Battling Obesity with Resistant Starch
The latest human research details ways in which less-digestible forms of starch may deliver important weight management benefits.
If everyone in the United States simply adopted the U.S. Dietary Guidelines of 2005 (http://www.cnpp.usda.gov/GAs2005Guidelines.htm), obesity would be on its way out. Exercising 30–90 minutes a day, eating at least half of our grains as whole grains, and eating four servings of fruits and five servings of vegetables a day are simple practices that are advised because of their ability to improve energy balance, keeping us from gaining weight and helping us to lose weight if we need to.
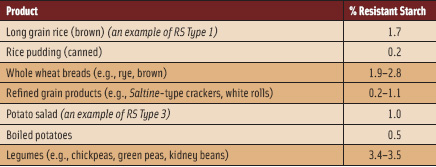
 How do whole grains and fruits and vegetables do this? They provide greater volume per calories than do diets that contain less of these foods, which helps us feel satisfied longer on fewer total calories, thus permitting weight loss and preventing weight gain (Rolls, 2009). What food components contribute to this effect? In part, the answer is resistant starch (RS). Whole grains and fruits and vegetables have plant cell wall materials that include dietary fibers, which interfere with our bodies’ ability to digest the starches that are the main energy source from these foods. These foods represent one form of resistant starch, starch that is physically inaccessible to human α-amylase, the enzyme responsible for starch digestion, because the vegetable and whole grain starch is surrounded by plant cell wall materials in the whole food or whole grain form. (See Table 1; in Table 2, note the example of brown rice vs rice pudding.)
How do whole grains and fruits and vegetables do this? They provide greater volume per calories than do diets that contain less of these foods, which helps us feel satisfied longer on fewer total calories, thus permitting weight loss and preventing weight gain (Rolls, 2009). What food components contribute to this effect? In part, the answer is resistant starch (RS). Whole grains and fruits and vegetables have plant cell wall materials that include dietary fibers, which interfere with our bodies’ ability to digest the starches that are the main energy source from these foods. These foods represent one form of resistant starch, starch that is physically inaccessible to human α-amylase, the enzyme responsible for starch digestion, because the vegetable and whole grain starch is surrounded by plant cell wall materials in the whole food or whole grain form. (See Table 1; in Table 2, note the example of brown rice vs rice pudding.)
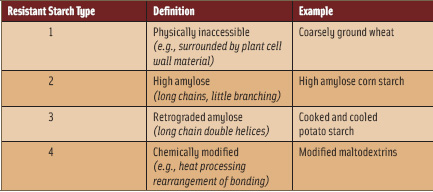
What do sushi rice and potato salad have in common? The starches in these foods, examples of RS Type 3 (Table 1), are less easily digested than hot steamed rice or boiled or mashed potatoes. (Note the difference between potato salad and boiled potatoes in Table 2.) This is due to less efficiency of α-amylase interacting with the long chains of amylose formed in the cooling and retrogradation of starch in these foods. Likewise, the development of a commercially available high-amylose corn starch gives consumers another option for eating more resistant starch. Various chemically modified resistant starches are under development, and some are in current use as food ingredients, such as resistant maltodextrins, which may be formed by heat processing, causing bond rearrangements that limit digestibility. How does eating less-digestible, i.e., digestion-resistant (a.k.a. resistant) starch benefit health?
Dietary Benefits of Resistant Starch
Let’s consider the case of someone changing their diet from refined to whole grains. Such a change would increase intake of resistant starch by roughly three-fold (Englyst et al., 2007), from ~0.7% to ~2.4% of carbohydrate intake. Thus, someone eating eight servings of bread/grain products per day, 532 kcal and 100 g carbohydrate (99.3 g digestible, based on 0.7 % RS) from white bread vs 492 kcal and 92 g carbohydrate (89.8 g digestible, based on 2.4% RS) from whole wheat bread would decrease their calorie intake from carbohydrates by about 40 kcal per day (99.7 g – 89.8 g = 9.9 g carbohydrates x 4 kcal/g (Axxya, 2008). This is the equivalent of ~4 lbs of fat per year (1 lb of fat = 3,500 kcal, ~350 days/year x 40 kcal/day = 14,000 kcal, or 4 lb of fat).
