The Paradox of Organic Ingredients
Are USDA organic regulations governing the use of nonorganic ingredients in organic products a valuable tool for growing organic packaged foods sales or an impossible standard?
Since its launch in 2002, the U.S. Dept. of Agriculture’s National Organic Program (NOP) has continued to evolve in response to an ongoing debate within the organic community regarding how organic foods should be defined and regulated. One of the key issues at the center of the debate is the “National List” of substances that are prohibited or permitted to be used in the production, processing, and handling of organic foods. The list identifies all approved exceptions to the assumption that all methods, contact materials, and ingredients used in the production of organic foods are in fact “organic.” Whether substances should be added to or removed from the National List has been a subject of debate and controversy since the inception of the NOP.
 The final section of the National List, §205.606, specifically defines “nonorganically produced agricultural products allowed as ingredients in or on processed products labeled as ‘organic.’” This section is specifically relevant to the production of multi-ingredient certified-organic foods with at least 95% (but less than 100%) organic contents by weight (excluding salt and water).
The final section of the National List, §205.606, specifically defines “nonorganically produced agricultural products allowed as ingredients in or on processed products labeled as ‘organic.’” This section is specifically relevant to the production of multi-ingredient certified-organic foods with at least 95% (but less than 100%) organic contents by weight (excluding salt and water).
Sales of the >95% segment of organic foods have been a major contributing factor to overall growth of the $22.9 billion U.S. organic food industry (OTA, 2009). Launches of organic versions of a wide range of packaged convenience foods, including prepared meals and snack products, have increased the adoption of organic foods among mainstream consumers. Sales of shelf-stable organic food and drink products grew 41% from 2006 to 2009 (in food, drug, and mass merchandisers, excluding Wal-Mart), and sales are estimated to exceed $3 billion in 2012 (Mintel, 2009). A 2008 study confirmed the growing trend of organic, multi-ingredient products using entropy metrics to show that organic food manufacturers are launching products in a broader range of food categories, which are increasingly eligible for USDA organic certification (Sporleder et al., 2008). Additional research has demonstrated that consumers value increasing levels of organic content in multi-ingredient foods. A survey of 300 traditional and specialty grocery store customers found that as organic content in breakfast cereal was increased, both the share of consumers willing to pay a premium and the average premium consumers were willing to pay increased (Batte et al., 2007). These findings indirectly emphasize the importance of regulations that define the criteria for certified organic foods, including the National List.
The National Organic Standards Board (NOSB), an independent, 15-member board appointed by the Secretary of Agriculture, is responsible for reviewing petitions and proposing amendments to the National List. The NOSB forwards recommendations to the NOP for rule making, guidance, or other action. The original thinking in creating §205.606 was that it would allow a wide range of USDA certified organic products to be quickly developed by removing the potential limitation of a lack of supply of organically produced minor ingredients. The NOSB and NOP assumed that handlers would benefit from a “market incentive” and inclusion in this section would “drive innovation” of organic alternatives (NOP, 2000; NOSB, 2009a). The original language published in the final rule establishing the National List stated: “The following nonorganically produced agricultural products may be used as ingredients in or on processed products labeled as ‘organic’ or ‘made with organic (specified ingredients or food group(s))’ only in accordance with any restrictions specified in this section. Any nonorganically produced agricultural product may be used in accordance with the restrictions specified in this section and when the product is not commercially available in organic form” (NOP, 2000; italics added for emphasis).
Changes to the National List
Five types of ingredients were initially added under this section, including native corn starch; water extracted gums (arabic, guar, locust bean, and carob bean); kelp (for use as a thickener and dietary supplement); lecithin (unbleached); and pectin (high methoxy). The hope was that as the organic food industry grew, demand for these minor ingredients would also grow and organic options would become available. The intent was to limit organic food processors to using only the nonorganically produced ingredients specified in this section (NOP, 2005). However, the NOP defined the term “commercially available” as “the ability to obtain a production input in an appropriate form, quality, or quantity to fulfill an essential function in a system of organic production or handling, as determined by the certifying agent in the course of reviewing the organic plan” (NOP, 2000; italics added for emphasis).
