Advances In Flavor Encapsulation
New flavor delivery technologies are creating significant opportunities for food product developers by supplying improved flavor character to finished products.
Flavor is a combination of aroma (volatile aromatic components) and taste or gustatory components (sweet, sour, umami, salty, and bitter) plus any oral sensation of chemical heat or cooling. To most professionals in the food industry, the concept of flavor delivery immediately suggests encapsulation as the preferred commercial modality. However, there is no one delivery (or encapsulation) system that can accept all types of flavors while supplying low cost, ease of production, and functionality with food-approved agents for all applications.
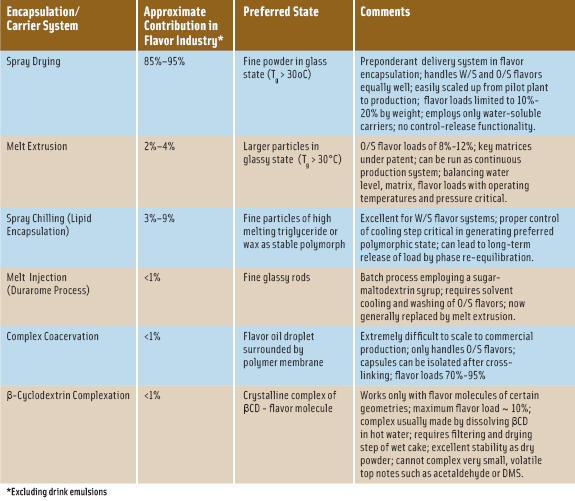 There are numerous reviews of flavor encapsulation technologies that give a general overview and introduction into technical aspects of specific systems (Gouin, 2004; Lakkis, 2007; Madene, Jacquot, Scher, and Desobry, 2006; Ubbink, 2003; Zuidam and Nedov, 2010). In Table 1, some of the important commercial encapsulation systems or delivery vehicles are noted with their relative contribution within the food-flavor industry in addition to key attributes achieved with their use. Two of the major encapsulation systems will be discussed, some significant considerations noted in their use, and potential options cited to extend their use.
There are numerous reviews of flavor encapsulation technologies that give a general overview and introduction into technical aspects of specific systems (Gouin, 2004; Lakkis, 2007; Madene, Jacquot, Scher, and Desobry, 2006; Ubbink, 2003; Zuidam and Nedov, 2010). In Table 1, some of the important commercial encapsulation systems or delivery vehicles are noted with their relative contribution within the food-flavor industry in addition to key attributes achieved with their use. Two of the major encapsulation systems will be discussed, some significant considerations noted in their use, and potential options cited to extend their use.
Spray Drying
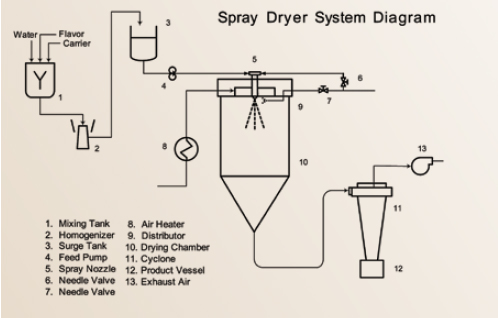 Spray drying has been the major standard process for preparation of dried food ingredients and encapsulated flavors during the last 100 years (Masters, 1997). This system is uniquely suited to handle both oil-soluble (O/S) and water-soluble (W/S) flavors and is the key production system for commercially encapsulated flavors (Porzio, 2007) (Figure 1). Standard emulsion carrier formulations consist mainly of a surface active polymer (gum arabic or OSAN starches) combined with intermediate molecular weight polymers (e.g., maltodextrins, polydextrose) and lower weight molecular packing agents such as mono- and disaccharides, corn syrup solids, and polyols.
Spray drying has been the major standard process for preparation of dried food ingredients and encapsulated flavors during the last 100 years (Masters, 1997). This system is uniquely suited to handle both oil-soluble (O/S) and water-soluble (W/S) flavors and is the key production system for commercially encapsulated flavors (Porzio, 2007) (Figure 1). Standard emulsion carrier formulations consist mainly of a surface active polymer (gum arabic or OSAN starches) combined with intermediate molecular weight polymers (e.g., maltodextrins, polydextrose) and lower weight molecular packing agents such as mono- and disaccharides, corn syrup solids, and polyols.
