Being Upfront With Front-of-Pack Labeling
The plethora of front-of-pack nutrition labels spurs the FDA and industry groups toward cooperation and standardization.
With the launch of First Lady Michelle Obama’s “Let’s Move” campaign in 2010 and the White House’s concern about obesity, America is more focused than ever on the impact diet has on health. An increasing number of shoppers are entering the supermarket looking for healthier food and beverage products to address specific disease states or to aide in their overall health and wellbeing.
Recognizing this opportunity to gain market share, food manufacturers are advertising their products’ health benefits on the front of packages. While the U.S. Food and Drug Administration (FDA) has begun to crack down on some of the messages that are considered health claims, the nutrient-content messages still abound.
The goal of most front-of-pack (FOP) nutrition messages is to provide consumers with a method to select more nutritious foods. However, in recent years the abundance of FOP messages has led to concerns about consumers being confused or even misled. In considering how FOP labeling should be used as a nutrition education tool in the future, in 2009 the U.S. Congress directed the Centers for Disease Control and Prevention (CDC) to undertake a study with the Institute of Medicine (IOM) on FOP nutrition rating systems and nutrition-related symbols. Once completed, the FDA will use the report to determine how best to proceed in potential regulation of FOP systems.
This article will examine the history of FOP systems, what the IOM has concluded after examining the strengths and weaknesses of current systems, and the ongoing research on consumers’ attitudes towards FOP systems. In addition, industry organizations are working to launch their own standardized approach to FOP systems. What will this look like and how will it affect the FDA’s decision?
Take Heart
In 1987, aiming to provide consumers with a single symbol that would indicate whether a food was “heart friendly,” the American Heart Association (AHA) created the Heart Guide symbol. In 1995, AHA began a new iteration of its FOP system, the Heart Check program, whose criteria were based on FDA coronary heart disease risk reduction claims, focusing first on levels of total and saturated fat and cholesterol, and later on fiber content (IOM, 2010). For a fee, food manufacturers can use this symbol on the front of food products that meet the AHA criteria.
--- PAGE BREAK ---
 When FOP systems first started appearing in the late 1980s and early 1990s, they were largely developed by nonprofit health organizations, such as the AHA. However, once General Mills launched its Goodness Center (to become Nutrition Highlights in 2007), and PepsiCo introduced its Smart Spot in 2004, many food manufacturers followed suit (Figure 1). With the increasing number of FOP systems being launched by retailers, manufacturers, and associations, concerns were raised about how a variety of systems would affect consumers. In 2006, the Center for Science in the Public Interest (CSPI) petitioned the FDA to design a national set of symbols to help consumers quickly identify healthier foods (CSPI, 2006).
When FOP systems first started appearing in the late 1980s and early 1990s, they were largely developed by nonprofit health organizations, such as the AHA. However, once General Mills launched its Goodness Center (to become Nutrition Highlights in 2007), and PepsiCo introduced its Smart Spot in 2004, many food manufacturers followed suit (Figure 1). With the increasing number of FOP systems being launched by retailers, manufacturers, and associations, concerns were raised about how a variety of systems would affect consumers. In 2006, the Center for Science in the Public Interest (CSPI) petitioned the FDA to design a national set of symbols to help consumers quickly identify healthier foods (CSPI, 2006).
“The supermarket is teeming with competing ‘healthy food’ symbols that run the gamut from highly helpful to fatally flawed,” said CSPI Executive Director Michael F. Jacobson in the 2006 press statement. “But a prominent and reliable symbol on the fronts of packages would be a tremendous help to those harried shoppers racing through the supermarket. Not everyone has the time or knowledge to scrutinize the Nutrition Facts labels or to decode the symbols Kraft, PepsiCo, General Mills, or other companies happen to be using.”
In response, the FDA held a public hearing to solicit information about the criteria used for displaying FOP symbols, as well as information regarding consumer experience with the FOP symbol programs currently in use. Then in 2008, the FDA issued guidance notifying the industry about the difference between FOP systems and implied nutrient content claims. The guidance reminded the industry that if a front-of-package symbol is viewed as an explicit or implied nutrient content claim, it must meet the current claim regulations. While the guidance aided in reinforcing regulations surrounding nutrient-content claims, the FDA still made no move to regulate FOP labeling as a whole.