--- PAGE BREAK ---
This definition in combination with the original language in §205.606 led many manufacturers and certifying agents to interpret the provision to mean that any ingredient approved as commercially unavailable by a certifying agent could be used in organic foods as long as the weight of nonorganic ingredients did not exceed 5% of the total weight of the food product (NOP, 2007).
The ambiguity of §205.606 generated much controversy and debate among organic consumers, producers, and ingredient suppliers. Most notably, it was one of the key issues in a two-year-long court case, Harvey v. Veneman. Harvey, an organic farmer, sued the USDA in 2003 for adopting provisions in the final NOP ruling that were inconsistent with the original language in the Organic Food Production Action (OFPA) of 1990 and which Harvey alleged “weaken[ed] the integrity of the organic program and the standards it sets forth.” Harvey contended that the language provided nonorganic products “not commercially available in organic form” with a blanket exemption from the review and recommendation process outlined in OFPA (hereafter also referred to as the Act). Sections 6517 and 6518 of the Act explicitly require that all specific exemptions to the Act’s ban on nonorganic substances undergo a notice and comment period prior to placement on the National List. In light of this evidence, a First District Court ultimately sided with Harvey on the count pertaining to §205.606 (one of nine original counts on which he sued). The USDA issued a Notice in the Federal Register on July 1, 2005, clarifying the meaning of §205.606 and stating that it does not establish a “blanket exemption” to the National List for nonorganic agricultural products that are not commercially available. The clarified language, which became effective on June 9, 2007, follows: “Only the following nonorganically produced agricultural products may be used as ingredients in or on processed products labeled as “organic,” only in accordance with any restrictions specified in this section, and only when the product is not commercially available in organic form” (NOP, 2007).
Ironically, this ruling ultimately led to a major expansion of the National List. Prior to the effective date of the clarified regulation, the NOP received approximately 99 petitions to add more than 600 nonorganically produced agricultural substances to §205.606 of the National List (NOP, 2007). This massive swell in petitions demonstrated the widespread use of nonorganic substances in the processing of organic foods (NOP, 2007).
The final rule added 38 nonorganically produced agricultural ingredients to the National List. Even though the U.S. organic food industry has grown rapidly since the implementation of the NOP, only one substance (rice starch) has been completely removed from §205.606. Petitions to remove ingredients from the National List as well as recent publications from leading organic associations suggest that organic alternatives to at least some ingredients, such as corn starch and lecithin, may have become available in sufficient quantity and quality (Soil Association, 2008; NOSB, 2009b; OTA, 2006). Yet these ingredients continue to be exempted for use in organic foods largely due to claims from manufacturers that supply is still fragile or that organic versions do not provide equal functionality. The NOSB continually struggles to find balance between promoting the widest possible adoption of the USDA organic certification and adhering to the standard of “organic preference” outlined in NOP regulations, which means that organic foods should have the highest possible amount of certified organic ingredients (CFR, 2010).
The following study examines this policy dilemma by determining the extent to which non-organically produced agricultural ingredients are used in organic processed food product introductions using data from a real-time product innovation database. The article concludes with a discussion of policy implications.
Use of Nonorganic Ingredients in Organic Products
A real-time food innovation resource, Mintel’s Global New Product Database (GNPD), was used to analyze all packaged food products with any organic ingredients released for sale in the United States in 2008. The product-level observations in the database were populated using information gathered by a network of field associates referred to as “shoppers” (GNPD, 2009). All key retail distribution channels were monitored by GNPD’s shopper network, including supermarkets, drug stores, natural food stores/health shops, gas stations, convenience stores, and other independent outlets. GNPD also gathers data on product innovations through trade shows, press releases, and company tracking (GNPD, 2009). Types of food innovations in the data set ranged from new packaging and new varieties to reformulated and novel products.