--- PAGE BREAK ---
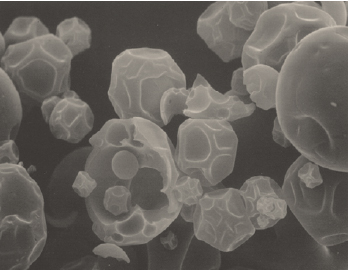 Employing either a nozzle (pneumatic) or wheel (centrifugal head) atomizer, a flavor emulsion is dispersed into a hot air plenum as fine droplets and rapidly dried. The atomized emulsions are initially dispersed as spherical liquid droplets into a dryer air stream. These aqueous emulsion droplets generally consist of up to 40% dissolved carrier(s) solids, approximately 10% flavor, and up to 60% water. The rapid drying process mandates that approximately 50% or more of the liquid droplet volume (as water) be rapidly removed. This operation leads the resulting semi-solid particles to undergo severe stress and distortion until a dried particle is finally obtained. Scanning electron microscopy (SEM) studies show that the spray-dried particles are in the form of collapsed soccer balls along with broken capsules, deformed particles, and cracked hollow particles (Rosenberg, 1988). When properly executed, spray drying yields a low-moisture powder in a glassy state.
Employing either a nozzle (pneumatic) or wheel (centrifugal head) atomizer, a flavor emulsion is dispersed into a hot air plenum as fine droplets and rapidly dried. The atomized emulsions are initially dispersed as spherical liquid droplets into a dryer air stream. These aqueous emulsion droplets generally consist of up to 40% dissolved carrier(s) solids, approximately 10% flavor, and up to 60% water. The rapid drying process mandates that approximately 50% or more of the liquid droplet volume (as water) be rapidly removed. This operation leads the resulting semi-solid particles to undergo severe stress and distortion until a dried particle is finally obtained. Scanning electron microscopy (SEM) studies show that the spray-dried particles are in the form of collapsed soccer balls along with broken capsules, deformed particles, and cracked hollow particles (Rosenberg, 1988). When properly executed, spray drying yields a low-moisture powder in a glassy state.
A patent disclosing use of specialized carrier solutes improves functionality in spray-dried products. In a very sophisticated approach for its time, J. Brenner obtained very high loads of flavor oils by identifying a polymer-plasticizer carrier formulation that remained in the plastic state during the particle drying process (Brenner et al., 1976). These formulas resulted in flavor loads of up to 40%–60% by volume. SEM photographs confirmed that a spherical particle with none of the collapsed character, cracked cell walls, or broken shells was present as had been noted with other formulas. The particle remained in a plastic state until the end of the drying process, forming a glassy shell with enclosed flavor oil.
The standard spray-drying encapsulation system carries with it several limitations. All encapsulation carriers are by design totally water soluble so that the resulting dried flavor particles are rapidly released by moisture. Increasing the dissolved carrier solids in the emulsion is desirable for production and recovery efficiency, but the increasing emulsion viscosity soon limits the nozzle or centrifugal head atomizers from efficient droplet dispersal and ultimately generates non-spherical droplets. These larger dispersed emulsion droplets are more likely to be incompletely dried, leading to caking on dryer walls.
An alternative is to identify an emulsion droplet dispersing process system that can handle increased solution viscosities in the range of 700–10,000 centipoise while retaining the flavor oil within dispersed droplets. Such a system could employ a variety of functional food polymers (e.g., alginates, carageenans, xanthans, pectins, methylcelluloses, guar gums, locust bean gum, gellans, curdlan, etc.) in combination with lower molecular weight solutes in the flavor emulsion. The rehydration character of these polymers should delay release by retarding hydration when placed in an aqueous product environment.
--- PAGE BREAK ---
One commercial drying system designed to efficiently disperse both slurries and high viscosity solutions for spray drying is the sonic dryer. This system uses a sonic horn to atomize and pulse very fine droplets of fluid slurry into a dryer at low pressures while making use of mild drying conditions. There is little doubt that high viscosity, increased solids solutions of water-soluble flavor systems would be very compatible with this system. However, the physics of sonic field atomization of flavor emulsions containing oil droplets is not well characterized for this spray-drying system. Several issues need consideration with a pulsed sonic dryer:
• Are the atomized droplets formed as spherical droplets with the flavor oil dispersed within?