--- PAGE BREAK ---
This is a modest effect, but certainly helpful in the “battle of the bulge.” Resistant starch intakes in the U.S. were estimated at 4.9 g per person per day, based on 1999–2002 National Health and Nutrition Examination Surveys (Murphy et al., 2008), so this intake could certainly be increased to benefit health. Increased intake of legumes may be a particularly effective approach to increasing dietary RS (Table 2).
Human intake studies have demonstrated that incorporating RS into the diet may deliver important health benefits. One of the main potential applications is in the creation of RS-containing foods for diabetics. Numerous studies have shown that RS decreases blood glucose response compared with a standard glucose meal or glucose beverage. Twelve healthy people who drank 50 g of a retrograded maltodextrin showed a 59% reduction in blood glucose and a 35% reduction in insulin response compared with a control glucose beverage (Brouns et al., 2007). Decreased blood glucose and insulin responses would limit adverse effects of hyperglycemia and hyperinsulinemia in diabetics. Healthy overweight subjects (>120% of ideal weight) who consumed a resistant corn starch with 27% amylose (40 g/day in a liquid supplement for 21 days compared with the same amount of conventional cornstarch) showed a 15% lower fasting blood glucose and a lower blood LDL cholesterol compared with baseline, changes not seen in the control group (Park et al., 2004). Ten normal weight and 10 obese women, all healthy, who ate muffins containing 5 g RS from high-amylose cornstarch showed decreased blood glucose and insulin responses compared with results when they consumed muffins containing conventional cornstarch (Behall et al., 2006). These studies suggest that overweight individuals, who are at greater risk for type 2 diabetes than lean individuals, would be protected from adverse effects of diabetes by RS, which could be incorporated into various foods. Twelve untreated borderline diabetics with fasting blood glucose of 100–140 mg/dL who ate bread containing 6 g RS from tapioca showed significantly decreased blood glucose and insulin responses compared with eating non-RS bread (Yamada et al., 2005). This demonstrated benefits of RS in pre-diabetics.
Resistant Starch and Satiety
In addition to direct effects of RS to lessen glucose and related insulin response, its ability to affect satiety responses has been investigated. Twenty healthy people who ate ~9 g of RS in a muffin reported greater satiety (using a visual analog scale questionnaire) over 180 min after this meal, compared to the same test group who consumed a non-RS muffin (Willis et al., 2009). The effect of RS may be mediated by its ability to be fermented in the lower gut, which produces short-chain fatty acids. In 15 healthy subjects, an evening meal containing 50 g available carbohydrate with 30 g RS from barley kernels increased satiety score measured after breakfast by questionnaire compared with an evening meal of 50 g available carbohydrate containing only 1.3 g RS (white bread). The barley meal also reduced plasma IL-6, a proinflammatory cytokine, after breakfast, compared with the white bread evening meal (Nilsson et al., 2008). In 14 subjects who ate barley kernels (15% RS) compared with white bread at an evening meal, blood glucose and insulin response to a breakfast of white bread was significantly lessened (Granfeldt, et al. 2006). Ten subjects who ate a breakfast sponge cake high in amylose showed lesser blood glucose response to a standard lunch than did subjects who ate non-RS sponge cake at breakfast (Brighenti et al., 2006). These studies indicate a role for RS fermentation products, which would be formed several hours after ingestion of the RS, and therefore exert their effects at a second meal (i.e., breakfast after an evening meal or lunch after breakfast) to influence satiety, glucose and insulin signaling, and other physiological processes such as inflammation.
