Testing for Gluten in Foods
FOOD SAFETY & QUALITY
People who suffer from celiac disease must avoid consumption of foods containing gluten, and the food industry is responding to this need by marketing an increasing number of gluten-free products. Manufacturers of such products must not only formulate their products without gluten but also check that there is no cross-contamination from other products and processes. This article will discuss gluten sensitivity, the available analytical tests for detecting gluten in food products, regulatory concerns, and challenges ahead for the food industry.
 Gluten Sensitivity
Gluten Sensitivity
Gluten is a mixture of prolamin (gliadin in wheat) and glutelin proteins naturally present in wheat, rye, barley, and related grains, including those wheat varieties known by such names as durum (semolina), spelt, einkorn, emmer, khorasan (Kamut), club wheat, triticale, and farro. It is most commonly present in products made from wheat flour and in certain other food products in which it is used as an ingredient, providing elasticity in baked goods, for example, as well as texture, moisture retention, and flavor.
Celiac disease, also referred to as celiac sprue, is a genetic disease that is said to affect about 1% of the people in North America and Europe. The immune system of people with the disease responds to the consumption of gluten by damaging the lining of the small intestine, thus interfering with absorption of nutrients. The disease has no cure but can be managed by avoiding gluten in the diet.
The number of products marketed as gluten-free is increasing worldwide, but even with the establishment of regulations allowing such labeling, it is possible that foods labeled as gluten-free may be contaminated during processing by equipment previously used for gluten-containing foods. Because of the high prevalence of wheat in the food supply, even products that are formulated or processed to not contain it may still contain enough trace amounts of gluten to produce symptoms in gluten-sensitive individuals. Consequently, reliable tests are required for the detection of gluten in foods.
Detection Methods
The majority of tests for gluten in food products are enzyme-linked immunosorbent assays (ELISAs). Microwell versions of ELISAs provide quantitative results. Lateral-flow devices generally provide qualitative results, indicating the presence of gluten above a threshold level, but in some instances can also provide semi-quantitative results. Other types of tests include polymerase chain reaction (PCR), which detects DNA rather than protein; adenosine triphosphate (ATP) swab tests for assessing cleanliness of equipment surfaces; and general protein swabs, which are not specific to gluten but detect all types of protein and can be used for assessing cleanliness. (See diagram for microwell illustration.)
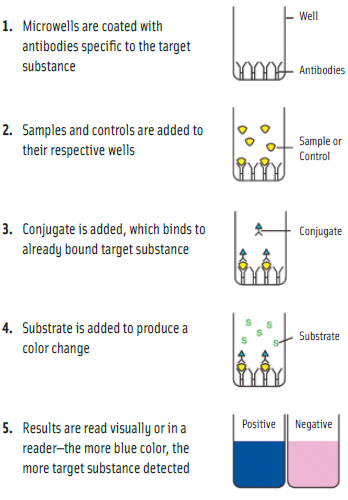
ELISAs are far more specific than the other methods, according to Steve Taylor, Professor in the Dept. of Food Science & Technology at the University of Nebraska-Lincoln, and Director of the Food Allergy Research & Resource Program (www.farrp.org) at the university. ATP testing usually won’t work for gluten if there are other sources of ATP present but is good for general cleanliness. A result positive for ATP would usually also mean positive for gliadin, but a result negative for ATP would not necessarily mean negative for gliadin because ATP tests are not as sensitive as ELISAs. The same comments apply to general protein tests, which are also not as reliable if other sources of protein are present.
--- PAGE BREAK ---
The most common form of ELISA for gluten detection is the sandwich format. As explained by Taylor, in sandwich ELISAs the antigen (gluten proteins in this case) bind to anti-gluten antibodies that are affixed to a surface, generally a microwell plate. Then a second gluten-specific antibody—this one linked to an enzyme—is applied over the surface and also binds to any gluten that is now affixed to the surface. Finally, a substance is added that the enzyme can convert into a detectable signal, such as a color change.
Lateral flow tests, also known as immunochromatographic assays, are usually available in dipstick format, in which the test sample flows along a solid substrate by capillary action, Taylor continued. When the sample is applied to the strip, it mixes with a colored reagent and moves with the substrate into specific zones on the strip that contain the specific antibodies. When liquid from a sample or wet equipment surface passes over this zone, the gluten will bind to the antibody. Color also forms as a line in this zone on the strip. A control zone is also usually included that will form a color that merely indicates that the strip has worked correctly. Thus, a negative test is the formation of one line while a positive test is the formation of two lines.