This is where the not-for-profit Keystone Center stepped in and at the beginning of 2009 created the Smart Choices FOP system. According to the Smart Choices Web site, the program “was motivated by the need for single, trusted, and reliable front-of-pack labeling program that U.S. food manufacturers and retailers could voluntarily adopt to help consumers make more nutritious food and beverage choices” (Smart Choices, 2008). Backed by many of the nation’s largest food manufacturers, the green checkmark would appear on qualifying products to identify more nutritious choices within specific product categories. However, when the checkmark started appearing on sugar-laden cereals such as Froot Loops, there was a backlash against the system by the media and nutritionists. The FDA and U.S. Department of Agriculture also weighed in and sent a letter to the program’s managers on Aug. 19, 2009 stating: “FDA and FSIS would be concerned if any FOP labeling systems used criteria that were not stringent enough to protect consumers against misleading claims; were inconsistent with the Dietary Guidelines for Americans; or had the effect of encouraging consumers to choose highly processed foods and refined grains instead of fruits, vegetables, and whole grains” (FDA, 2009).
With the increasing criticism and ongoing consumer confusion, in October 2009, FDA Commissioner Margaret Hamburg announced that the agency planned to develop standardized criteria on which future front-of-package nutrition or shelf labeling will be based. Given this information, the Smart Choices program voluntarily postponed active operations and the participating manufacturers removed the Smart Choices symbol from products at the end of October.
--- PAGE BREAK ---
IOM Findings
Once directed by Congress to study FOP nutrition rating systems, the IOM formed the Committee on Examination of Front-of-Package Nutrition Rating Systems and Symbols and held its kick-off meeting in February 2010. It was decided that the study would be completed in two phases by an appointed IOM committee. Phase 1, which was completed in October 2010, reviewed the current systems and examined the strength and weaknesses of the nutrition science that underlies them to reach conclusions based on a nutrition perspective. Phase 2, to be completed Fall 2011, involves assessing consumer use and understanding of FOP symbols and determining which rating systems and symbols best promote public health (IOM, 2010).
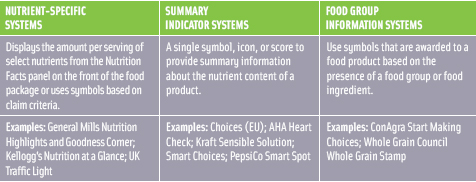 Given the vast number of FOP systems on the market, the IOM committee wasn’t able to evaluate each system, so they selected three category types to help compare systems (Figure 2) (IOM, 2010). In all, the committee examined 20 systems, each of which uses different nutrition criteria, resulting in differences in the ratings that the products receive. After analyzing the strengths and weaknesses of each system, the IOM reached some major decisions. First, the committee decided that the goal of FOP systems should be to help consumers identify and select food based on nutrients most linked to public health concerns for Americans. Given this goal and the rising rates of obesity in the United States, it was determined that in addition to calorie and serving size, the most critical nutritional components to include in FOP systems are saturated fat, trans fat, and sodium.
Given the vast number of FOP systems on the market, the IOM committee wasn’t able to evaluate each system, so they selected three category types to help compare systems (Figure 2) (IOM, 2010). In all, the committee examined 20 systems, each of which uses different nutrition criteria, resulting in differences in the ratings that the products receive. After analyzing the strengths and weaknesses of each system, the IOM reached some major decisions. First, the committee decided that the goal of FOP systems should be to help consumers identify and select food based on nutrients most linked to public health concerns for Americans. Given this goal and the rising rates of obesity in the United States, it was determined that in addition to calorie and serving size, the most critical nutritional components to include in FOP systems are saturated fat, trans fat, and sodium.
“Calories, saturated fat, trans fats, and sodium present the most serious diet-related risks to people’s health, and many Americans consume far too much of these nutrients,” said Committee Chair Ellen Wartella, Director, Center on Media and Human Development, School of Communication, Northwestern University. “As Americans grapple with increasing rates of serious health problems connected to their diets, it’s important that the nutritional information they receive is clear, consistent, and well-grounded in nutrition science.”
In addition, the committee concluded that “there is insufficient evidence to support inclusion of the following nutrients on FOP labels: total fat, cholesterol, total carbohydrate, total or added sugars, protein, fiber, vitamins, and minerals other than sodium” (IOM, 2010). This finding is based on the relative importance of these nutrients to the most pressing diet-related health concerns and challenges for measuring compliance. In addition, the IOM committee believes including nutrients (e.g., fiber and certain vitamins and minerals) in FOP systems may encourage over-fortification or the addition of these nutrients to food systems in which the nutrient is unstable or not biologically available, which would contradict FDA fortification policy.