• What percentage of flavor oil will be found on the dried flavor powder as surface oil?
• Do oxidation-sensitive terpene oils show greater or lesser levels of oxidative off-flavors compared to standard spray-dried systems?
In one pilot plant test, a pulsed sonic drying system was employed to dry a standard spray carrier system consisting of gum arabic/maltose/cinnamon oil, resulting in 77% recovery of the encapsulated flavor. When substituting three other nontraditional polymer carriers with identical co-carrier and oil levels, the resulting dried products exhibited recoveries of 22% to 61% of oil flavor. Because the dryer operating variables were not optimized, some improvements in recovery and yield might be attained. Also, an alternative practical option would be use of surfactants to form liquid crystal membranes at the oil-water interface to retard lipid droplets from separating from the aqueous phase with sonic horn dispersion.
Melt Extrusion: Glass Encapsulation
Melt extrusion, also referred to as extrusion encapsulation or glass encapsulation, has become a standard encapsulation process used by a number of flavor houses (Porzio, 2008) (Figure 2). This system utilizes a co-rotating twin screw extruder, employing various carbohydrate-polymer matrices to generate a glassy matrix with encapsulated flavors. However, the high internal temperatures developed by mechanical friction, or by block heating in cooking extruders, leads to high internal pressures and exit temperatures of molten exudate with a resulting puffing and flashing off of moisture and flavors.
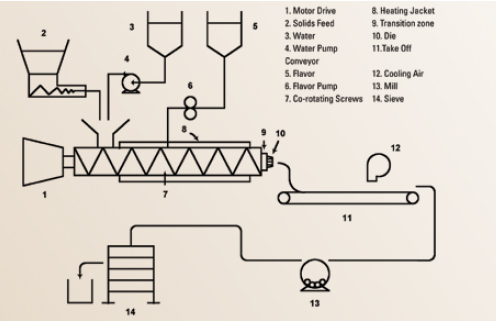 Optimized melt extrusion systems employed for flavor encapsulation must balance lower melt temperatures, reduced internal pressures, limited water levels with shaft RPM, and die geometries to generate a plastic melt that exits the die without flashing. This melt must be rapidly solidified via air cooling to generate a fractural glassy solid within a few seconds on the receiving/take-off belt. A balanced melt extruder encapsulation system can be run continuously by feeding flavor, plasticizer (water), and matrix streams; melting, mixing, and dispersing flavor; expelling; cooling; milling; sieving; and packaging. In one process variation the flavor can be injected under pressure into the melt zone and dispersed before being conveyed through the die.
Optimized melt extrusion systems employed for flavor encapsulation must balance lower melt temperatures, reduced internal pressures, limited water levels with shaft RPM, and die geometries to generate a plastic melt that exits the die without flashing. This melt must be rapidly solidified via air cooling to generate a fractural glassy solid within a few seconds on the receiving/take-off belt. A balanced melt extruder encapsulation system can be run continuously by feeding flavor, plasticizer (water), and matrix streams; melting, mixing, and dispersing flavor; expelling; cooling; milling; sieving; and packaging. In one process variation the flavor can be injected under pressure into the melt zone and dispersed before being conveyed through the die.
--- PAGE BREAK ---
A key function of the extruder is to generate the glassy state (Levine and Slade, 1988; Levine, 2002). A glass is the higher energy, amorphous solid in contrast to the rubbery amorphous state. This solid is characterized by its glass transition temperature, which is routinely determined by differential scanning calorimetry (DSC) or modulating differential scanning calorimetry (MDSC) analyses. The glass transition temperature is normally identified as a temperature range, but by convention, reported as a single midpoint temperature in that range with the notation Tg. Desirable glass transition temperatures of the cooled solid exudate glass should be 30oC–45oC.
An alternative option with melt extrusion is to take a molten flavormatrix and cool the exiting melt under pressure to prevent flash distillation of moisture and flavor. This concept of pressure cooling an extruded melt has been described in a patent (Fulger and Popplewell, 1997). For example, acetaldehyde (a key low-boiling flavor top-note) was added to the melt, cooled under pressure, and retained in the resulting glass matrix. The same process but cooled at ambient pressure exhibited reduced yields and numerous pores in the matrix from the flashing of the volatile agents, decreasing the density of solid particles.