--- PAGE BREAK ---
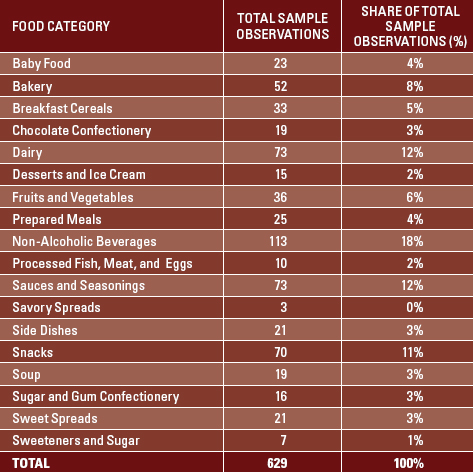
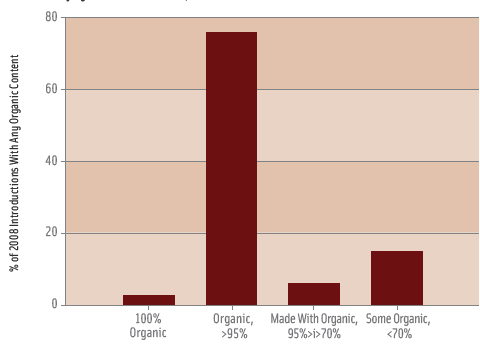 A total of 1,017 food and beverage products from 18 of GNPD’s 19 predefined food and beverage categories were examined; observations in the alcoholic beverages category were not included in the scope of this study. Each of the foods was classified according to the four USDA-defined levels of organic content (Figure 1). Seventy-five observations were not classified because the information in the database was not sufficient to determine the level of organic content. In total, 747 products were classified as “organic,” including 28 100% organic products and 719 >95% organic products. Observations missing ingredient information were removed (n=118) to obtain a final sample size of 629 organic food and beverage products (Table 1).
A total of 1,017 food and beverage products from 18 of GNPD’s 19 predefined food and beverage categories were examined; observations in the alcoholic beverages category were not included in the scope of this study. Each of the foods was classified according to the four USDA-defined levels of organic content (Figure 1). Seventy-five observations were not classified because the information in the database was not sufficient to determine the level of organic content. In total, 747 products were classified as “organic,” including 28 100% organic products and 719 >95% organic products. Observations missing ingredient information were removed (n=118) to obtain a final sample size of 629 organic food and beverage products (Table 1).
 The ingredient labels of the remaining products were searched for items in §205.606 as of January 1, 2009. Due to the variability in presentation of ingredient information, the broadest plausible terms were used in search functions for each ingredient. Labels containing ingredients present in more than 10 products (or ~2% of all products) were then searched by hand to determine if the ingredient used was consistent with the form on the National List, and whether it was used in organic or conventional form. Section 205.606 includes several coloring agents that may also serve as flavor ingredients, such as annatto and turmeric. Therefore, all products using the basic ingredient, i.e., “turmeric,” were counted. Resultant statistics of the use of National List ingredients and organic versions were summarized by type of ingredient and category. For the most commonly used ingredients in §205.606, the share of all organic products containing the ingredient was calculated to compare the relative use. The proportion of all products containing each ingredient that used an organic form of said ingredient was also calculated to compare the relative use of the organic alternative (Figure 2).
The ingredient labels of the remaining products were searched for items in §205.606 as of January 1, 2009. Due to the variability in presentation of ingredient information, the broadest plausible terms were used in search functions for each ingredient. Labels containing ingredients present in more than 10 products (or ~2% of all products) were then searched by hand to determine if the ingredient used was consistent with the form on the National List, and whether it was used in organic or conventional form. Section 205.606 includes several coloring agents that may also serve as flavor ingredients, such as annatto and turmeric. Therefore, all products using the basic ingredient, i.e., “turmeric,” were counted. Resultant statistics of the use of National List ingredients and organic versions were summarized by type of ingredient and category. For the most commonly used ingredients in §205.606, the share of all organic products containing the ingredient was calculated to compare the relative use. The proportion of all products containing each ingredient that used an organic form of said ingredient was also calculated to compare the relative use of the organic alternative (Figure 2).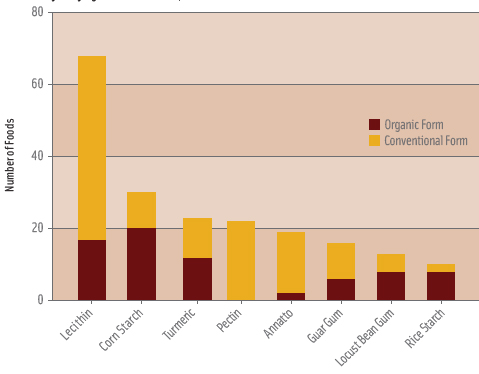
Mixed Adoption of Minor Organic Ingredients
Figure 1 shows the relative importance of the >95% organic content level of organic foods, with this level accounting for 76% of all 2008 introductions with any organic content. Only eight ingredients in §205.606 were present in 2% or more of food and beverage observations including (in descending order of use): lecithin, corn starch, turmeric, pectin, annatto, guar gum, locust bean gum, and rice starch (Figure 2). Products in the bakery, dairy, snack, and prepared meals categories most commonly used these eight ingredients. Lecithin, mainly in the form of soy lecithin, was present in 11% of observations and was the most widely used ingredient followed by corn starch at 5%. Table 2 provides detailed information on the eight most commonly used ingredients and shows there is essentially no correlation between the share of product introductions which used an ingredient from §205.606 and the share of products which used the ingredient in organic form.
