Clostridium Difficile: An Emerging Food Safety Risk
Traditionally associated with healthcare environments, C. difficile has been increasingly isolated from packaged foods and food animals, raising concerns about foodborne and zoonotic transmission to humans.
Once a relatively obscure organism to the general public, Clostridium difficile has gained recognition as a highly virulent intestinal microorganism capable of causing protracted hospital-associated diarrheal illnesses, usually in situations when antibiotics are administered (Taubes, 2008). Contributing factors to this greater awareness are a corresponding increase in the incidence of C. difficile infections (CDI), heightened severity of disease symptoms with higher reoccurrence rates, and an increase in antimicrobial resistance in the Unites States (Wiegand et al., 2012). The increase in CDI cases has spurred increased concern regarding the growing segment of community-associated infections distinct from infections acquired in healthcare settings (Limbago et al., 2009). Further, molecular studies in various regions indicate that contaminated foods might serve as a contributing vector for an increasing number of CDIs. 
Understanding the Bacterium
Like Clostridium botulinum, C. difficile is known as a mesophilic, spore-forming bacterium requiring anaerobic conditions for growth, although tolerance and growth have been noted under hypoxic conditions across various strains. Unlike C. botulinum, C. difficile does not produce paralytic neurotoxins nor does it replicate opportunistically in moist, low-acid foods (Cato et al., 1986). The predominant reservoir of C. difficile is the gastrointestinal tract of humans and warm-blooded animals. C. difficile was first named Bacillus difficilis due to its difficulty in isolation. In the gut, it is a relatively slow-growing bacterium when compared to other intestinal bacteria (Cato et al., 1986).
Reported growth temperature ranges from 25–45°C with optima between 30 and 37°C; however, some strains grow faster at higher temperatures. Similar to other pathogenic spore formers, C. difficile is relatively unaffected by exposure to cooking temperatures ≤72oC (Rodriguez-Palacios et al., 2010). Spores are inactivated by 5–6 log10 at 85oC for 15 min, but higher temperatures are needed to ensure spore elimination (Rodriguez-Palacios et al., 2011). Phenotypically, C. difficile closely resembles C. sporogenes; however, C. difficile cannot digest meat or milk and does not produce lipases (Cato et al., 1986).
Understanding the Disease
First isolated from the stools of healthy newborns in 1935, the pathogenic potential of C. difficile went unnoticed until the mid-1970s. Once the association of C. difficile to pseudomembranous colitis (PMC) was established, the epidemiology of C. difficile infections was studied in detail.
--- PAGE BREAK ---
Elderly and immune-compromised individuals stand the highest risk for CDI and PMC. The severity of PMC is highly variable. The disease gets its name from characteristic patchy lesions that appear on the lining of the large intestine. Toxins produced by C. difficile kill the tissues lining the bowel. PMC often progresses to life-threatening conditions, including toxic megacolon and bowel perforation. Toxic megacolon involves rapid widening of the colon that can also occur as a result of ulcerative colitis and Crohn’s disease. Most toxic megacolons require immediate surgery and removal. Less frequently, C. difficile reaches other parts of the body causing abscesses or local and generalized infections.
CDI do not always manifest as PMC. The most common symptoms are watery, foulsmelling stools with an odor similar to horse manure (due to p-cresol production by C. difficile) and abdominal pain and fever that may continue for weeks (Bartlett, 2008). Recurrence of CDI occurs in up to 36% of cases; this percentage has increased in recent years in the U.S. It is estimated that C. difficile is responsible for at least one-quarter of all antibiotic-associated diarrheas, half of antibiotic-associated colitis, and virtually all cases of PMC. Only Campylobacter jejuni is thought to cause more cases of bacterial diarrhea; however, numbers of CDI are on the rise. In 2005, there were nearly 300,000 hospitalizations for CDI in the U.S. per annum, an increase of almost 160,000 since 2000. Current estimates are around 500,000. Infection control measures have helped, but with exceptions, the rising trend remains.