A modified practical version of the system would require significant capital expenditures to design and manufacture. Installing a manifold to divert the molten exudate while under pressure into cooling chambers and then cooling the melt would yield a pressurized glassy state solid. Using a pressure release lock to uncouple the chamber, equalizing the pressures to ambient conditions, would release the glassy product in an efficient manner to allow for continuous or semi-continuous production.
Critical aspects of any melt extrusion system are the specific extruder system, manufacturer, size, L/D, and screw configurations. More importantly, oil (flavor) loads are limited to a maximum of 12%, at which increasing surface oils on the solid particles become significant. The use of gelatin (and hydrolyzed gelatins) as a polymer melt yields oil flavor loads up to 20% in a glass matrix with very limited amounts of surface oil (Porzio and Popplewell, 1997; Porzio and Zasypkin, 2004). Unfortunately, with gelatins (or hydrolyzed gelatins), flavors containing aldehydes and ketones react with protein amines, forming adducts and unbalancing flavors.
For those familiar with this technique, there is another option to avoid current restricting patent barriers while obtaining flavor oil loads of up to 20%–25%. This system would still employ similar functional polymer matrices (avoiding gelatin) to yield a glassy solid matrix. Here these benefits come with an increase in processing costs. Water-soluble flavors would be equally adaptable to this alternate system.
--- PAGE BREAK ---
New Encapsulation Opportunities
There are many successful approaches which have been developed that yield unique encapsulated flavor functionalities by understanding flavormatrix-processing dynamics. Four such vehicles follow.
Thermal Stable (Bake Proof) Flavors
The volatile components of most flavors are quite sensitive to temperatures above ambient conditions. Not only are the key top-notes lost by heating, but the intermediate volatile flavor compounds dissipate at different rates to unbalance flavor sensory character. Encapsulation designed for thermal release has been applied to nonvolatile agents. By using higher melting lipids or waxes as coating agents on organic acid particles, the baking industry can release the acid upon heating to react with leavening agents in products such as refrigerated dough. This is a simple, effective approach designed for that specific technical problem.
Another option is to place the flavor in an encapsulation media, which retards flavor release during the heating process even in the presence of moisture. After the heating process is completed and the food system cools, the flavor is then released. One innovative technique takes advantage of specific, thermally reversible polymer transitions. Polymers with this functionality are the methylcelluloses and hydroxypropyl-methylcelluloses (HPMC). These polymers are water-soluble and surface active, show significant viscosity at low concentrations, and exhibit a uniquely reversible thermal response curve when transitioning from solution to gel upon heating and returning to a solubilized hydrated polymer upon cooling (Dow, 1986).
Unfortunately, soluble methylcelluloses are quite sensitive to carbohydrate oligomers and food polymers in solution and will phase out at ambient temperatures in the presence of some of the standard co-agents.
Thus a practical route employing thermally reversible gelling polymers can be achieved by means of a sophisticated understanding of polymer phase responses. By controlling solution and phase inversion properties of methylcelluloses and HPMC with flavor carrier systems, it is possible to encapsulate flavors in a commercial system, resulting in both thermal protection and controlled release in most food applications. The final product is a dry powder that can be pre-added to bake mixes, cooked half products, and doughs to protect flavors during the mixing and baking steps but releases flavors upon cooling for increased flavor impact.
--- PAGE BREAK ---
Modulating Flavor Carriers
The term modulating flavor carrier (MFC) refers to an encapsulating flavor carrier that effects transduction of flavor and taste sensory signals at cellular receptor sites by amplifying or muting specific flavor and taste sensory signals. This technique allows use of much lower levels of flavor agents as spray dried flavors or full replacement of other functional flavor-contributing ingredients. When employed, the MFC is perceived as having the full flavor character of the original. Modulating flavor systems have been developed and commercialized over the last 30 years by different flavor houses.
