the effect that inclusion of a substance in the diet on a regular basis “may help to reduce the risk “ of a named disease.
FDA has evaluated health claims using a standard of significant scientific agreement (SSA): the conclusion that a sufficient body of sound, relevant scientific evidence shows consistency across different studies and among different researchers. According to the agency, “although SSA is not consensus in the sense of unanimity, it represents considerably more than an initial body of emerging evidence” (FDA, 1999). When SSA has been achieved, qualified experts would agree that the evidence supports the substance–disease relationship and the validity of the relationship is not likely to be reversed by new and evolving science, although the exact nature of the relationship may need to be refined.
In December 2002, FDA announced that it would consider allowing “qualified health claims” that would be evaluated using a weight of the scientific evidence (WOSE) standard. The WOSE standard is less stringent than the SSA standard. These qualified health claims would not be formally codified as health claims, but would be allowed under “enforcement discretion” (FDA, 2002). The agency has published interim guidelines that will be used until the formal rulemaking is complete (FDA, 2003).
Different levels of scientific evidence would trigger qualifying language that describes the evidence supporting the claim. The agency has developed an evidence-ranking system that is used to assign a final rank to the evidence in support of the claim. The first-level scientific ranking is SSA, used for health claims; claims that meet this standard do not require any clarifying language. The second level may require a disclaimer—appropriate qualifying language—such as “Although there is scientific evidence supporting the claim, the evidence is not conclusive.” The third-level disclaimer may read “Some scientific evidence suggests . . . However, FDA has determined that this evidence is limited and not conclusive.” The fourth-level disclaimer notes that “Very limited and preliminary scientific research suggests . . . FDA concludes that there is little scientific evidence supporting this claim.”
--- PAGE BREAK ---
Another type of claim that may apply to functional foods (including dietary supplements) is known as a structure/function claim. The current requirements for these claims differ slightly for foods vs dietary supplements, but in general, a structure/function claim describes the dietary impact of the substance on the structure or function of the human body. Structure/function claims may not claim to treat a disease or its symptoms, and they must not trigger some other requirement for FDA preclearance (e.g., the claim could be regarded as a health claim). Examples of structure/function claims include “Calcium helps build strong bones” and “Protein helps build strong muscles.”
The Expert Panel took issue with the restrictive nature of structure/function claims, noting that “sometimes compliance with the regulations results in misleading (if not outright false) statements of the underlying science.” To avoid drug classification, some claims may not accurately convey the actual effects of the food and may confuse consumers. For example, a claim that a food lowers cholesterol would be considered a drug claim because it implies abnormal cholesterol levels. Thus, functional foods that affect cholesterol levels state that the food “maintains normal cholesterol levels,” which is a permissible structure/function claim. However, such a statement is potentially misleading if the food in fact lowers cholesterol levels.
The Expert Panel recommends that product labeling and health claims should be allowed to accurately reflect the scientific evidence. As long as claims are scientifically valid, enormous public health benefits would result from having consumers understand and act on the claimed product benefit.
Another area of current controversy is FDA’s policy regarding nutritive value, which requires that the health benefit of a food component be derived from its nutritive value. FDA states, in 21 CFR 101.14(a)(3), that “nutritive value means a value in sustaining human existence by such processes as promoting growth, replacing loss of essential nutrients, or providing energy.” There is no consensus on the meaning of this definition, and conflicts exist between legislation, regulations, and other agency documents.
The Expert Panel recommends that the benefits for functional foods be based on nutritive value or through the provision of a physical or physiological effect that has been scientifically documented or for which a substantial body of evidence exists for a plausible mechanism.