--- PAGE BREAK ---
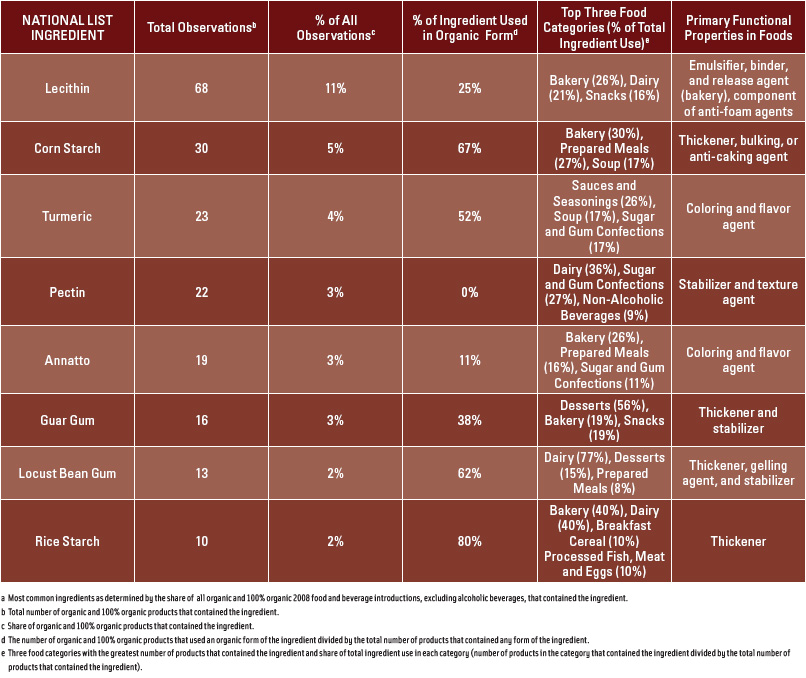 The central reason for including an ingredient in §205.606 of the National List is that the ingredient is commercially unavailable in organic form. One common explanation for an ingredient being commercially unavailable is a lack of sufficient demand to entice a supplier to manufacture the ingredient. The results of this study offer support for this argument, as it is clear that most of the ingredients in this section were only used in a very small share of organic products. However, the results of this study also support two reasons for the removal of some ingredients from the National List. First, this study provides several specific examples of minor ingredients that are available and used in organic form in multiple food categories. For example, rice starch was only present in 2% of products examined, but 80% of the products that contained any rice starch used an organic form of the ingredient. This is likely due to the fact that rice starch expired from the National List in June of 2009. Therefore, manufacturers had an incentive to begin sourcing organic versions of the ingredient. Corn starch and lecithin offered additional examples of mixed adoption. Corn starch was used by 9 products in the bakery category, with 5 using a nonorganic form and 4 using an organic form of the ingredient. Conversely, lecithin was present in 11% of products, but only 25% of the products containing lecithin utilized an organic form of the ingredient. These examples illustrate that the frequency of use of an ingredient may not be correlated with organic availability. Second, the relatively low proportion of organic products using nonorganic agricultural ingredients demonstrates that removing these substances from the National List may not cause “significant business disruptions” in the organic food industry, which was a critical concern of the NOP and used as an argument for expanding §205.606 (NOP, 2007).