AOAC International has approved the method “Gliadin as a Measure of Gluten in Foods” as Official Method 991.19. This ELISA uses the R5 monoclonal antibody, which binds to gliadin/gluten and similar prolamins from related grains. AOAC’s Research Institute, which provides an independent evaluation of test kits, has certified R-Biopharm’s RidaScreen® Gliadin method as a Performance Tested Method. The test uses the R5 monoclonal antibody, which is recommended by the Codex Alimentarius. Two other methods are also in the Performance Tested Method process but have not yet been certified.
In late December 2010, Neogen introduced its Veratox® for Gliadin R5 rapid test for gluten to better meet the needs of the international testing community. It differs from its predecessor, Veratox for Gliadin, by using the R5 gliadin antibody. According to Neogen’s Vice President for Food Safety, Ed Bradley, the test conforms to the Codex Alimentarius recommendation, especially for use to test products destined for the export market, where this type of test is often favored. Testing has shown the performance of both the existing and revised versions to be comparable. Like its predecessor, the new test is intended for the quantitative analysis of in-process ingredients, clean-in-place solutions, and finished products.
Taylor said that the R5 antibody tests are the most popular and reliable, but there are other antibody sources. Romer Labs, for example, offers its AgraStrip® Gluten G12 Test Kit , a semi-quantitative lateral-flow test that uses a new-generation monoclonal antibody called G12 that specifically targets the toxic fragment (33-mer) of the gliadin protein in gluten that triggers the autoimmune reaction in celiac patients. According to Elisabeth Halbmayr-Jech, Technical Manager at Romer Labs, the company is developing a microwell ELISA using the G12 antibody. Work is being done on new extraction methods for it, and the company hopes to launch the test later this year.
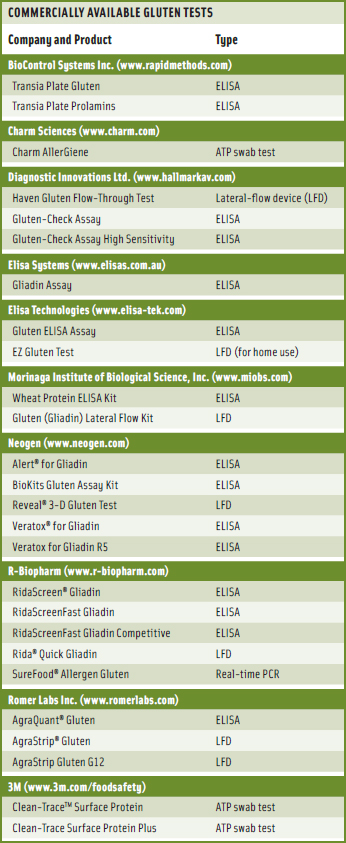
Numerous tests for the detection of gluten are commercially available (see table on p. 79) for companies to purchase for in-house use. Taylor pointed out that many of the companies that wish to enter the gluten-free market have limited in-house resources and need to send samples to external laboratories. He said that the FARRP Analytical Laboratory previously made its own test methods but now provides testing services to the food industry, using commercially available ELISA tests (usually the R5 ELISA tests). The laboratory also provides advice on validation and verification of gluten-free status. Other laboratories offer similar services.
Bradley said that among the most recent advances in gluten testing is the ability to conduct real-time testing to confirm that manufacturing lines have been properly cleaned to eliminate any allergenic residue prior to starting production of another product. Neogen’s Reveal 3-D line, for example, has been rapidly adopted, he said, because it is easy to use, provides accurate results, and does not disrupt normal manufacturing schedules, especially important when time to results is of greatest concern.
--- PAGE BREAK ---
Eric A.E. Garber, Research Chemist at the U.S. Food and Drug Administration (FDA) Center for Food Safety and Applied Nutrition (CFSAN), said that the FDA has been doing a lot of research on testing for gluten. Several years ago, when the commercial market for ELISA detection methods first started to grow, the FDA conducted systematic evaluations regarding grain specificity, using pure grains supplied by the U.S. Dept. of Agriculture’s Agricultural Research Service and examined the ability of the various methods to detect gluten in processed/cooked foods. Whenever the FDA analyzes a novel food, it performs a battery of controls to demonstrate the reliability of the methods being used and the level of error associated with the analysis.
Furthermore, he said, rather than just relying on currently available methods, three groups at CFSAN are working on novel multiplex methods with different endpoints based on different extraction and analytical principles. Garber noted that until the clinical pathology and biochemistry of celiac disease are fully understood, the development and application of analytical methods will consist of a series of successive approximations, constantly being refined to meet the consumer needs as they are better understood. It should be remembered, he said, that an estimated one in 133 Americans suffers from celiac sprue, and avoidance of doses that trigger the immunogenic response is currently the only method for managing the disease, hence the need for analytical methods to assure accurate and meaningful labeling.