Bringing Functional Foods to Market
The Expert Panel identified a seven-step process that addresses critical aspects in the design, development, and marketing of functional foods (Fig. 2). Although all seven steps will be undertaken for each new bioactive substance and the resulting functional foods, the specific requirements within each step will vary depending on the physical, chemical, and biological characteristics of the functional component, the applicable regulatory requirements, and the health claims to be made.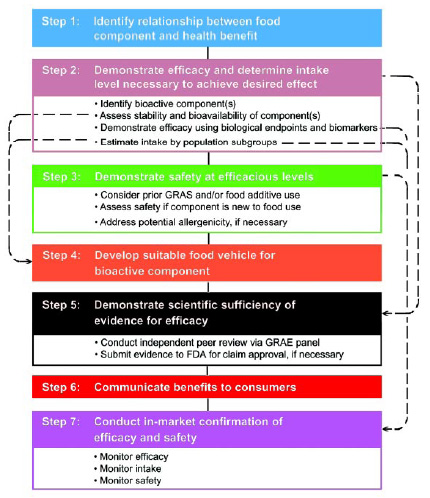
--- PAGE BREAK ---
1. Identify the relationship between the food component and the health benefit. The future success of functional foods relies on establishing a sound scientific basis for the relationship between a functional food and the claimed health benefit(s). A wealth of scientific literature describes the types of research that can be used to identify potential relationships between functional components and health benefits.
2. Demonstrate efficacy and determine the intake level necessary to achieve the desired effect. Demonstrating the efficacy of the bioactive components is critical in building a strong scientific basis for claims related to the intake of a functional food. Unfortunately, it is not an easy task.
First, researchers attempt to identify the bioactive components that result in the health benefit observed in step 1. In some cases, the bioactive component may be unidentified or partially identified. For example, the component may be part of a group of substances (e.g., terpene or alkaloid group), but the specific component may not be identified. Sometimes the chemical identity is unknown, and a defined surrogate compound is used to establish efficacy.
Next, the stability and bioavailablity of the components must be demonstrated. This analysis includes the physical and chemical form of the food component, the effects of the total diet, the effects of food processing, and the effects of environmental factors during crop production.
Stable and bioavailable components also must produce the identified health benefit. Reliable measures of the effects of bioactive components in functional foods are critical. In some cases, it is possible to measure the health or disease-prevention endpoint directly. However, it is often necessary to identify a biomarker that serves as a reliable surrogate for the underlying biological effects.
To establish a credible health claim, the evidence of efficacy must be critically evaluated for adequacy. The strength and relevance of the data supporting the bioactive component’s specific role in improving the health outcome of interest must be explicitly addressed. The Expert Panel believes that Hill’s criteria (Hill, 1971; Keystone, 1996) provide an effective approach to this evaluation:
Strength of association. How statistically significant and convincing are the data that support the relationship?
Consistency of the observed association. How well do the available data from different sources, areas, and types of studies support the relationship?
Specificity of the association. Do the data demonstrate a predictable relationship between the bioactive component and the proposed effect?
Temporal relationship of the observed association. Is the proposed effect observed following treatment with the bioactive component?
--- PAGE BREAK ---
Dose–response relationship. Do the data demonstrate a magnified effect of the bioactive component with increasing dose?
Biological plausibility. Is there a plausible mechanism to explain the effects of the bioactive component?
Coherence of the evidence. Does the relationship help explain the available data, when viewed as a whole?
In applying each of the Hill criteria to the research findings, it is also necessary to consider the amount and type of evidence, the quality of the evidence, the totality of the evidence, and the relevance of the evidence to the specific claim.
To receive a health benefit from consuming a functional food, it must be consumed in adequate quantities to achieve the desired effect. Dietary intake assessments must address intake levels for the targeted population, potential high- and low-intake consumers, and population subgroups with special risks or benefits.
3. Demonstrate safety at efficacious levels. Functional components must be determined to be safe at their projected use levels, using an objective, science-based evaluation process. The assessment of safety should be based on the long-standing principle that foods are safe. As such, the prior safe use of the component should play an important role in the demonstration of safety. Substances new to food use must undergo a safety assessment, including the potential for allergenicity.
4. Develop a suitable food vehicle for the bioactive component. The food vehicle must be appropriate for the intended consumer and deliver the bioactive component at the desired compliance levels. The food vehicle must be consumed at the level necessary to achieve the benefit but not consumed at amounts so great as to be toxic. In addition, the selection of a vehicle depends on the stability and bioavailability of the bioactive components in that particular food.
5. Demonstrate sufficiency of the scientific evidence for efficacy. The Expert Panel believes that the evaluation of efficacy will be most effective and cost-efficient if undertaken by panels of independent scientists with appropriate expertise. This approach has been successfully applied to Generally Recognized as Safe (GRAS) determinations for many substances. A parallel process should be used to establish efficacy by conducting a review of the evidence demonstrating efficacy. The process could be called a Generally Recognized as Efficacious (GRAE) determination.