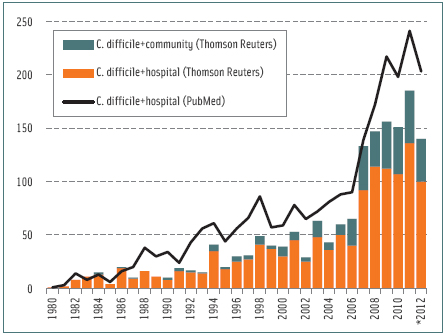 Because its association with the administration of antibiotics was recognized early on in studies involving C. difficile infections, one of the original references to PMC was as “clindamycin colitis” (Tedesco et al., 1974). However, virtually any antibiotic can disturb the intestinal biota and predispose CDI. CDI seldom occur without prior antibiotic therapy. Metronidazole and vancomycin are first-choice anti-C. difficile antibiotics used to treat infections, but CDI at times occur following therapy with those antibiotics. It appears that a number of intestinal flora imbalances in susceptible individuals can lead to CDI, not just antibiotic therapy. Nasogastric tube insertion, enteral feeding, enema administration, surgery, inflammatory bowel diseases, cancer, HIV, chemotherapy, and antacids can increase the risk of CDI. Alarmingly, CDI have also worsened the prognosis and outcome of patients suffering inflammatory bowel diseases (Navaneethan et al., 2012). Overall, the incidence and severity of CDI in various countries have made C. difficile a global public health challenge; often surpassing the importance of methicillin-resistant Staphylococcus aureus (Lessa et al. 2012).
Because its association with the administration of antibiotics was recognized early on in studies involving C. difficile infections, one of the original references to PMC was as “clindamycin colitis” (Tedesco et al., 1974). However, virtually any antibiotic can disturb the intestinal biota and predispose CDI. CDI seldom occur without prior antibiotic therapy. Metronidazole and vancomycin are first-choice anti-C. difficile antibiotics used to treat infections, but CDI at times occur following therapy with those antibiotics. It appears that a number of intestinal flora imbalances in susceptible individuals can lead to CDI, not just antibiotic therapy. Nasogastric tube insertion, enteral feeding, enema administration, surgery, inflammatory bowel diseases, cancer, HIV, chemotherapy, and antacids can increase the risk of CDI. Alarmingly, CDI have also worsened the prognosis and outcome of patients suffering inflammatory bowel diseases (Navaneethan et al., 2012). Overall, the incidence and severity of CDI in various countries have made C. difficile a global public health challenge; often surpassing the importance of methicillin-resistant Staphylococcus aureus (Lessa et al. 2012).
--- PAGE BREAK ---
Mechanism of Disease
The two primary agents of CDI are toxins A and B (Kuehne et al., 2010). These are among the largest polypeptide toxins known (250–300 kilodaltons). Toxin A is a potent enterotoxin with slight cytotoxic activity while toxin B is an extremely potent cytotoxin. Both cause damage to the gut lining by disrupting the cell cytoskeleton causing cell rounding, impaired function, and death. Some C. difficile also produce a third, unrelated toxin, called the binary toxin or C. difficile transferase (CDT). CDT modifies G-actin by ADP-ribosylation to inhibit actin polymerization (Schwan et al., 2009). Although associated with recent epidemic strains, CDT appears significantly less of a contributing disease-causing factor than toxins A and B. CDT may facilitate colonization by inducing the formation of cell protrusions, but its contribution to disease remains unclear.
It is estimated that 3–5% of healthy adults are asymptomatic carriers of toxigenic C. difficile. This number increases to 20% in hospitalized adults, but 60–70% of infants less than one year of age harbor the pathogen intestinally. Most infants can have toxigenic C. difficile and detectable toxins A and B in their stools, yet be completely asymptomatic. Although the reasons for such silent colonization in infants are not well understood, they seem to confer protection as children grow older (Jangi and Lamont, 2010). In adults, colonization with nontoxigenic C. difficile seems to protect against CDI.
Since the early 2000s, several C. difficile strains associated with outbreaks of severe CDI appear to be more virulent, resistant to more antibiotics, and capable of producing much more toxins (Warny et al., 2005). Such strains of C. difficile were found in food animals and retail foods (Rodriguez-Palacios et al., 2006; 2007; 2009), raising earlier questions of foodborne and zoonotic CDI transmission (Gould and Limbago, 2010).
Probiotic Treatments
Ironically, the best treatment for CDI involves the administration of antimicrobials (e.g., metronidazole, vancomycin) which cannot replace lost gut biota. Further, C. difficile is gaining resistance against metronidazole and vancomycin (Pelaez et al., 2008). Consequently, therapeutic microbiology (e.g., probiotic use as supplements or suppositories) has gained popularity. Typical probiotic organisms studied include specific strains of lactobacilli and bifidobacteria, and the yeasts Saccharomyces boulardii and S. cerevisiae.
