Ensuring the Safety of Sweeteners from Stevia
Successful documentation of the safety of high-purity stevia-derived sweeteners has paved the way for acceptance and regulatory authorizations by FDA and several international bodies.
Due in part to the escalating incidence of obesity and diabetes, consumers and food manufacturers have substantial interest in the increased availability of a wide array of good-tasting foods with reduced caloric content. Advances in producing high-purity stevia-derived sweeteners in recent years respond directly to this market interest. For the addition of stevia-derived sweeteners into various foods to occur, the regulatory and safety considerations with the sweeteners extracted from stevia leaves had to be addressed to ensure that such food offerings met the regulatory requirements. The U.S. Food and Drug Administration (FDA) and numerous regulatory bodies and expert panels worldwide have undertaken rigorous evaluations of the composite safety information to protect the well-being of consumers.
 Regulatory History
Regulatory History
Sweeteners derived from stevia have been permitted in foods in South America and in several countries in Asia including China, Japan, and South Korea for years. More recently, the stevia sweeteners have received food usage approvals in Mexico, Australia, New Zealand, Switzerland, France, and Hong Kong. Steviol glycosides have been used as a dietary supplement in the United States since 1995 (Geuns, 2003), and since 2008, high-purity steviol glycosides and high-purity rebaudioside (Reb) A compositions that have been determined to be GRAS are permitted in foods in the U.S.
The international community has been closely involved in advancing key safety assessments for stevia sweeteners, and several such initiatives merit discussion. The Joint Expert Committee on Food Additives (JECFA) reviewed the safety of steviol glycosides for more than a decade. In 2000, JECFA published the original safety review on steviol glycosides, and JECFA established a temporary ADI (acceptable daily intake) of 0–2 mg/kg body weight (bw)/day (on a steviol basis) at its 63rd meeting (WHO, 2000). JECFA continued its review and deliberations on the safety of steviol glycosides over the next few years, making the temporary status of the ADI permanent while raising the ADI to 0–4 mg/kg bw/day (on a steviol basis) following JECFA’s favorable review of additional clinical studies on steviol glycosides (FAO, 2008; WHO, 2008). In 2009, JECFA published a fi nal monograph addendum on steviol glycosides (WHO, 2009).
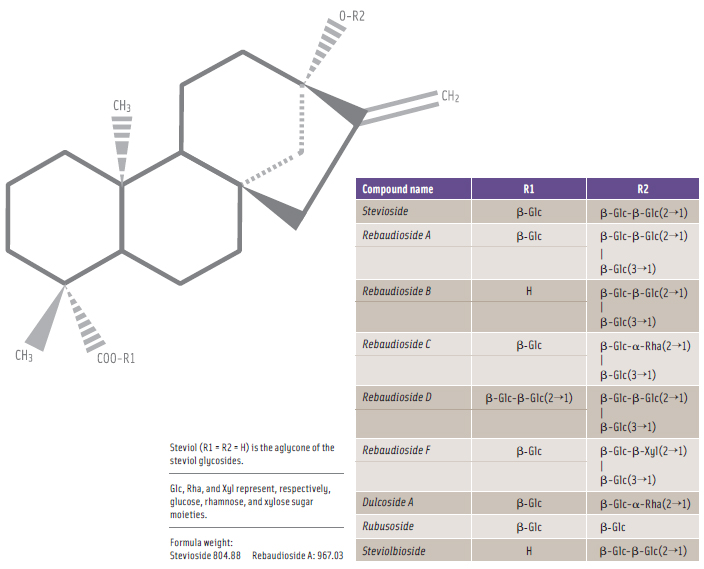 In early 2009, a number of parties—including the Australian government—submitted a request to the Codex Committee on Food Additives in which they proposed that the JECFA specifi cations for acceptable steviol glycosides should be expanded beyond the initial seven named steviol glycosides to include Reb D and Reb F (see Figure 1) in constituting the minimum 95% steviol glycosides composition (CCFA, 2009). This proposed modifi cation to include Reb D and Reb F as requested was endorsed by the Codex Alimentarius Committee, and JECFA approved the modifi ed steviol glycosides specifi cations to include Reb D and Reb F (FAO, 2010).