A GRAE determination would achieve public confidence while conserving government resources. The Expert Panel believes that FDA should establish a procedure for GRAE notification similar to that used for GRAS substances.
--- PAGE BREAK ---
6. Communicate benefits to consumers. The results of steps 1–5 should form the basis of the messages used to inform consumers of the relationship between the consumption of the functional food and its intended benefits. The health claims should be accurate and not misleading. The International Food Information Council Foundation guidelines (IFIC, 2004; Reinhardt, 2004) provide information about how to appropriately communicate scientific information to consumers.
7. Conduct in-market confirmation of efficacy and safety. Once the functional food is on the market, its manufacturer should monitor the actual consumption patterns and, when possible, the resulting health benefit. In addition, information about any adverse effects should be collected.
Appreciating the Role of Research
The vast potential for functional foods will not be achieved without extensive scientific research to ensure the safety and efficacy of these products.
Basic and applied research is necessary to identify the entire range of potential functional foods, from new roles for traditional nutrients to currently unidentified bioactive components to nutrigenomics applications. Improved knowledge of biomarkers is essential to understanding and documenting the effects of these functional foods.
Privacy of genetic information and the limitations of decisions that can be made based on knowledge of an individual’s genetic profile have been debated within the context of pharmaceutical applications. The Expert Panel supports efforts to develop a legal, ethical, and societal framework to address the availability and use of nutritional genomic data.
Appropriate incentives to the food industry would greatly enhance the development of functional foods. Food companies have traditionally funded research for new food product formulations, but for functional foods the stakes are higher—for both the food companies and consumers. The research required for a functional food to meet scientific standards for efficacy is a substantial investment, but the return on that investment is not exclusive to that company. As soon as the health claim is adequately documented, competing companies can use the claim. Incentives such as a period of exclusivity or tax incentives would encourage food companies to pursue functional food development as a profitable venture.
--- PAGE BREAK ---
Moving Forward
The Expert Panel identified several areas where changes are needed to further encourage the development of functional foods. The following recommendations are particularly critical:
• Modify the current definition and application of the term “nutritive value.”
• Expand basic and applied nutritional research into known nutrients, bioactive food components, and the intersection of genomics and molecular nutrition.
• Expand research on biomarkers and physiological endpoints.
• Use GRAE panels to evaluate health claims and streamline the regulatory approval process.
• Allow health claims based on significant scientific agreement and qualified health claims based on the weight of the scientific evidence. Do not allow claims when the scientific support is preliminary or very limited.• Allow product labeling and health claims to accurately reflect the scientific data without triggering drug status.
• Develop incentives for companies to invest in functional foods research and development.
The future development of functional foods will require contributions from basic and applied scientists in academia, government, and industry. Consumers want and need these products, and mechanisms must be found to foster their availability.
How to Obtain the Report
The full report, The Promise of Functional Foods: Opportunities and Challenges,” will be available in the near future on IFT’s Web site at www.ift.org.
--- PAGE BREAK ---
IFT Expert Panel on Functional Foods
Fergus Clydesdale, Ph.D., (Chair), Distinguished Professor and Department Head, Dept. of Food Science, University of Massachusetts, Amherst.
Wayne R. Bidlack, Ph.D., Dean, College of Agriculture, California State Polytechnic University, Pomona
Diane F. Birt, Ph.D., Distinguished Professor, Dept. of Food Science and Human Nutrition; Director, Iowa Center for Research on Botanical Dietary Supplements, Iowa State University, Ames.
Bruce R. Bistrian, M.D., Ph.D, Professor of Medicine, Harvard Medical School, Boston, Mass.
Joseph F. Borzelleca, Ph.D., Professor Emeritus, Dept. of Pharmacology and Toxicology, Medical College of Virginia/Virginia Commonwealth University, Richmond
Roger A. Clemens, Dr.P.H., Director, Laboratory for Analytical Research and Services in Complementary Therapeutics; Associate Director, Regulatory Science; Adjunct Professor, Dept. of Molecular Pharmacology and Toxicology, University of Southern California School of Pharmacy, Los Angeles
Mark L. Dreher, Ph.D., Vice President, Research and Development, McNeil Nutritionals, LLC, a Johnson & Johnson company, New Brunswick, N.J.