Consumer Behavior Research
The IOM kicked off Phase 2 of its examination of FOP systems and symbols on Oct. 26, 2010 with a public meeting to review consumer research and hear public comments. The goals for the committee in Phase 2 is to consider: 1) which systems and symbols are most effective with consumer audiences; 2) how to maximize their use; and 3) the potential benefits of a single, standardized FOP labeling system regulated by the FDA.
--- PAGE BREAK ---
At the Oct. 26 meeting, Chung-Tung Jordan Lin and Alan Levy from the FDA’s Center for Food Safety and Applied Nutrition presented their findings from recent FDA consumer studies of FOP nutrition rating systems. The goals of the FDA’s studieswere to assess: the ability of FOP schemes to encourage healthy choices; consumer perceptions of trustworthiness and usefulness of FOP schemes; and noticeability of different types of schemes.
The FDA found that participants were most likely to choose the healthier food when using the traffic light symbol—using green, yellow, and red to indicate nutrient levels as “high,” “medium,” and “low,” respectively. Of the four FOP schemes tested, the FDA researchers ranked the perceived utility in descending order as follows: traffic light symbol, nutrition tips (amounts of calories, total fat, saturated fat, sodium, and sugars, in addition to words characterizing the level of each nutrient), calories only symbol, and Smart Choice checkmark. However, Lin and Levy also concluded that “no FOP system was able to inspire consumers to focus on nutrition when making product decisions,” and that only the Nutrition Facts label was able to do this. The researchers mentioned that this effect may be due to the “brand identity” of Nutrition Facts as a symbol of thinking about health. Therefore, it is important to note that whatever FOP system is adopted will require consumer education and a positive marketplace experience to make the system effective (Lin and Levy, 2010).
Industry Initiatives
At the same time that the IOM launched Phase 2 of its research, the Grocery Manufacturers Association (GMA) and the Food Marketing Institute (FMI) announced that they are working together to develop a FOP nutrition labeling system. The GMA is an industry association with more than 300 food, beverage, and consumer product companies as members. FMI is an association that conducts programs in public affairs, food safety, research, education, and industry relations on behalf of its 1,500 member companies made up of food retailers and wholesalers. The FDA Guidance released in October 2009 called upon the industry to voluntarily utilize a common, credible approach to nutrition-related FOP system. This sparked the GMA and FMI to begin a dialog with the FDA and establish a goal of developing a unified FOP labeling system.
“More than a year ago we pledged to the administration that we would develop a front-of-pack labeling system that could be broadly adopted by industry and that would be effective with consumers. We solicited input from the FDA, public health professionals, packaging experts, and consumers and that’s all led to our announcement on Oct. 27,” explained Scott Faber, Vice President of Federal Affairs, GMA.
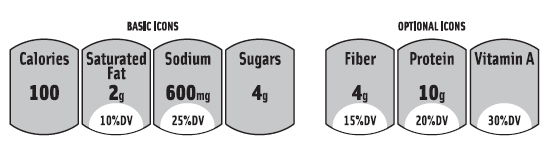 At press time, the proposed GMA/FMI FOP labeling system will add important nutrition information on calories and other nutrients to limit (saturated fat, sodium, and sugars) to the front of the food and beverage packages. It might even have the option of listing up to three beneficial nutrients, such as fiber, protein, and vitamin A. (Figure 3 shows a mock up of what the GMA/FMI label might look, as presented to the IOM in October 2010.) “We believe the labeling program we are developing is consistent with the FDA’s front-of-pack guidance,” said Leslie Sarasin, FMI President and CEO. “We are in agreement with the FDA on the purpose and general principles of the labeling program and continue to work towards finalizing the details.”
At press time, the proposed GMA/FMI FOP labeling system will add important nutrition information on calories and other nutrients to limit (saturated fat, sodium, and sugars) to the front of the food and beverage packages. It might even have the option of listing up to three beneficial nutrients, such as fiber, protein, and vitamin A. (Figure 3 shows a mock up of what the GMA/FMI label might look, as presented to the IOM in October 2010.) “We believe the labeling program we are developing is consistent with the FDA’s front-of-pack guidance,” said Leslie Sarasin, FMI President and CEO. “We are in agreement with the FDA on the purpose and general principles of the labeling program and continue to work towards finalizing the details.”
--- PAGE BREAK ---
To ensure consumer comprehension and understanding of FOP labeling systems, in the summer of 2010, the GMA provided a grant to the International Food Information Council (IFIC) Foundation to conduct consumer research. Marianne Smith Edge, IFIC’s Senior Vice President of Nutrition and Food Safety, presented the findings from their GMA-funded research study at the Oct. 26 IOM meeting.