The central reason for including an ingredient in §205.606 of the National List is that the ingredient is commercially unavailable in organic form. One common explanation for an ingredient being commercially unavailable is a lack of sufficient demand to entice a supplier to manufacture the ingredient. The results of this study offer support for this argument, as it is clear that most of the ingredients in this section were only used in a very small share of organic products. However, the results of this study also support two reasons for the removal of some ingredients from the National List. First, this study provides several specific examples of minor ingredients that are available and used in organic form in multiple food categories. For example, rice starch was only present in 2% of products examined, but 80% of the products that contained any rice starch used an organic form of the ingredient. This is likely due to the fact that rice starch expired from the National List in June of 2009. Therefore, manufacturers had an incentive to begin sourcing organic versions of the ingredient. Corn starch and lecithin offered additional examples of mixed adoption. Corn starch was used by 9 products in the bakery category, with 5 using a nonorganic form and 4 using an organic form of the ingredient. Conversely, lecithin was present in 11% of products, but only 25% of the products containing lecithin utilized an organic form of the ingredient. These examples illustrate that the frequency of use of an ingredient may not be correlated with organic availability. Second, the relatively low proportion of organic products using nonorganic agricultural ingredients demonstrates that removing these substances from the National List may not cause “significant business disruptions” in the organic food industry, which was a critical concern of the NOP and used as an argument for expanding §205.606 (NOP, 2007).
Practical Implications
It is important to note that the data highlight the frequency of ingredient use in product introductions and cannot be used to extrapolate the quantity demanded of the ingredient or the use of the ingredient in all commercially available organic products. However, in the context of the current literature, the data reveal an interesting perspective on the use of nonorganic agricultural ingredients in organic foods. For example, the concurrent use of organic and conventional corn starches in bakery, meal, and snack products provides a salient example of the dilemma of the National List. Corn starch is commonly used as a bulking, thickening, or anti-caking agent in a wide range of products. Starches also help to regulate the moisture content and texture of a food product and may be used in combination with gums as a fat replacer. The functionality of a particular corn starch can vary depending on the specific product application even among products in the same food category.
Given the inevitable variability of the applications of corn starch, to what lengths must suppliers of organic corn starches go to prove the ingredient is available in sufficient quality and quantity? Corn starch was one of the first items petitioned for removal from the National List (NOSB, 2004). The NOSB denied the requests for the removal of corn starch and other ingredients, citing that the requests did not provide sufficient information such as “supply source, supply quantity, functionality, performance, test data, and name and address of producers who have used this material under similar circumstances” (NOSB, 2005). Under the current structure of the NOP regulations to remove an ingredient from the National List, the petitioner must provide sufficient evidence to prove that the ingredient is commercially available in a form that is functionally equivalent to the nonorganic alternative. This process places an excessive burden on the petitioner and provides little incentive for organic food manufacturers to seek out or even collaboratively develop organic alternatives. As one member on the NOSB summarized the dilemma, “I feel like I am stuck in a chicken-and-an-egg thing … until it is off the list, industry won’t be incented to use it” (NOSB, 2008). Ultimately, potentially insignificant differences in functionality that allow ingredients to remain on §205.606 may be deterring certifiers and food manufacturers from fully complying with the principle of “organic preference.”
An Evolving Definition of Organic
As the multi-ingredient organic food sector continues to grow, the fundamental definition of organic food itself is evolving. In addition to the debate over commercial availability, functional ingredients and new technologies have created complex debate over how to define whether a substance is synthetic or nonsynthetic and agricultural or nonagricultural, so as to determine which criteria should be applied in determining whether or not to include the ingredient on the National List (NOSB, 2010a). The recent popular and lucrative practice of adding functional ingredients, such as active microbial cultures and fructooligosaccharides (an ingredient now listed in §205.606), to foods has also challenged the traditional definition of organic. The NOP is also facing the issue of how to regulate the use of “accessory nutrients” such as certain types of omega fatty acids and phytochemcials in organic foods. These ingredients are not “naturally” present in many food products, but research has shown that they may have numerous health benefits and improve the overall nutritional quality of a product (McEvoy, 2010a). Additionally, recent research on the food applications of nanotechnology has caused debate regarding the extent to which new technologies should be allowed in the production of organic foods (NOSB, 2009c). As the functional foods industry and food technologies continue to evolve, the organic community will have to continually adapt to compete with an increasingly diverse and innovative range of food products.