John W. Erdman Jr., Ph.D., Professor, Dept. of Food Science and Human Nutrition, University of Illinois, Urbana
Nancy Fogg-Johnson, Ph.D., Principal, Life Sciences Alliance/Technology & Business Ventures, Inc., Villanova, Pa.
Loren Israelsen, J.D., President, LDI Group, Inc., Salt Lake City, Utah Marge Leahy, Ph.D., Senior Manager of Health and Nutrition, Ocean
Marge Leahy, Ph.D., Senior Manager of Health and Nutrition, Ocean Spray Cranberries, Inc., Lakeville/Middleboro, Mass.
Gilbert A. Leveille, Ph.D., Senior Consultant, Cargill, Inc.,Wayzata, Minn.
Diane B. McColl, Esq., Hyman, Phelps, and McNamara, Washington, D.C.
Stephen H. McNamara, Esq., Hyman, Phelps, and McNamara, Washington, D.C.
Kenneth C. Mercurio, Director of Regulatory and Nutrition, Nestlé USA, Inc., Glendale, Calif.
John A. Milner, Ph.D., Chief, Nutrition Science Research Group, Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, Rockville, Md.
Shridhar K. Sathe, Ph.D., Professor, Dept. of Nutrition, Food, and Exercise Sciences, Florida State University, Tallahassee
John E. Vanderveen, Ph.D., Scientist Emeritus, Center for Food Safety and Nutrition, Food and Drug Administration, San Antonio, Tex.
by Fergus Clydesdale, Ph.D.
The author, Chair of the IFT Expert Panel on Functional Foods, is Distinguished Professor and Head, Dept. of Food Science, University of Massachusetts, 100 Holdsworth Way, Amherst, MA 01003 ([email protected]).
References
Arthur, M.H. 2002. Emerging microbiological food safety issues: Implications for control in the 21st century. Food Technol. 56(2): 48-51.
Clydesdale, F.M. 1998. Science, education and technology: New frontiers for health. Crit. Rev Food Sci. Nutr. 38: 397-419.
FDA. 1999. Guidance for industry: Significant scientific agreement in the review of health claims for conventional foods and dietary supplements. Dec. 22. Office of Special Nutritionals, Center for Food Safety and Applied Nutrition, Food and Drug Admin., Washington, D.C. www.cfsan.fda.gov/~dms/ssaguide.html.
FDA. 2002. Guidance for industry: Qualified health claims in the labeling of conventional foods and dietary supplements. Dec. 18. Office of Nutritional Products, Labeling, and Dietary Supplements, Center for Food Safety and Applied Nutrition, Food and Drug Admin., Washington, D.C. www.cfsan.fda.gov/~dms/hclmgui2.html.
FDA. 2003. Guidance for industry and FDA: Interim procedures for qualified health claims in the labeling of conventional human food and human dietary supplements. July 10. Center for Food Safety and Applied Nutrition, Food and Drug Admin., Washington, D.C. www.cfsan.fda.gov/~dms/hclmgui3.html.
Hill, A.B. 1971. Statistical evidence and inference. In “Principles of Medical Statistics,” 9th ed., pp. 309-323. Oxford University Press, New York.
IFIC. 2002. Functional foods attitudinal research. Aug. Intl. Food Information Council, Washington, D.C. http://ific.org/research/funcfoodsres02.cfm.
IFIC. 2004. Guidelines for communicating the emerging science of dietary components for health. Intl. Food Information Council, Washington, D.C. www.ific.org/nutrition/functional/guidelines.
IFT. 2000. IFT expert report on biotechnology and foods. Inst. of Food Technologists, Chicago, Ill. www.ift.org/pdfs/biotech/report.pdf.
IFT. 2004. Managing food safety: Use of performance standards and other criteria in food inspection systems. Inst. of Food Technologists, Chicago, Ill. www.ift.org/pdfs/scitech/managing_food_safety.pdf.
Keystone. 1996. Keystone national policy dialogue on food, nutrition and health. Final rept. Keystone Center, Keystone, Colo., and Washington, D.C.
Reinhardt, W. 2004. Communicating the benefits of functional foods. Food Technol. 58(12):100.