For CDI, the primary goals of probiotic use are to prevent infection and repopulate lost gut biota with beneficial organisms to reduce the risk of recurrent infections. There is a wide range of responses in clinical trials involving probiotics. Results are affected by different conditions of application, patient populations, probiotic strains, and lengths of study. A review of randomized studies during 1967–2007 showed only two reported beneficial effects (Pillai and Nelson, 2008). Clearly, the most effective strains and treatment parameters are under development.
--- PAGE BREAK ---
In addition to the use of lactic acid bacteria, the use of non-toxigenic (and thus non-pathogenic) strains of C. difficile has been long proposed as a reasonable probiotic strategy (Wilson and Sheagren, 1983). Their presence and growth in the intestinal tract may protect against toxigenic strains and CDI. Non-toxigenic strains of C. difficile appear to be widely present in natural environments and animals. The protective role of naturally present non-toxigenic strains, also found in foods, is uncertain.
A more unconventional principle of improving the intestinal ecology of humans suffering protracted CDI has been borrowed from veterinary medicine, there called ruminal transfaunation. In humans, the comparable approach is fecal transplantation (also called human probiotic infusion or fecal bacteriotherapy), which has been applied to some individuals as a last resort to treat severe CDI and inflammatory bowel diseases. Treated patients receive either a series of fecal transplants composed of biota from healthy close relatives via nasogastric intubations or enemas. Results have been promising.
Animal Reservoirs and Zoonotic Potential
In textbooks, CDI is still described as a disease acquired via exposure to environments contaminated with C. difficile, namely healthcare settings. However, there is increasing evidence to suggest that environments outside hospitals, and particularly animals and foods, are an unrecognized source for exposure to C. difficile; i.e., no official comprehensive preventive measures are publicly available to reduce such risk. Established habitats for C. difficile are the human bowel and genital tract, marine sediments and salt water, soil, sand, fresh water, and hospital environments. C. difficile has also been isolated from rivers and estuaries, soils, and root vegetables since the 1980s (Borriello et al., 1983; Janezic et al., 2012). Additionally, C. difficile has been found in camels, horses, donkeys, dogs, cats, birds, food animals such as swine, chickens, and cattle, and wildlife species (French et al., 2010; Thakur et al., 2011). Since 2005, there is increasing molecular evidence that isolates from animals and humans are likely to be shared, indicating the risk for zoonotic transmission (Arroyo et al., 2005).
About 7% of healthy horses carry C. difficile (Medina-Torres et al., 2011), and since horse manure can contain viable spores of C. difficile for years, use of horse manure as a traditional organic fertilizer can be considered a zoonotic concern. In most food animals (poultry, cattle, and pigs), young animals appear to be more frequently colonized by C. difficile than older animals. For example, over 60% of poultry early in production carried C. difficile, but by the time of slaughter rates dropped between 6 and 12% (Zidaric et al., 2008).
Occurrence in Foods
Bacterial endospores can persist for years even under extreme environments (Baverud, 2003), and as expected, a range of different foods carry spores of C. difficile (Gould and Limbago, 2010). The first food products examined and found incidentally positive for C. difficile were spoiled gas-blown packages of raw ground beef and pork (Broda et al., 1996). The spoilage population was described as psychrotrophic clostridia; further testing isolated C. difficile that proved negative for gas production. Since 2006, epidemic toxigenic strains of C. difficile responsible for disease in humans have been found in various retail meats. The occurrence of C. difficile ranged widely from 0–42% in various categories of packaged meats. In a sampling study at the retail store level, 6% of establishments across three provinces of Canada had at least one product with C. difficile (Rodriguez-Palacios et al., 2009). In Europe, the highest rate of contamination was reported this year in edible mollusks in Italy, 49% (Pasquale et al., 2012).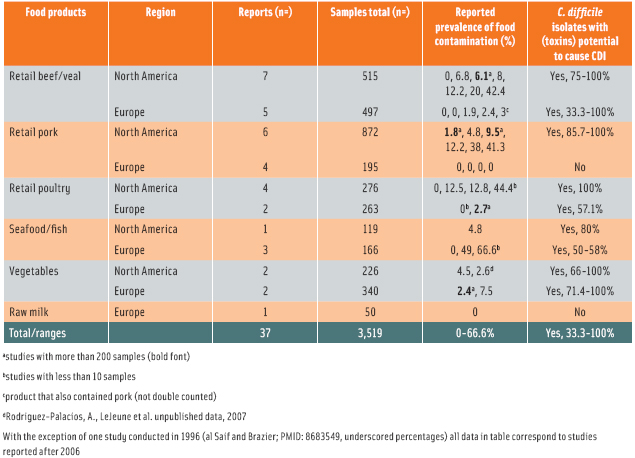