In early 2009, a number of parties—including the Australian government—submitted a request to the Codex Committee on Food Additives in which they proposed that the JECFA specifi cations for acceptable steviol glycosides should be expanded beyond the initial seven named steviol glycosides to include Reb D and Reb F (see Figure 1) in constituting the minimum 95% steviol glycosides composition (CCFA, 2009). This proposed modifi cation to include Reb D and Reb F as requested was endorsed by the Codex Alimentarius Committee, and JECFA approved the modifi ed steviol glycosides specifi cations to include Reb D and Reb F (FAO, 2010).
The Food Standards Australia New Zealand (FSANZ) completed its evaluation of an application for use of steviol glycosides in foods in 2008. FSANZ recommended that the Australia and New Zealand Food Regulation Ministerial Council amend its Food Standards Code to allow food uses of steviol glycosides (FSANZ, 2008). In 2008, Switzerland’s Federal Office for Public Health (2008) approved the use of stevia as a sweetener, citing the favorable actions of JECFA, and France published its approval for the food uses of Reb A with a purity of 97% (AFSSA, 2009).
--- PAGE BREAK ---
In September 2009, based on a review of the international regulation of Stevia rebaudiana and the clinical evidence for safety and efficacy, Canada’s Natural Health Products Directorate adopted guidelines for use of stevia and steviol glycosides in Natural Health Products (NHPs) (Health Canada, 2009). The revised recommendation for the maximum limit for steviol glycosides in NHPs is in accordance with the full ADI of 4 mg steviol/kg bw established by JECFA. In August 2010, Hong Kong authorized the use of steviol glycosides as a sweetener for use in foods (Hong Kong, 2010).
The European Food Safety Authority (EFSA) examined the safety information of steviol glycosides in light of JECFA’s 2008 findings and in response to a request by the European Commission to deliver a scientific opinion on the safety of the steviol glycosides as a sweetener for use in several designated food categories (EFSA, 2010). After considering the data on stability, degradation products, metabolism, and toxicology, the EFSA Panel endorsed the ADI for steviol glycosides of 4 mg/kg bw/day (as steviol). On the basis of this favorable safety opinion by EFSA, approval by the European Commission is widely expected in 2011 for use in foods and beverages.
The U.S. safety evaluations of steviol glycosides advanced in concert with international actions. The specific regulatory vehicle pursued in the U.S. to enable broad-based addition to foods consisted of establishing GRAS status. Prior to 2008, at least two GRAS petitions seeking authorization for the addition of stevia products to foods were submitted to FDA but were unsuccessful, presumably because the previously available safety data for the stevia products—including purity considerations—were inadequate.
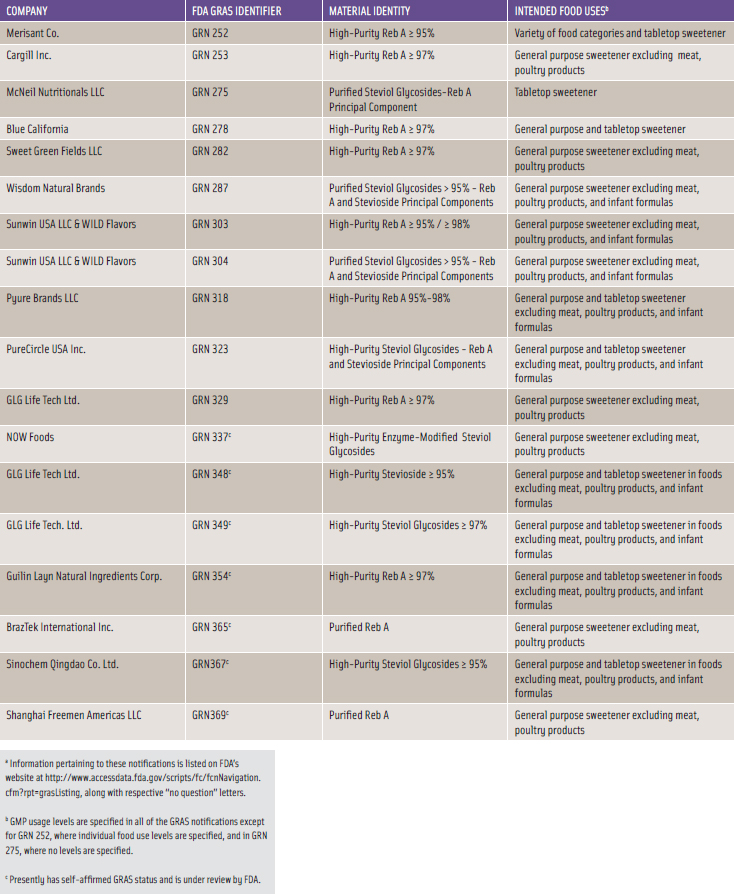 Several companies have obtained GRAS status since 2008. Wisdom Natural Brands convened an independent expert panel to prepare a GRAS dossier for high-purity steviol glycosides. It then requested an independent review by a second expert panel, and by March 2008, both expert panels embraced Wisdom’s self-affi rmed GRAS declaration. In May 2008, Merisant and Cargill submitted their own GRAS notifi cations to FDA for their respective high-purity Reb A compositions. On December 17, 2008, FDA issued “no question” letters for each of these GRAS notices. Since December 2008, several GRAS notifi cations were voluntarily submitted to FDA for stevia-derived sweetener products as shown in Table 1.