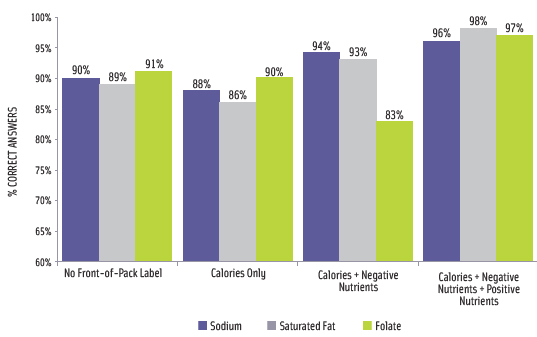 IFIC conducted an online survey in August and September 2010 that tested an FOP symbol that included icons for calories, saturated fat, sodium, and sugars, as well as optional icons for beneficial nutrients. The survey found that consumers were more frequently able to accurately find and state nutritional content when the relevant information appeared on the front of package (Smith Edge, 2010). Importantly, it appeared that the presence of positive nutrients on the FOP label did not interfere with the consumer’s ability to accurately find and state caloric or negative nutrient content (Figure 4). “When consumers were asked to find specific nutrition information that was available on the front of the package, they viewed the Nutrition Facts panel far less often, with no or improved impact on accuracy,” said Smith Edge. Across all labeling systems tested and for all product categories, a large majority of participants were able to select the product considered to be “high health.” Very few stated that the products were the same or they were unsure.
IFIC conducted an online survey in August and September 2010 that tested an FOP symbol that included icons for calories, saturated fat, sodium, and sugars, as well as optional icons for beneficial nutrients. The survey found that consumers were more frequently able to accurately find and state nutritional content when the relevant information appeared on the front of package (Smith Edge, 2010). Importantly, it appeared that the presence of positive nutrients on the FOP label did not interfere with the consumer’s ability to accurately find and state caloric or negative nutrient content (Figure 4). “When consumers were asked to find specific nutrition information that was available on the front of the package, they viewed the Nutrition Facts panel far less often, with no or improved impact on accuracy,” said Smith Edge. Across all labeling systems tested and for all product categories, a large majority of participants were able to select the product considered to be “high health.” Very few stated that the products were the same or they were unsure.
Using the insight garnered from the IFIC studies, the GMA and FMI will work on finalizing the label design and expect it to begin to appear on packages in grocery stores in early 2011. At press time, Sarasin explained that several companies, representing thousands of products, have already pledged to participate in the FOP program. “We expect the number of companies and products participating will grow once the program is in place,” said Sarasin. To build consumer awareness and promote use of the new label, the participating food and beverage manufacturers and retailers have agreed to support the change to their product labels with a $50 million consumer education campaign.
In a similar initiative, the American Beverage Association (ABA) announced in February 2010 that America's nonalcoholic beverage companies are voluntarily coming together to put the calorie information on the front of all their packages, vending machines, and fountain machines.
The industry has already begun implementing the initiative and expects to be completed in 2012 (ABA, 2010).
--- PAGE BREAK ---
What the Future Holds
After the IOM completes Phase 2, the final report will be given to the FDA to consider possible regulation of FOP labeling. It is apparent from the crackdown of front-of-pack nutrient claims that the FDA is very aware of the importance of FOP labeling and will therefore place it high on its priority list. However, it is well known that the regulatory process can move very slowly. “The FDA decision is going to take years in terms of getting a final rule,” said Karen Duester, President and Regulatory Specialist for Food Consulting Co. “I wouldn’t expect to see a definite industry compliance deadline before 2014 at the very earliest.” In addition, it is possible that any FDA guidance or rule on FOP labeling might come out in conjunction with a reformatted Nutrition Facts panel. According to Duester, this new Nutrition Facts panel might take into account the new Daily Values that the IOM has had for a quite a while now, in addition to the prominence and types of nutrients included on the panel.
Meanwhile, at press time the goal of the GMA/FMI is to have its label on packages in stores in early 2011. And the ABA’s calories label is already appearing on many non-alcoholic beverages. How will the industry’s FOP labels affect or interact with the FDA’s future decision? While it is a bit too early to speculate on the FDA’s final decision for FOP labeling, many regulatory specialists, lawyers, and consultants agree that it is a step in the right direction for industry to be involved and stay active in the process.