This advanced flavor delivery technology employs and combines several techniques:
• using flavor enhancer(s) (and, for masking, flavor maskers),
• employing compounded flavors, balanced for the sensory profile requirements of the MFC,
• identifying and employing cell wall signal transducers,
• matching unique flavor activators for the MFC, and
• using key flavor adjuvants matched to the flavor.
In addition, this technology requires critical processing control of the phase states in the original dispersion and during drying. Examples of modulated flavor systems include dairy ingredient replacers, fruit solids replacers, heat (capsaicin) amplification, and salty character enhancement. Also, MFCs can mask bitterness, potato earthiness, metallic notes, and musty notes as well as artificial sweetener aftertastes, the taste of plastic, and bitter notes from emulsifiers.
Any route to MFC formula development is predicated on effective sensory evaluations by trained sensory panels. The flavor system can be tested in water or in a final product and rated in relation to some gold-standard flavor or current product. The subtleties of the key ingredient interactions and states are extremely sensitive, requiring critically balanced ratios of key ingredients. The subtlety and potency of an MFC was exhibited in formulating a seasoning mix with a specific flavor adjuvant. Samples were prepared and evaluated by trained sensory panelists. The sample with the flavor adjuvant at 15 parts per trillion as consumed gave improved flavor impact over the control (i.e., no adjuvant) on a statistically relevant basis. When properly developed, MFCs can replace up to 95% of standard, compounded, spray-dried flavors. Key dairy and fruit powders can be replaced at a 100% level. Sodium chloride’s salty character (Na+ signals) can be amplified to yield an MFC that can function for up to 95% Na+ replacement. Potassium chloride bitter notes can be masked to yield a sodium-free but organoleptically equivalent NaCl replacer. Heat capsaicin levels can be amplified 5- to 10-fold with the concomitant decrease in formulary use. The limitations of MFCs are noted in terms of specific product applications. Heat and moisture sensitivities limit use of MFC systems in retorted or other sterilized liquid systems.
--- PAGE BREAK ---
Complex Coacervation
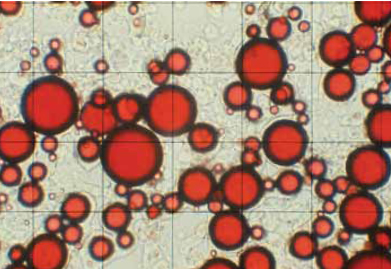 Complex coacervation is based on studies of oppositely charged, solvated polymers phasing out from solution (Bungenburg de Jong, 1949) and the first commercialization patent (Green, 1957). This encapsulation system functions by depositing the co-polymer gel, which phases out slowly, wetting and enrobing a dispersed, immiscible oil droplet. The process is a kinetically controlled system in which the polymer coacervate slowly phases out of solution. By balancing the phase dynamics of wetting, coating, and deposition, each oil droplet is ultimately surrounded by a coacervate polymer membrane (Thies, 1977; 1985). The negative aspects of the process are numerous:
Complex coacervation is based on studies of oppositely charged, solvated polymers phasing out from solution (Bungenburg de Jong, 1949) and the first commercialization patent (Green, 1957). This encapsulation system functions by depositing the co-polymer gel, which phases out slowly, wetting and enrobing a dispersed, immiscible oil droplet. The process is a kinetically controlled system in which the polymer coacervate slowly phases out of solution. By balancing the phase dynamics of wetting, coating, and deposition, each oil droplet is ultimately surrounded by a coacervate polymer membrane (Thies, 1977; 1985). The negative aspects of the process are numerous:
• the system is extremely difficult to scale from laboratory to production volumes (Cainich,1975);
• surface active and water-soluble agents in the flavor can inhibit the flavor system from being completely enrobed by polymer;
• commercial sources of oleoresins containing added surfactants to aid in normal aqueous dispersion will interfere completely and prevent membrane formation;
• water-soluble flavors are not encapsulated by this system.
Another significant issue is that each flavor system, flavor oil, or oleoresin must be re-developed in lab tests to verify it can be encapsulated by complex coacervation and the new process conditions identified before commercialization. A clever method to avoid these issues has been disclosed (Soper, 2000). Here bland-tasting oils such as medium-chain triglycerides, hydrogenated, high-stability oils, or fractionated vegetable oils are encapsulated by complex coacervation, forming a flavorless oil capsule. Then a desired flavor is partitioned into the oil through the porous, hydrated membrane before sealing the flavor oil-coacervate membrane. These flavor-free coacervated capsules can be sold as a commercial product to other customers as is or used as an intermediate to generate a finished encapsulated flavor.