Several companies have obtained GRAS status since 2008. Wisdom Natural Brands convened an independent expert panel to prepare a GRAS dossier for high-purity steviol glycosides. It then requested an independent review by a second expert panel, and by March 2008, both expert panels embraced Wisdom’s self-affi rmed GRAS declaration. In May 2008, Merisant and Cargill submitted their own GRAS notifi cations to FDA for their respective high-purity Reb A compositions. On December 17, 2008, FDA issued “no question” letters for each of these GRAS notices. Since December 2008, several GRAS notifi cations were voluntarily submitted to FDA for stevia-derived sweetener products as shown in Table 1.
The component steviol glycosides compositions tend to vary among the notifiers with high-purity Reb A and high-purity steviol glycosides predominating; however, the compositions and purities all fall within the JECFA specifications. Table 1 also highlights rather broad food uses with use levels tending to be governed by adherence to good manufacturing practices. As of mid-March 2011, FDA had received 18 GRAS notices on Reb A or steviol glycosides compositions, of which 11 notices received “no question” letters from FDA while seven notices remain under review.
--- PAGE BREAK ---
As seen with the initial regulatory actions undertaken by Wisdom Natural Brands, GRAS status can legally be established with GRAS self-affirmation with appropriate reliance on qualified independent Expert Panels. FDA’s GRAS notification process allows for the voluntary submission of a firm’s independent GRAS determination that FDA will rigorously review. Despite the absence of a legal requirement that self-affirmed GRAS determinations must be shared with FDA or otherwise made known to the general public, several companies voluntarily elected to submit their GRAS determinations to FDA in part because stevia-based materials are subject to detention during import proceedings unless 1) identified for dietary supplement use or 2) documented as having received a “no question” letter from FDA. Once a company’s stevia product appears on FDA’s GRAS inventory, they are no longer subject to these importation complications.
Identity and Intended Food Uses of Stevia-Derived Sweeteners
The substances listed in Table 1 are characterized by a high degree of chemical purity. Some products consist of high-purity Reb A with designated purities of 95% or higher. Other sweetener mixtures containing a higher percentage of stevioside are designated as high-purity steviol glycosides with percentages specified as ≥ 95%.
As noted in Table 1, the NOW Foods sweetener described in GRN 337 consists of enzyme-modified steviol glycosides; the GLG Life Tech sweetener described in GRN 348 consists of high-purity stevioside; and one of the sweetener formulations described in GRN 304 by Sunwin and WILD Flavors consists of 90% pure stevioside.
To be sure, the chemical structures of the subject steviol glycosides as depicted in Figure 1 are quite similar. This chemical similarity among the steviol glycosides is due to the common steviol backbone with glucose, rhamnose, and xylose moieties attached in two specific steviol locations.
The purity of the sweetener components is a critical aspect in addressing the safety of the stevia sweeteners. Safety concerns surfaced during the earlier evaluations of stevia leaves and the crude extracts. During JECFA’s safety deliberations, the importance of high purity was recognized, and this prompted JECFA to impose the minimum purity requirement on the component steviol glycosides as noted earlier.