--- PAGE BREAK ---
More recently, poultry have also been shown to carry toxigenic strains (Weese et al., 2010; Harvey et al., 2011). The frequency of C. difficile contamination oscillates between 3 and 18% with variability across different parts of the chicken. A newly emerging toxigenic strain for CDI (PCR ribotype 078) appears to predominate in various foods in the U.S. and Canada. Additionally, C. difficile is also isolatable from vegetables, seafood, and fish in North America (Metcalf et al., 2010; 2011). From the studies that have been published in the past 10 years, it is discomforting to observe that hyper-virulent strains of C. difficile (e.g., PCR ribotypes 027 and 078; Ackerlund et al., 2008) are the most common strains isolated from foods. The reasons for this predominance are unknown, although differential heat resistance favoring predominant strains can contribute (Rodriguez-Palacios, 2011). Recent genome-wide molecular studies testing pork products in the U.S. indicate that C. difficile contamination can occur before or during meat processing (Curry et al., 2012). Although C. difficile isolation protocols vary, a summary of reports on C. difficile and foods (see Table) indicates that contamination seems higher in North America (U.S./Canada) than in Europe. Standardized protocols are needed for risk assessment across regions and food products.
Disinfection
Because C. difficile spores could be transferred to susceptible hosts or food-handling surfaces via contaminated hands, there are efforts to enhance the removal or elimination of spores from human skin. Thus far, hand-washing is the most effective means to eliminate (i.e., rinse away) C. difficile from hands, which is especially important for individuals in healthcare centers.
Bleach (1:10 diluted solutions of 5.3–6.2% sodium hypochlorite) is commonly used for routine environmental disinfection and along with glutaraldehyde (for specialized use) are highly effective contact disinfectants. Effectiveness is related to humidity and exposure time. Guidelines to combat C. difficile have been prepared and constantly updated by professional organizations such as the Society for Hospital Epidemiologists of America, the Infectious Diseases Society of America, and the Association for Professionals in Infection Control and Epidemiology.
Comparisons to Established Foodborne Pathogens
It is estimated that 20–27% of CDI cases are acquired and occur in the community, not in a hospital environment. There have been no confirmed cases of foodborne disease caused by C. difficile. In part, it was the purpose of this article to raise awareness to the possibility that such an event or events could occur. Relevant questions to be answered relate to aspects of the infective dose of C. difficile in humans, factors that favor the presence of C. difficile in foods, and the promising role of therapeutic microbiology.
As we know, bacteria have a track record for mutating and adapting and consequently emerging as a serious threat to human health. In our food supply, C. difficile appears to be another organism on which we need to keep close tabs.
--- PAGE BREAK ---
Acknowledgement
This article evolved from the IFT symposium, Clostridium difficile: Is it a foodborne pathogen?, presented at the 2011 IFT Annual Meeting & Food Expo®, New Orleans, LA, and sponsored by the Food Microbiology Div.
Alex Rodriguez-Palacios, Ph.D., ([email protected]), former student member of IFT, is Postdoctoral Scholar, Division of Gastroenterology and Liver Disease, Dept. of Medicine, Case Western Reserve University School of Medicine, Cleveland, OH 44106.
Jeffrey T. LeJeune, Ph.D., ([email protected]) a member of IFT, is Professor, Food Animal Health Research Program, The Ohio State University, Wooster, OH 44691.
Dallas G. Hoover, Ph.D., ([email protected]) a Professional member of IFT, is Professor, Dept. of Animal & Food Sciences, University of Delaware, Newark, DE 19716-2150.
References
Ackerlund, T., Persson, I., Unemo, M., Noren, T., Svenungsson, B., Wullt, M., and Burman, L.G. 2008. Increased sporulation rate of epidemic Clostridium difficile Type 027/NAP1. J. Clin. Microbiol. 46: 1530-1533.