The Need for a More Adaptive NOP
Under the current regulations, the minimum estimated time required for the NOSB to review a petition is 145 days, which does not include the additional time that would be needed for the ruling making process if the NOSB recommended an ingredient be removed from the List (NOP, 2010a). If no petitions for removal are filed, the substances on the National List are reviewed only once every five years under the sunset review process as specified in the OFPA.
--- PAGE BREAK ---
Furthermore, although the NOSB has access to a Technical Advisory Panel (TAP), the panel is not required, and rarely requested, to review substances being considered for §205.606 (NOSB, 2007). The NOSB itself is not required to have a member with any food science experience, and in considering questions of functionality, the board seems to lack unbiased food science expertise in some circumstances. For example, in a recent debate over whether some form of lecithin should be removed from the National List, the NOSB had to rely on information brought forward by manufacturers and suppliers to learn about the functionality differences of regular vs de-oiled, unbleached vs bleached, and dry vs fluid lecithin (NOSB, 2009b). These parties all had a vested interest in the outcome of the board’s decision and in some cases brought forward conflicting information. The NOSB may be better served by conducting an independent, scientific review of all substances petitioned for addition or removal from the National List and possibly engaging the academic community in complex discussions of the functionality of ingredients in various applications.
Collectively, the evidence of this study suggests that the current review and petition process is at best not supporting the development of organic alternatives and at worst may actually be an impediment. All parties involved in the production, processing, certification, and consumption of organic foods would be better served by a simpler, more streamlined process to determine which substances may be used in organic foods. As the multi-ingredient organic food sector continues to grow, the NOP will be faced with ever more complex issues. Perhaps a better approach would be for the NOP to incentivize the development of organic ingredients and processes by setting clear guidelines and deadlines and providing development support to food manufacturers. This approach would be similar to the current model used by the United Kingdom’s Soil Assn. For example, the Soil Assn. announced in spring of 2008 that it would require all organic food manufacturers to source organically produced versions of lecithin, locust bean gum, and guar gum (all ingredients which currently remain on the National List) by 2009. The Soil Assn. additionally offered support to link manufacturers with potential suppliers (Soil Assn., 2008). This type of model may allow the NOP to both incentivize the creation of new organic ingredients and enforce the highest standard of “organic preference.”
Promises of Change
Under the current administration, there has been a renewed focus on the NOP and improving its regulatory processes. In March 2010, the Office of the Inspector General released a report that evaluated NOP policies and procedures from October 2003 to July 2009. Among its 14 recommendations for improvement, one of the key issues highlighted in the report was better guidance and communication between the NOP and accredited certifying agents (ACAs) to ensure that organic standards are consistently and uniformly applied to all organic manufacturers. The NOP has promised to publish a Program Manual this fall to offer better guidance to ACAs, which will hopefully include standardized guidelines for determining commercial availability of nonorganic ingredients.
The NOP has also vowed to work closely with the NOSB in developing further guidance and regulations. The NOSB has recently made a recommendation of revised definitions for the classification of materials (e.g., synthetic/nonsynthetic, agricultural/nonagricultural) on the National List, and the NOP will likely incorporate these new classifications into new rulings and guidance documents. Additionally, the NOSB is currently reviewing 45 of the 46 substances currently included in §205.606, which are due to expire from the National List in 2012 (NOP, 2010b). As of this publication, one of the ingredients, hops, has been recommended by the NOSB Handling Committee for removal from the National List on January 1, 2013. In this recommendation, the Committee reiterated the need for the NOP to offer additional guidance on commercial availability criteria and emphasized the critical role of certifying agents by saying: “Their action (or inaction) contributes substantially to the degree of functionality of commercial availability perceived by industry and the public at large” (NOSB, 2010b). The outcome of this review and public rule-making process could dramatically change the use of nonorganic ingredients in organic foods. Finally, the NOP must also re-evaluate its standards as it seeks an equivalency agreement with the European Union. To support these tasks, the Administration has increased funding for the NOP from $2.65 million in 2008 to a proposed $10.1 million budget in 2011 (McEvoy, 2010b). The staff of the NOP has proportionally increased in size, from 14 in 2008 to a proposed 32 members in 2011, including the addition of a food scientist as a member of the NOP’s Standards Division (McEvoy, 2010b). Time will tell if these additional resources and enhanced oversight will lead to more transparent regulations that will encourage innovations in the organic industry.