The intended food uses of the stevia-derived sweeteners that have attained FDA-acknowledged GRAS status are quite similar. Nearly all of the GRAS notifications refer to use of stevia sweeteners as a general purpose non-nutritive sweetener for use in a broad-based selection of food categories and as a tabletop sweetener. Some high-volume food categories such as baked goods and nonalcoholic beverages are specifically identified for stevia-derived sweeteners uses. Several notifications specifically exclude use in meat and poultry products while others exclude use in infant formulas.
Use levels are identified for individual food categories in some of the earlier GRAS notifications, but in many notifications, reference is made to use levels that comply with Good Manufacturing Practices (GMP) levels, which means that the amounts of ingredients to be added are to be no more than that required to accomplish the intended technical effect.
--- PAGE BREAK ---
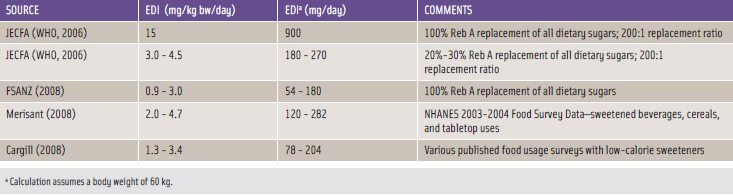 Food category selections, together with respective food usage levels of the sweeteners, are needed in calculating estimated daily intake (EDI) levels, which are critical when making food safety determinations. Multiple rigorous approaches have been undertaken by diff erent parties in estimating consumption levels of the stevia-derived sweeteners, and Table 2 summarizes the EDI assessments made for steviol glycosides as expressed in terms of Reb A.
Food category selections, together with respective food usage levels of the sweeteners, are needed in calculating estimated daily intake (EDI) levels, which are critical when making food safety determinations. Multiple rigorous approaches have been undertaken by diff erent parties in estimating consumption levels of the stevia-derived sweeteners, and Table 2 summarizes the EDI assessments made for steviol glycosides as expressed in terms of Reb A.
Very conservative consumer intake estimates undertaken by JECFA were utilized to gauge the potential human exposures of the subject steviol glycosides in foods in the U.S. and other countries. JECFA assumed that steviol glycosides would replace all dietary sugars at the lowest reported relative sweetness ratio for steviol glycosides and sucrose, which is 200:1. This evaluation yielded a highly conservative dietary exposure that overestimates likely consumption.
JECFA reasoned that true dietary intakes of steviol glycosides would more realistically be 20%–30% of the full replacement values of all dietary sugars, which translates to a daily consumption of 180–270 mg Reb A for a 60 kg individual (WHO, 2006).
FSANZ (2008) likewise estimated steviol glycosides dietary intake for adult consumers in New Zealand. Both Merisant and Cargill independently calculated dietary estimates for Reb A using different approaches. Merisant (2008) relied upon NHANES 2003–2004 Food Survey data for designated food categories; Cargill (2008) utilized actual intake data with a large number of foods containing low-calorie sweeteners, which were directly compared to aspartame consumption levels in those foods.
By considering the independent composite dietary intake estimates summarized in Table 2, the anticipated human exposures resulting from the different approaches tend to converge with Reb A EDIs of about 100–300 mg/day for a 60 kg individual.
As described more fully below, the consensus ADI for steviol glycosides expressed as steviol is 4 mg/kg bw/day, which corresponds to 12 mg/kg bw/day for Reb A or 720 mg/day for a 60 kg individual. When compared with the calculated EDIs in Table 2, the ADI exceeds the EDI by a factor of more than two, thereby supporting the conclusion that reasonably anticipated levels of high-purity Reb A or high-purity steviol glycosides in the diet fall within consumption levels that comply with FDA’s operational definition of safe.
The subject of acceptable food usage levels continues as a topic of interest with regulatory agencies. In October 2009, Cargill applied to FSANZ to increase the maximum usage levels of high-purity steviol glycosides in the high-volume food categories of ice cream and various beverages. Cargill supported its application with increased usage levels by presenting market share analyses which overestimate actual intake while remaining well below the generally accepted ADI. In December 2010, FSANZ recommended accepting the increased usage levels as requested since no public health and safety issues were identified. Final actions may materialize in 2011 (FSANZ, 2010).