Arroyo, L.G., Kruth, S.A., Willey, B.M., Staempfli, H.R., Low, D.E., and Weese, J.S. 2005. PCR ribotyping of Clostridium difficile isolates originating from human and animal sources. J. Med. Microbiol. 54: 163-166.
Bartlett, J.G. 2008. Historical perspectives on studies of Clostridium difficile and C. difficile infection. Clin. Infect. Dis. 46 Suppl 1: S4-11.
Baverud, V., Gustafsson, A., Franklin, A., Aspan, A., and Gunnarsson, A. 2003. Clostridium difficile: Prevalence in horses and environment, and antimicrobial susceptibility. Equine Vet. J. 35: 465-471.
Borriello, S.P., Honour, P., Turner, T., and Barclay, F. 1983. Household pets as a potential reservoir for Clostridium difficile infection. J. Clin. Pathol. 36: 84-87.
Broda, D.M., DeLacy, K.M., Bell, R.G., Braggins, T.J., and Cook, R.I. 1996. Psychrotrophic Clostridium sp. associated with ‘blown pack’ spoilage of chilled vacuum-packed red meats and dog rolls in gas-impermeable plastic casings. Int. J. Food Microbiol. 29: 335-352.
Cato, E.P., George, W.L., and Finegold, S.M. 1986. Genus Clostridium. in Bergey’s Manual of Systematic Bacteriology, volume 2, Sneath, P.H.A., Mair, N.S., Sharpe, M.E., and Holt, J.G. (eds.). Williams & Wilkins, Baltimore, pp. 1141-1200.
Curry, S.R., Marsh, J.W., Schlackman, J.L., and Harrison, L.H. 2012. Prevalence of Clostridium difficile in uncooked ground meat products from Pittsburgh, PA. Appl. Environ. Microbiol. 78: 4183-4186.
French, E., Rodriguez-Palacios, A., and LeJeune, J.T. 2010. Enteric bacterial pathogens with zoonotic potential isolated from farm-raised deer. Foodborne Pathog. Dis. 7(9): 1031-7.
Gould, L.H. and Limbago, B. 2010. Clostridium difficile in food and domestic animals: A new foodborne pathogen? Clin. Infect. Dis. 51: 577-582.
Harvey, R.B., Norman, K.N., Andrews, K., Hume, M.E., Scanlan, C.M., Callaway, T.R., Anderson, R.C., and Nisbet, D.J. 2011. Clostridium difficile in poultry and poultry meat. Emerg. Infect. Dis. 8: 1321-1323.
Janezic, S., Ocepek, M., Zidaric, V., and Rupnik, M. 2012. Clostridium difficile genotypes other than ribotype 078 that are prevalent among human, animal and environmental isolates. BMC Microbiol. Doi: 10.1186/1471-2180-12-48.
Jangi, S. and Lamont, J.T. 2010. Asymptomatic colonization by Clostridium difficile in infants: Implications for disease in later life. J. Pediatr. Gastroenterol. Nutr. 51: 2-7.
Kuehne, S.A., Cartman, S.T., Heap, J.T., Michelle, K., Cockayne, L., Minton, A., and Nigel, P. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467(7316): 711-713.
Lessa, F.C., Gould C.V., and McDonald, L.C. 2012. Current status of Clostridium difficile infection epidemiology. Clin. Infect. Dis. 55(suppl-2): S65–S70.
Limbago, B., Long, C., Thompson, A., Killgore, G., Hannett, G., Havill, N., Mickelson, S., Lathrop, S., Jones, T., Park, M., Harriman, K., Gould, H., McDonald, L.C., and Angelo, F.J. 2009. Clostridium difficile strains from community-associated infections. J. Clin. Microbiol. 47: 3004-3007.
Medina-Torres, C.F., Weese, J.S., and Staempfli, H.R. 2011. Prevalence of Clostridium difficile in horses. Vet. Microbiol. 152: 212-215.
Metcalf, D.S., Avery, B.P., Janecko, N., Matic, N., Reid-Smith, R., and Weese, J.S. 2011. Clostridium difficile in seafood and fish. Anaerobe 17: 85-86.
Metcalf, D.S., Costa, M.C., Dew, M.W., and Weese, J.S. 2010. Clostridium difficile in vegetables, Canada. Lett. Appl. Microbiol. 51: 600-602.