Debra Van Camp is a graduate student in the Dept. of Food Marketing, Saint Joseph’s University, 5600 City Ave., Philadelphia, PA 19131 ( [email protected] ). Pauline Ie, a member of IFT, is a graduate student in the Dept. of Food Science and Technology, The Ohio State University, 227 Parker Food Science and Technology Bldg., 2015 Fyffe Rd., Columbus, OH 43210 ( ie [email protected]). Noah Muwanika is a former undergraduate student, Dept. of Agricultural Environmental and Development Economics, The Ohio State University ([email protected]). Yael Vodovotz, Ph.D., a Professional Member of IFT, is an Associate Professor, Dept. of Food Science and Technology, The Ohio State University ([email protected]). Neal H. Hooker, Ph.D., is the C.J. McNutt Professor, Dept. of Food Marketing, Saint Joseph’s University ([email protected]).
References
Batte, M.T., Hooker, N.H., Haab, T.C., and Beaverson, J. 2007. Putting their money where their mouths are: Consumer willingness to pay for multi-ingredient, processed organic food products. Food Policy 32: 145–159.
CFR. 2010. Code of Federal Regulations. 205.270 b(1). Accessed June 5, 2010.
GNPD. 2009. Global New Products Database: How we do it. Mintel International Group Ltd., London, England. http://www.gnpd.com. Accessed June 5, 2010.
Harvey v. Veneman. 396 F.3d 28 (1st Cir. 2005).
McEvoy, M. 2010a. Action memorandum for the chair of the National Organic Standards Board–Scope of nutrient vitamins and minerals in organic food, April 26. National Organic Program, Washington, D.C. http://www.ams.usda.gov/nop. Accessed June 5, 2010.
McEvoy, M. 2010b. Report to the National Organics Standards Board, April 26. National Organic Program. Accessed June 5, 2010.
Mintel. 2009. Organic Food and Drink Retailing—U.S. November. Mintel International Group Ltd. http://academic.mintel.com. Accessed June 5, 2010.
NOP. 2000. Final rule. National Organic Program, Fed. Reg. 65: 80548-80684.
NOP. 2005. Notice. Fed. Reg. 70: 38090-38091.
NOP. 2007. Notice. Fed. Reg. 72: 35137-35141.
NOP. 2010a. National List materials review process. National Organic Program. http://www.ams.usda.gov. Accessed June 5, 2010.
NOP. 2010b. Notice. National Organic Program, Fed. Reg. 75: 14500-14508.
NOSB. 2004. Meeting transcripts, April 29. National Organic Standards Board. National Organic Program. Accessed June 5, 2010.
NOSB. 2005. Meeting transcripts, Nov. 16. Accessed June 5, 2010.
NOSB. 2007. Meeting transcripts, Nov. 29. Accessed June 5, 2010.
NOSB. 2008. Meeting transcripts, May 20. Accessed June 5, 2010.
NOSB. 2009a. Meeting transcripts, Nov. 3. Accessed June 5, 2010.
NOSB. 2009b. Meeting transcripts, May 4. Accessed June 5, 2010.
NOSB. 2009c. Nanotechnology in organic production, processing and packaging, Sept. 8. Accessed June 5, 2010.
NOSB. 2010a. Addendum to Nov. 6, 2006 recommendation on classification of materials, March 1. Accessed June 5, 2010.
NOSB. 2010b. Handling committee response to petition to remove hops from §205.606, Oct. 8.
Office of the Inspector General. Oversight of the National Organic Program. Audit Report 01601-03-Hy.
Organic Food Production Act. 1990. Pub. L. No. 101-624, 110 Stat. 3935.
OTA. 2006. Comments to the National Organic Standards Board, Oct. 17. Organic Trade Assn., Greenfield, Mass. http://www.ota.com. Accessed June 5, 2010.
OTA. 2009. Organic Trade Assn. releases its 2009 organic industry survey. Accessed June 5, 2010.
Soil Assn. 2008. Certification News 62 (Spring). Soil Association Certification Limited. www.soilassociation.org. Accessed June 5, 2010.
Sporleder, T.L., Hooker, N.H., Shanahan, C.J., and Bröring, S. 2008. Innovation in food products: First-mover strategy and entropy metrics. Intl. Food and Agribusiness Mgmt. Rev. 11(3): 49-65.