--- PAGE BREAK ---
Safety Evaluations
The U.S., international regulatory agencies, and international food safety panels have thoroughly reviewed the biological, toxicological and clinical data on stevia and steviol glycosides. Most notably, JECFA over the years has evaluated steviol glycosides multiple times. The majority of these safety reviews focused on mixtures of steviol glycosides. Some of the earliest toxicology reviews revealed possible adverse health effects such as decreased fertility and kidney effects, but results from better toxicology studies with modern test protocols and use of purer test materials resolved these earlier safety concerns. Additionally, JECFA raised questions about clinical effects on blood pressure and glucose metabolism in hypertensive and diabetic individuals, respectively, in comparison to normal human subjects. These uncertainties prompted additional clinical testing on high purity test materials, and by 2006, sufficient favorable data were generated to resolve concerns about these health matters.
Merisant and Cargill strengthened JECFA’s safety assessments of steviol glycosides with well-conducted investigations on Reb A that were incorporated into their respective GRAS notifications.
Pharmacokinetic work revealed that stevioside and Reb A are not absorbed per se but are converted to steviol in the gastrointestinal tract. In both humans and rats, steviol is rapidly converted to the glucuronide, and the glucuronide is not further metabolized but is efficiently excreted. It is important to recognize that Reb A is handled pharmacokinetically similarly to stevioside.
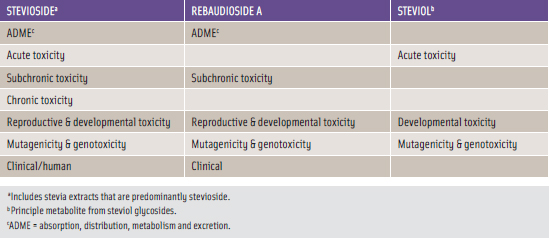 Table 3 contains a summary of the types of scientifi c documentation that directly addresses the safety aspects of the stevia-derived sweeteners.
Table 3 contains a summary of the types of scientifi c documentation that directly addresses the safety aspects of the stevia-derived sweeteners.
Based on the composite safety studies on steviol glycosides blends, stevioside, and Reb A, JECFA established the ADI to be 4 mg/kg bw/day as steviol, which corresponds on a molecular weight adjusted basis to 8 mg/kg bw/day as stevioside and 12 mg/kg bw/day as Reb A. This determination has been accepted by the broader scientific community.
For a substance to attain GRAS status, it must also meet two common knowledge elements that further validate the safety conclusion. The first common knowledge element for a GRAS determination requires that data and information relied upon to establish safety must be generally available; this is most commonly established by utilizing studies published in peer-reviewed scientific journals. The majority of the studies relied upon by expert panels have been published in the scientific literature or have otherwise been made public by JECFA and various regulatory bodies during their safety deliberations. Thus, the foundational safety studies have become generally available to the scientific community, and this requirement has been fulfilled.
--- PAGE BREAK ---
The remaining common knowledge element for GRAS determinations requires that consensus must exist among qualified scientists about the safety of the substance with its intended use. The 2008 JECFA final opinion largely meets the common knowledge test by itself since JECFA is composed of expert scientists from various regulatory agencies around the world including direct, substantive participation by FDA. As noted earlier, JECFA’s safety conclusions have been reviewed and validated by other respected authorities, including FSANZ, EFSA, and Switzerland, Canada, France, and Hong Kong.
The views of several well-respected scientists are consistent with the finding that high-purity steviol glycosides are safe for human consumption at doses in the range of the JECFA ADI (Xili et al., 1992; Toyoda et al., 1997; Geuns, 2003; Carakostas et al., 2008). Favorable safety conclusions have been provided by a large number of scientists who served on GRAS expert panels, including those notifications detailed in Table 1, and FDA’s issuance of several “no question” letters further supports the safety conclusions.
Robert S. McQuate, Ph.D., a Professional Member of IFT, Chief Executive Officer and Co-Founder of GRAS Associates LLC, 20482 Jacklight Lane, Bend, OR 97702 ( [email protected] ).