A more preferred method avoids all the physical-chemical dynamics involved in coacervate phase formation by means of a non-kinetic process. An additional positive benefit of this system is that it can encapsulate flavors containing water-soluble fractions that normally interfere with the standard complex coacervation process. This novel route has been commercialized by both a flavor and a pharmaceutical house. The key technical insights needed resulted from a sophisticated understanding and control of interfacial dynamics, wetting energies, and mechanical input along with adjunct agents to generate this complex coacervate-flavor encapsulation system.
--- PAGE BREAK ---
Extrusion Reaction Flavors
Reaction flavors attempt to duplicate the complex flavors prepared during frying, baking, roasting, or sautéing. Using flavor precursors with the appropriate heating processes, manufactured reaction flavors avoid use of whole tissue. These reaction flavors are usually manufactured as liquid systems with preselected ingredients at controlled pH and reaction temperature-time conditions in either oil, water, or oil-water binary reaction media. By employing a predetermined heating profile, the maximum flavor character/intensity is obtained. The liquid system is separated from any resulting precipitates, filtered, and packed off. If emulsions are employed, any reaction flavors found in one phase must be separated from the other liquid phase. In selected cases, both water- and oil-soluble flavors constitute the complete flavor system and must remain combined.
In these cases, two options are available. One is to emulsify and spray dry the combined phases. The other option is to add both surfactants and alcoholic agents to form a stable single liquid phase as a microemulsion.
The melt extrusion process system can be employed to produce a number of reaction flavors. These reaction flavor systems depend on Maillard and Amadori reactions between specific reducing sugars and amino acids. Flavors that can be developed are characterized as nutty, chocolate, butterscotch, graham, savory (chicken, pork, beef), or caramel and have been manufactured employing a twin-screw extruder. The advantage of this extrusion system lies in continuous high throughput production along with the added benefit of encapsulating the resultant flavor in a glassy matrix. The amino acid-reducing sugar precursors can be added to the dry feed going into the extruder. Higher levels of these agents may be limited to prevent off-flavor development.
If the short residence time and heating profile do not produce stronger reaction flavors, other options are available. For example, the reducing sugar-amino acid materials can be pre-dissolved in water and added as the plasticizing agent to the extruder.
If more complex or kinetically limited reaction intermediates are needed, the sugar-amino acid system can be dissolved in water, pH adjusted, heated to generate intermediates, and spray dried. The dried intermediates can then be added to the feed stream in the extruder.
Fried flavor is a much desired reaction flavor system that can also be manufactured by a melt extrusion process. In one study, a researcher used preheated oil injected into a plasticized flour matrix in the melt zone of an extruder (Pitchon, 1980). Evaluation of this process reveals more of a cooked note and not a true fried flavor.
For true extrusion fried flavors, a unique flavor precursor is added to a specific carbohydrate matrix formula. The extruder operating variables (i.e., heating profile, screw configuration, and feed rate) are adjusted and optimized to generate fried notes. The product is a solid glass encapsulated fried flavor that can be used as a surface enrobing flavor system for snacks and baked products.
--- PAGE BREAK ---
Flavor Delivery Programs and Novel Technical Vehicles
A specific R&D program model, which has worked successfully over the last 40 years in the food-flavor industry, has developed significant new flavor delivery technologies and technical platforms. A program is initiated with a business plan supported and funded by upper management. This plan requires input and buy-in from key related business functions including sales, marketing, and manufacturing as well as R&D.
Once a plan is established, sufficient technical expertise must be assembled in the form of an internal R&D matrix organization. Many technical specialties will be required to support such a program, including sensory testing, applications labs, analytical support for both physical and chemical analyses, process engineering systems and staff, and critically, flavorists.
The physics and chemistry of flavor delivery and encapsulation meet in the arena of physical chemistry. Involvement in any new or modified encapsulation technique requires familiarity with concepts involving the glassy state, amorphous lipids and lipid polymorphs, surfactant mesophases, phase diagrams, solution chemistry, interfacial and colloid chemistry, partitioning responses, flavor molecular interactions, reaction kinetics, polymer responses in solutions and melts, and the chemical and sensory aspects of flavors.