“I think it’s very important for industry to be involved in this process and I think the GMA, FMI, and ABA are doing exactly the right thing,” said Anthony Pavel, Partner at K&L Gates. “The more robust a program the industry can develop that is a fair, even-minded program that relates critical information in a clear manner to consumers is beneficial and smart.”
Ricardo Carvajal, Counsel at Hyman, Phelps & McNamara P.C., agrees: “The Commissioner of the FDA has expressed a desire to address FOP labeling issues in cooperation with industry. As long as a spirit of cooperation and information sharing prevails, the end result should be something everyone can work with.”
By cooperating with the FDA, the food industry demonstrates that it doesn’t want to confuse consumers with a plethora of FOP labeling. However, it also hopes to sway the agency to allow other FOP messages that help differentiate a product at the point of sale. In Phase 1, the IOM recommends listing only nutrients associated with diet-related health risks. Obviously, the industry is still going to want to also highlight positive aspects of the product. At press time, the proposed GMA/FMI FOP label included the option to list up to three beneficial nutrients in addition to the nutrients to limit. As the IOM works on Phase 2 of its report, it’s possible that the committee will keep a watchful eye on the launch of the GMA/FMI FOP label and assess its potential impact on, and acceptance by, consumers. In any case, it is highly likely that the FDA will study the IOM report and industry initiatives such as the GMA/FMI label before making its final decision on FOP labeling.
In the end, whatever FOP system is chosen by FDA, whether voluntary or mandatory, needs to be widely embraced in order for manufacturers to use it and consumers to ultimately trust it. “If the industry can help shape this program and the program is in line with a good portion of what the industry is looking for, it definitely puts some weight behind whatever system is ultimately developed,” said Pavel. After all, the goal of a standardized FOP label is to help consumers more readily understand the nutrition facts of food and beverages, hopefully making it easier for them to choose healthier options.
Kelly Frederick is Digital Media Editor, Institute of Food Technologists
([email protected]).
References
ABA. 2010. Beverage Industry Will Make Calories More Clear and Useable for Consumers. February. American Beverage Association, Washington, D.C. http://www.ameribev.org/news--media/news-releases--statements/more/180/.
CSPI. 2006. FDA Urged to Create New “Healthy Food” Labeling System. November. Center for Science in the Public Interest, Washington, D.C. http://www.cspinet.org/new/200611301.html.
FDA. 2008. Guidance for Industry: Dear Manufacturer Letter Regarding Front-of-Package Symbols. December. U.S. Food and Drug Administration, Washington, D.C. http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodLabelingNutrition/ucm120274.htm.
FDA. 2009. Letter to the Smart Choices Program. August. U.S. Food and Drug Administration, Washington, D.C. http://www.fda.gov/Food/LabelingNutrition/LabelClaims/ucm180146.htm.
FDA. 2009. Guidance for Industry: Letter Regarding Point of Purchase Food Labeling. October. U.S. Food and Drug Administration, Washington, D.C. http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodLabelingNutrition/ucm187208.htm.
GMA. 2010. Food and Beverage Industry Announces Front-of-Pack Nutrition Labeling Initiative to Inform Consumers and Combat Obesity. October. Grocery Manufacturers Association, Washington, D.C. http://www.gmaonline.org/news-events/newsroom/food-and-beverage-industry-announces-front-ofpack-nutrition-labeling-initi/.
IFIC. 2010. Front of Pack Labeling Consumer Research Project. October. International Food Information Council Foundation, Washington, D.C. http://www.iom.edu/~/media/Files/Activity%20Files/Nutrition/NutritionSymbols/Marianne%20Smith%20Edge%20IFIC%20FOP%20SLIDES%20for%20IOM%201026101.pdf.
IOM. 2010. Examination of Front-of-Package Nutrition Rating Systems and Symbols: Phase I Report. October. Institute of Medicine, Washington, D.C. www.iom.edu.
Jordan Lin, C. and Levy, A. 2010. Food and Drug Administration Front-of-Pack Consumer Research. October. U.S. Food and Drug Administration, Washington, D.C. http://www.iom.edu/~/media/Files/Activity%20Files/Nutrition/NutritionSymbols/Jordan%20Lin%20FDA%20FOP%20studies%20for%20IOM%2010262010ppt%20Compatibility%20Mode.pdf.
Smart Choices Program. 2008. New Front-of-Pack Nutrition Labeling Program Designed to Help Shoppers Make More Nutritious Food and Beverage Choices. October. The Keystone Center, Keystone, Colo. www.smartchoicesprogram.com.