Thanks are extended to several colleagues—Richard Kraska, Madhusudan Soni, Wayne Bidlack, James Coughlin, Robin Guy, Michael Falk, Walter Glinsmann, and Robert Nicolosi—for their services on steviol glycosides GRAS expert panels convened by GRAS Associates LLC, which contributed substantially to the development of this article.
References
AFSSA. 2009. Rept. Agence Francais De Securite Sanitaire Des Aliments, France. http://www.afssa.fr/Documents/AAAT2009sa0119.pdf; http://www.foodnavigator.com/Legislation/France-aproves-high-Reb-A-stevia-sweeteners.
Carakostas, M.C., Curry, L.L., Boileau, A.C., and Brusick, D.J. 2008. Overview: The history, technical function and safety of rebaudioside A, a naturally occurring steviol glycoside, for use in food and beverages. Food Chem. Toxicol. 46(7)(Suppl.1): S1-10.
Cargill. 2008. GRAS notification 253 for Rebaudioside A. U.S. Food and Drug Administration, Washington, D.C. http://www.cfsan.fda.gov/~rdb/opa-grsn.html.
CCFA. 2009. Proposals for additions and changes to the priority list of food additives proposed for evaluation by JECFA (CL 2008/26-FA). Codex Alimentarius Commission E, FAO/WHO/JECFA CX/FA 09/41/11. Codex Committee on Food Additives.
EFSA. 2010. Scientific opinion on the safety of steviol glycosides for the proposed uses as a food additive. EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). European Food Safety Authority. EFSA Jour. 8(4): 1537, 1-84.
FAO. 2008. Steviol glycosides. FAO JECFA Monographs 5. Food and Agriculture Organization.
FAO. 2010. Steviol glycosides. FAO JECFA Monographs 10. Food and Agriculture Organization. http://www/fao/org/ag/agn/jecfaadditives/specs/monograph10/additive-442-m10.pdf.
FSANZ. 2008. Application A540, steviol glycosides as intense sweeteners. Final assessment rept. Food Standards Australia New Zealand.
FSANZ. 2010. Application A1037, steviol glycosides—increase in permitted use levels. Assessment rept. Dec. 15. Food Standards Australia New Zealand.
Geuns, J.M.C. 2003. Molecules of interest stevioside. Phytochem. 64: 913-921.
Health Canada. 2009. Revised guidelines for the use of stevia in natural health products. http://www.hc-sc.gc.ca/dhp-mps/prodnatur/legislation/docs/noticeavis-stevia-eng.php.
Hong Kong. 2010. Legislative council brief. Sweeteners in food (amendment) regulation. May. http://www.cfs.gov.hk/english/programme/programme_rafs/files/2010_amendment_to_sweeteners_in_food_regulation_Legco_brief.pdf.
Merisant. 2008. GRAS notification 252 for Rebaudioside A by Whole Earth Sweetener Co. LLC. U.S. Food and Drug Administration, Washington, D.C. http://www.accessdata.fda.gov/scripts/fc/fcnNavigation.cfm?rpt=grasListing.
Switzerland Federal Office of Public Health. 2008.http://www.bag.admin.ch/themen/lebensmittel/04861/04972/index.html?lang=fr.
Toyoda, K., Matsui, H., Shoda, T., Uneyama, C., and Takahashi, M. 1997. Assessment of the carcinogenicity of stevioside in F344 rats. Food Chem. Toxicol. 35: 597–603.
WHO. 2000. Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additive Series, 42. Safety evaluation of certain food additives. Stevioside.
WHO. 2006. Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additive Series, 54. Safety evaluation of certain food additives. Steviol Glycosides, pp.117-144.
WHO. 2008. Joint FAO/WHO Expert Committee on Food Additives. 69th Meeting. Summary and conclusions. Steviol Glycosides. July 4.
WHO. 2009. Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additive Series, 60. Safety evaluation of certain food additives. Steviol Glycosides (addendum).
Xili, L., Chengjiany, B., Eryi, X., Reiming, S., Yuengming, W., Haodong, S., and Zhiyian, H. 1992. Chronic oral toxicity and carcinogenicity study of stevioside in rats. Food Chem. Toxicol. 30: 957-965.