Additionally, unique materials and ingredient interactions rarely studied but noted in the literature may be utilized: polymer-polymer melt incompatibility, polymer-flavor fixation, organogels, hydrogels, clathrates, conjoined crystals, liquid crystals, crystalline-flavor complexes, supercritical fluids, eutectics, inorganic phases, bi-phasic aqueous systems (water-in-water [w/w] emulsions), CO2-induced capillary condensation of flavors, and new rationally designed flavors.
There are multiple benefits in the development of these new, uniquely functional flavor delivery systems. They include proprietary insulation, reduced costs with flavor use, improved products, customer loyalty, consumer friendly nutritional labels, and leverage with ingredient supply chain issues of quality, cost, and availability.
Michael Porzio, Ph.D., a Professional Member of IFT, is Consultant, Flavor Delivery Systems, P.O. Box 382, Hunt Valley, MD 21030 ([email protected]).
References
Brenner, J., Henderson, G., and Bergenster, R. 1976. Process for encapsulating oil and products produced thereby. U.S. Patent 3,971,852.
Bungenburg de Jong, H.G. 1938. Proc. Acad. Sci., Amsterdam, 41 pg. 646.
Cainich, V.A. 1975. Microencapsulation: scale-up considerations and production. Drugs Pharm. Sci. 41: 221–255.
Dow Chemical Company. 1986. Methocel premium food gums [technical brief].
Fulger C. and Popplewell, M. 1997. Flavor encapsulation. U.S. Patent 5,601,865.
Gouin, S. 2004. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 15: 30-347.
Green, B.K. and Schleicher, L. 1957. U.S. Patent 2,800,457.
Lakkis, J., ed. 2007. Encapsulation and Controlled Release Technologies in Food Systems. Blackwell Publishing, Oxford, UK.
Levine, H. 2002. Amorphous Food and Pharmaceutical Systems. Royal Society of Chemistry, Cambridge, U.K.
Levine, H. and Slade, L. 1988. Thermomechanical properties of small carbohydrate-water-glasses and rubbers. J. Chem. Soc. Faraday Trans. 184: 2169–2633.
Madene, A., Jacquot, M., Scher, J., and Desobry, S. 2006. Flavor encapsulation and controlled release—a review. Intl. J. Food Sci. Technol. 41: 1-21.
Masters, K. 1979. Spray Drying Handbook. John Wiley and Sons Inc., New York.
Pitchon, E. 1980. Food extrusion process. U.S. Patent 4,225,630.
Porzio, M. 2007. Spray drying. Perfumer & Flavorist 32(11): 34–39.
Porzio, M. 2008. Melt extrusion and melt injection. Perfumer and Flavorist 33(6): 48–53.
Porzio, M. and Popplewell, M. 1997. Encapsulation compositions. U.S. Patent 5,603,971 and continuation patents: U.S. Patent 5,897,897; U.S. Patent 6,187,351; U.S. Patent 6,652,895; U.S. Patent 6,790,453; U.S. Patent 6,416,799
Porzio, M. and Zasypkin, D. 2004. Encapsulation compositions and process for preparing the same. U.S. Patent 6,790,453 and continuation patents: U.S. Patent 7,488,503; U.S. Patent 7,779,341.
Rosenberg, M., Talmon, Y., and Kopelman, I.J. 1988. Microstructure of spray dried microcapsules. Food Microstruct. 7(1): 15–23.
Soper, J.C., Kim, Y.D., and Thomas, M.T. 2000. Method of encapsulating flavors and fragrances by controlled water transport into microcapsules. U.S. Patent 6,045,835.
Thies, C. 1975. Physiochemical aspects of microencapsulation. Polymer and Plastics Technology Engineering 5(1): 1–22.
Thies, C. 1987. Microencapsulation. Encyclopedia of Polymer Science and Engineering 9: 724–745.
Ubbink, J. 2003. Flavor delivery systems. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons.
Zuidam, N.J. and Nedov, V., eds. 2010. Encapsulation and Control Release Technologies for Active Food Ingredients and Food Processing. Springer, New York.