The ability of RS to prevent or treat obesity is implied in the studies showing increased satiety after RS intake (Willis et al., 2009; Granfeldt et al., 2006). Studies in humans showing treatment of obesity using products formulated with RS would require several months to years to be considered valid, let alone studies that link RS with obesity prevention. Groups of 107-month-old female Sprague Dawley rats fed a diet with RS showed decreased body fat and increased serum PYY and GLP-1, putative satiety factors produced by the intestine, compared with rats fed conventional cornstarch (Keenan et al., 2006). This study represents an extreme of RS intake, but it is intriguing in its implications that RS might lessen body fat in humans. Thirty overweight humans taking a Phaseolus vulgaris extract that inhibited α-amylase showed significantly greater reductions in body mass index and other measures of body fat compared with similar subjects taking a placebo over 30 days (Celleno et al., 2007). This suggests that inhibiting carbohydrate digestibility, as would occur with RS intake, might be an effective weight reduction strategy.
--- PAGE BREAK ---
Studying Side Effects
Although the benefits of RS are compelling, one might wonder if a food ingredient that is fermented in the lower gut would potentially cause unpleasant side effects of excessive gas, pain, or other gastrointestinal symptoms. This was not observed in one 21-day study of people eating 40 g RS (Park et al., 2004). In several trials of RS food ingredients, my laboratory has not observed increased gastrointestinal symptoms in subjects eating RS compared with conventional starches. For example, we studied three resistant maltodextrins compared with a non-resistant maltodextrin given as beverages in 25 g portions to 20 men as single breakfast meals in a randomized crossover design. Three of 20 subjects fed the non-resistant maltodextrin reported mild gastrointestinal pain and bloating in response to a questionnaire asking about symptoms over a 24-hr period after the test meals. This result was similar to two of the resistant maltodextrins, which showed three of 20 and two of 20 subjects reporting mild adverse gastrointestinal effects. The most highly resistant maltodextrin (least digestible) caused mild gastrointestinal symptoms in six of 20 subjects, suggesting a somewhat greater adverse effect as more carbohydrates were fermented in the lower gut. Grabitske and Slavin (2008, 2009) examined human studies reporting gastrointestinal effects of low-digestible carbohydrates such as inulin and fructooligosaccharides; several but not all studies showed greater flatus and pain, looser stools, and mild to moderate bloating with these carbohydrates. RS needs more study for its possible adverse gastrointestinal effects.
Future directions for food scientists and the food industry with respect to RS include design of new foods that include significant RS content, development of a wider variety of RS-based food ingredients, and much more testing of RS, particularly with respect to the mechanisms of the second meal effect, efficacy in obesity treatment and prevention, and investigation of gastrointestinal side effects. Inter-individual variation in responses to RS should be taken into account in this work. For example, Brouns et al. (2007) showed a range of response to a resistant starch across individual subjects. When consuming a resistant starch, one subject had only 10% less digestibility than for the highly digestible control starch, whereas another subject had 90% less digestibility than for the control starch. What is responsible for such variability? This might prove very important to learn in order to help to optimize health more individually, and as people become more aware of resistant starches and want to try to use these starches to benefit their weight and health. (Results may vary, as many weight loss ads caution!)
Gut microbes may be key to unraveling many of the factors involved in the human body’s response to resistant starches. Turnbaugh et al. (2006) identified differences between the gut microbiomes of lean and obese individuals. Mice got fatter when their guts were colonized with an “obese” microbiota. Thus, our gut microbes might help or hinder our ability to ferment resistant starch residues. The effects of these fermentation products on body weight needs much exploration. Understanding the role of the gut microbiota in responding to RS and mediating RS fermentation is a frontier that is very likely to help in understanding RS, obesity, and inflammatory and satiety signaling. Efforts by food technologists to blend next-generation starches and new probiotic microbes may provide relief for the obesity epidemic.
by Suzanne Hendrich, Ph.D., a Professional Member of IFT, is Professor, Food Science and Human Nutrition, Iowa State University, 220 MacKay Hall, Ames, IA 50011-1123 ([email protected]).
References
Axxya. 2008. Nutrient Composition of Foods, Nutritionist Pro™ Knowledge Base, Axxya Systems, Stafford, Texas. www.axxya.com.