 Challenges Ahead
Challenges Ahead
Among the challenges in analyzing foods for the presence of gluten are the complexity of gluten proteins, the diversity of analytical results provided by different test kits, the complexity of processed food samples, the variety of extraction procedures, and the lack of reference materials for validation.
Bradley said that one of the challenges ahead is the testing of complex food matrices without time-consuming and/or complicated extraction procedures and without sacrificing accuracy. Testing to protect consumers as well as commercial brands, he said, will increase as ease of use improves and time to results is decreased, as long as accuracy is not compromised.
Garber said that the most important challenge ahead comes from the revolution in modern analytical technology such that, with the necessary resources, almost anything can be achieved. This means that defining the questions and goals becomes more important with an appreciation of the resources and limitations that serve as context.
Taylor said that the biggest challenge ahead is validating existing methods. The various test methods may not give the same result on the same sample in every case, probably because antisera are different. But problems also exist with the standards. There needs to be an international reference standard for gluten, he said. This sounds easy but is incredibly complex. Cereal chemists, he noted, can’t really tell how much gluten is in wheat flour because it is variable and there is no gold-standard way to measure it. They have always used functional methods and not chemical methods. The MoniQA Network of Excellence (www.moniqa.org) and others are working on developing an international reference standard.
Additional challenges, he said, are harmonization—different test kit companies might use different criteria for judging the success of their methods—and obtaining consensus on threshold levels, setting a baseline for analytical sensitivity for these tests.
--- PAGE BREAK ---
Regulatory Status
The international standards-setting organization Codex Alimentarius Commission allows food products containing less than 20 ppm of gluten to be labeled as “gluten-free” and products containing 20–100 ppm to be labeled as “very low gluten.”
According to the FDA, data from the peer-reviewed scientific literature demonstrate that current analytical technology can reliably and consistently detect gluten in wheat, rye, and barley at levels of 20 ppm in a variety of food matrices. The commission has not yet defined “gluten-free” but has not objected to the use of the term in food labeling as long as it is not misleading.
The FDA proposed in January 2007 (Update: Final Rule passed August 2013) to define a gluten-free food as one that does not contain the following: any species of wheat, rye, barley, or their crossbred hybrids; any ingredient (such as wheat flour) derived from these grains that has not been processed to remove gluten; an ingredient (such as wheat starch) derived from any of these grains that has been processed to remove gluten but whose use results in 20 ppm or more of gluten in the food; or 20 ppm or more of gluten.
In its proposed rule, the FDA stated that it intended to conduct a safety assessment on gluten exposure in individuals with celiac disease for consideration in developing a final rule. According to FDA Policy Analyst/Press Officer Sebastian Cianci, the agency subsequently completed a lengthy safety assessment and submitted a draft report for review by an independent panel of scientific experts. The FDA has now revised its safety assessment report to address the experts’ comments and will soon publish a Federal Register notice to announce the report’s availability and solicit public comments on it and its potential use in the final rule on gluten-free food labeling. Release of the safety assessment report will be announced on the FDA’s website (http://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/allergens/default.htm). After the comment period closes and FDA has reviewed and considered the comments, Cianci said, the agency will issue the final rule on gluten-free food labeling as quickly as possible.
Additional Resources
The Food Allergy Research and Resource Program at the University of Nebraska-Lincoln (http://farrp.unl.edu), in cooperation with the Food Allergy and Anaphylaxis Network (www.foodallergy.org), has produced a publication called “Allergen Control in the Food Industry.” It discusses such topics as components of an effective allergen control plan for food processors; product design; segregation of allergenic foods or ingredients during receiving, storage, handling, and processing; supplier control programs for ingredients; prevention of cross-contamination during processing; and other topics. It is available at http://farrp.unl.edu/allergencontrolfi.
The Institute of Food Technologists developed a five-session online course called “Food Allergens: Regulations, Risks, and Controls” that covered mechanisms of allergenicity, food allergen regulations, risk in the global food supply, allergen control in food processing, plant management, and consumer communication. The program was initially presented in November 2010 and may be offered again in the near future.
The Fifth Food Technology, Innovation & Safety Forum 2011, “Building a Better Allergen Management Program,” will be held May 17–18, 2011, in Chicago, Ill. More information is available at www.thefoodsummit.com/programme.asp.
The Third MoniQA International Conference, “Food Safety and Consumer Protection,” will be held September 27–29, 2011, in Varna, Bulgaria. More information is available at http://varna2011.moniqa.org.
Neil H. Mermelstein, a Fellow of IFT, is Editor Emeritus of Food Technology
[email protected]