Navaneethan, U., Mukewar, S., Venkatesh, P.G., Lopez, R., and Shen, B. 2012. Clostridium difficile infection is associated with worse long-term outcome in patients with ulcerative colitis. J. Crohns Colitis 3: 330-336.
Pasquale, V., Romano, V., Rupnik, M., Capuano, F., Bove, D., Aliberti, F., Krovacek, K., and Dumontet, S. 2012. Occurrence of toxigenic Clostridium difficile in edible bivalve molluscs. Food Microbiol. 31: 309-312.
Pelaez, T., Cercenado, E., Alcala, L., Marin, M., Martin-Lopez, A., Martinez-Alarcon, J., Catalan, P., Sanchez-Somolinos, M., and Bouza, E. 2008. Metronidazole resistance in Clostridium difficile is heterogeneous. J. Clin. Microbiol 46: 3028-3032.
Pillai, A. and Nelson, R. 2008. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev (1): CD004611.
Rodriguez-Palacios, A., Staempfli, H.R., Duffield, T., Peregrine, A.S., Trotz-Williams, L.A., Arroyo, L.G., Brazier, J.S., and Weese, J.S. 2006. Clostridium difficile PCR ribotypes in calves in Canada. Emerg. Infect. Dis. 12: 1730-1736.
Rodriguez-Palacios, A., Staempfli, H.R., Duffield, T., and Weese, J.S. 2007. Clostridium difficile in retail ground meat in Canada. Emerg. Infect. Dis. 13: 485-487.
Rodriguez-Palacios, A., Reid-Smith, R.J., Staempf li, H.R., Daignault, D., Janecko, N., Avery, B.P., Martin, H., Thompson, A.D., McDonald, L.C., Limbago, B., and Weese, J.S. 2009. Possible seasonality of Clostridium dif ficile in retail meat in Canada. Emerg. Infect. Dis. 15: 802-805.
Rodriguez-Palacios, A., Reid-Smith, R. J., Staempfli, H. R., and Weese, J. S. 2010. Clostridium difficile survives minimal temperature recommended for cooking ground meats. Anaerobe 16: 540-542.
Rodriguez-Palacios, A. and LeJeune, J.T. 2011. Moist-heat resistance, spore aging, and superdormancy in Clostridium difficile. Appl. Environ. Microbiol. 77: 3085-3091.
Rodriguez-Palacios, A. 2011. Ecology and Epidemiology of Human Pathogen Clostridium difficile in Foods, Food Animals and Wildlife. Ph.D. thesis dissertation. The Ohio State University.
Schwan, C., Stecher, B.R., Tzivelekidis, T., van Ham, M., Rohde, M., Hardt, W-D., Wehland, J., and Aktories, K. 2009. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathogens, 5: e1000626.1
Tedesco, F.J., Stanley, R.J., and Alpers, D.H. 1974. Diagnostic features of clindamycin-associated pseudomembranous colitis. N. Engl. J. Med. 290: 841-843.
Taubes, G. 2008. Collateral damage. The rise of resistant Clostridium difficile. Science 321(5887): 360.
Thakur, S., Sandfoss, M., Kennedy-Stoskopf, S., and Deperno, C.S. 2011. Detection of Clostridium difficile and Salmonella in feral swine populations in North Carolina. J. Wildlife Dis. 47: 774-776.
Warny, M., Pepin, J., Fang, A., Kilgore, G., Thompson, A., Brazier, J., Frost, E., and McDonald, L.C. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366: 1079-1084.
Weese, J.S., Reid-Smith, R.J., Avery, B.P., and Rousseau, J. 2010. Detection and characterization of Clostridium difficile in retail chicken. Lett. Appl. Microbiol. 50: 362-365.
Wiegand, P.N., Nathwani, D., Wilcox, M.H., Stephens, J., Shelbaya, A., and Haider, S. 2012. Clinical and economic burden of Clostridium difficile infection in Europe: A systematic review of healthcare-facility-acquired infection. J. Hosp. Infect. 81: 1-14.
Wilson, K.H. and Sheagren, J.N. 1983. Antagonism of toxigenic Clostridium difficile by nontoxigenic C. difficile. J. Infect. Dis. 147: 733-736.
Zidaric, V., Zemljic, M., Janezic, S., Kocuvan, A., and Rupnik, M. 2008. High diversity of Clostridium difficile genotypes isolated from a single poultry farm producing replacement laying hens. Anaerobe 14: 325-327.