Behall, K.M., Scholfield, D.J., Hallfrisch, J.G., and Liljeberg-Elmståhl, H.G. 2006. Consumption of both resistant starch and beta-glucan improves postprandial plasma glucose and insulin in women. Diabetes Care 29(5): 976-81.
Brighenti, F., Benini, L., Del Rio, D., Casiraghi, C., Pellegrini, N., Scazzina, F., Jenkins, D.J., and Vantini, I. 2006. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am. J. Clin. Nutr. 83(4): 817-22.
Brouns, F., Arrigoni, E., Langkilde, A.M., Verkooijen, I., Fässler, C., Andersson, H., Kettlitz, B., van Nieuwenhoven, M., Philipsson, H., Amado, R. 2007. Physiological and metabolic properties of a digestion-resistant maltodextrin, classified as type 3 retrograded resistant starch. J. Agric. Food Chem. 55(4): 1574-81.
Celleno, L., Tolaini, M.V., D’Amore, A., Perricone, N.V., and Preuss, H.G. 2007. A dietary supplement containing standardized Phaseolus vulgaris extract influences body composition of overweight men and women. Int. J. Med. Sci. 4(1): 45-52.
Englyst, K.N., Liu, S., and Englyst, H.N. 2007. Nutritional characterization and measurement of dietary carbohydrates. Eur. J. Clin. Nutr. 61 (Suppl. 1): S19–S39.
Grabitske, H.A. and Slavin, J.L. 2008. Low-digestible carbohydrates in practice. J. Am. Diet. Assoc. 108(10): 1677-81.
Grabitske, H.A. and Slavin, J.L. 2009. Gastrointestinal effects of low-digestible carbohydrates. Crit. Rev. Food Sci. Nutr. 49(4): 327-60.
Granfeldt, Y., Wu, X., and Björck, I. 2006. Determination of glycaemic index; some methodological aspects related to the analysis of carbohydrate load and characteristics of the previous evening meal. Eur. J. Clin. Nutr. 60(1): 104-12.
Keenan, M.J., Zhou, J., McCutcheon, K.L., Raggio, A.M., Bateman, H.G., Todd, E., Jones, C.K., Tulley, R.T., Melton, S., Martin, R.J., and Hegsted, M. 2006. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity 14(9): 1523-34.
Murphy, M.M., Spungen, D.J., and Birkett, A. 2008. Resistant starch intakes in the United States. J. Am. Diet. Assoc. 108: 67-78.
Nilsson, A.C., Ostman, E.M., Holst, J.J., and Björck, I.M. 2008. Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. J. Nutr. 138(4): 732-9.
Park, O.J., Kang, N.E., Chang, M.J., and Kim, W.K. 2004. Resistant starch supplementation influences blood lipid concentrations and glucose control in overweight subjects. J. Nutr. Sci. Vitaminol. 50(2): 93-9.
Rolls, B.J. 2009. The relationship between dietary energy density and energy intake. Physiol. Behav. 97: 609-615.
Turnbaugh, P.J., Ley, R.E., Mahowald, M.E., Magrini, V., Mardis, E.R., and Gordon, J.I. 2006. An obesityassociated gut microbiome with increased capacity for energy harvest. Nature 444: 1027-31.
USDA. 2005. U.S. Dietary Guidelines. http://www.cnpp.usda.gov/DGAs2005Guidelines.htm.
Willis, H.J., Eldridge, A.L., Beiseigel, J., Thomas, W., and Slavin, J.L. 2009. Greater satiety response with resistant starch and corn bran in human subjects. Nutr. Res. 29(2): 100-5.
Yamada, Y., Hosoya, S., Nishimura, S., Tanaka, T., Kajimoto, Y., Nishimura, A., and Kajimoto, O. 2005. Effect of bread containing resistant starch on postprandial blood glucose levels in humans. Biosci. Biotechnol. Biochem. 69(3): 559-66.
