Testing Nanomaterial Safety
FOOD SAFETY & QUALITY
Nanotechnology—the study of matter measuring 100 nanometers or less in at least one dimension—has become increasingly investigated for more than a decade. Organizations in the United States and other countries have been actively supporting and conducting research on nanotechnology, including the unique properties, potential applications, and safety of these materials and how they relate to food. The following describes some of what’s being done by U.S. federal agencies with regard to nanotechnology and food.
National Nanotechnology Initiative
The U.S. National Nanotechnology Initiative (NNI) (nano.gov) is the U.S. government’s multiagency, multidisciplinary research and development program on nanotechnology. It brings together the expertise of 27 federal agencies and supports collaborative research and development in academic, government, and industry laboratories. Speaking at IFT’s 2012 Annual Meeting & Food Expo last June, Sally Tinkle, Deputy Director and Environmental, Health, and Safety Coordinator of the National Nanotechnology Coordination Office of the NNI, said that nanotechnology holds great promise for food applications, including food safety and security, maintaining product freshness, identifying contaminants, improving food packaging and food processing technologies, and improving nutrition through enhanced bioavailability and efficacy. She described some of the results that are already occurring with respect to food safety, such as the use of Raman spectroscopy to differentiate Salmonella strains and the development of biosensors that use antibodies to identify contaminants with unprecedented sensitivity.
She described the NNI’s research efforts to provide information that regulatory agencies need to perform risk assessments that protect public health and the environment. These are as follows: research on nanoscale phenomena, structures, and processes; research on nanomaterial properties so that nanostructures with targeted properties can be designed; research and development to create novel devices and systems or improve existing ones; research and development of instruments for characterization, measurement, synthesis, and design of nanomaterials, structures, devices, and systems; research and development related to manufacturing of nanoscale materials, structures, devices, and systems; establishment of major research facilities and acquisition of instrumentation; development of educational materials for schools, undergraduate programs, technical training, and public engagement and communication; and research on understanding the environmental, health, and safety impacts of nanotechnology development and corresponding risk assessment, risk management, and risk mitigation.
National Toxicology Program
 The U.S. National Toxicology Program (NTP) (http://ntp.niehs.nih.gov) is an interagency program whose mission is to develop and apply tools of modern toxicology and molecular biology to evaluate agents of public health concern. It involves conducting toxicological evaluations, developing and validating improved testing methods, developing approaches and generating data to strengthen the science base for risk assessment, and communicating with all stakeholders. The NTP has a broadbased research program to address potential human health hazards associated with the manufacture and use of nanoscale materials. The NTP Nanotechnology Safety Initiative (http://ntp.niehs.nih.gov/go/nanotech) is conducting research on several classes of nanoscale materials, including metal oxides, fluorescent crystalline semiconductors (quantum dots), carbon-based fullerenes, carbon nanotubes, nanoscale silver, and nanoscale gold.
The U.S. National Toxicology Program (NTP) (http://ntp.niehs.nih.gov) is an interagency program whose mission is to develop and apply tools of modern toxicology and molecular biology to evaluate agents of public health concern. It involves conducting toxicological evaluations, developing and validating improved testing methods, developing approaches and generating data to strengthen the science base for risk assessment, and communicating with all stakeholders. The NTP has a broadbased research program to address potential human health hazards associated with the manufacture and use of nanoscale materials. The NTP Nanotechnology Safety Initiative (http://ntp.niehs.nih.gov/go/nanotech) is conducting research on several classes of nanoscale materials, including metal oxides, fluorescent crystalline semiconductors (quantum dots), carbon-based fullerenes, carbon nanotubes, nanoscale silver, and nanoscale gold.
U.S. Food and Drug Administration
The Center for Food Safety and Applied Nutrition (CFSAN) at the U.S. Food and Drug Administration (FDA) has a research program to explore the safety assessment of nanomaterials in food and cosmetic products. It focuses on understanding the interaction of a variety of matrices and nanomaterials and exploring the potential use of electron spin resonance spectroscopy as a rapid screening tool, determining in vitro the dermal penetration of nanomaterials in cosmetics, examining the possibility of nanomaterial leaching from food packaging materials and determining whether there is a safety concern, and investigating approaches to study the potential toxicity of nanomaterials. The FDA’s National Center for Toxicological Research is developing analytical tools and procedures to quantify nanomaterials in complex matrices and conducting toxicity studies on nanomaterials.
--- PAGE BREAK ---
The FDA has also issued draft guidance documents on nanotechnology for the food and cosmetic industries. On June 14, 2011, the agency issued “Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology,” and on April 20, 2012, it issued “Safety of Nanomaterials in Cosmetic Products” and “Assessing the Effects of Significant Manufacturing Process Changes, including Emerging Technologies, on the Safety and Regulatory Status of Food Ingredients and Food Contact Substances, Including Food Ingredients that are Color Additives.” The latter document describes what food manufacturers should consider when determining whether a significant change in manufacturing process for a food substance already in the market—particularly intentional alterations of particle-size distribution on the nanometer scale—affects the food substance’s identity, safety, or regulatory status and warrants a regulatory submission to FDA. The agency expects to issue a final guidance document by the end of this year.
Since the FDA has not yet established formal definitions of “nanotechnology,” “nanoscale,” and related terms, the draft guidance documents address particles with sizes of 1 nm – 1,000 nm. The agency is considering whether the definition should be based on size alone or whether additional criteria such as shape, charge, the ratio of surface area to volume, or other physical or chemical properties should be included.
U.S. Dept. of Agriculture
In 2011 the U.S. Dept. of Agriculture’s National Institute of Food and Agriculture (NIFA), through a joint solicitation with the Environmental Protection Agency and the National Science Foundation, funded four projects on environmental, health, and safety aspects of nanotechnology as part of NIFA’s Agriculture and Food Research Initiative (AFRI) competitive grants program. The grant recipients of three of the four projects presented updates on their research during a symposium titled “Safety Evaluation of Nanodelivery Systems and Nanoparticles in Foods” at the 2012 IFT Annual Meeting & Food Expo. Hongda Chen, co-organizer of the symposium and National Program Leader, Bioprocessing Engineering/Nanotechnology, at NIFA, said that the safety assessment of nanoparticles in foods has mostly been speculation based on little data so far and that the symposium was organized to present recent data and fill the knowledge gaps regarding safety evaluation of nanoparticles relevant to food applications.
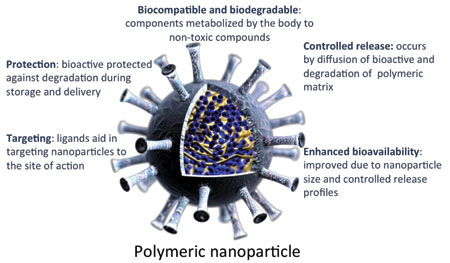
Cristina M. Sabliov, co-organizer of the symposium and Scott and Ruth Bergeron Associate Professor in the Biological and Agricultural Engineering Dept. at Louisiana State University and LSU AgCenter, described the methods used to measure the biodistribution of polymeric nanoparticles as a first step in assessing their safety. Nanoparticles were designed to protect lutein from degradation during storage, ensure a constant release of the bioactive in the gastrointestinal (GI) tract, and be effectively taken up by enterocytes, thus improving the bioavailability of lutein.
Polymeric nanoparticles 100 nm – 120 nm in diameter were made by emulsion evaporation. First, an emulsion was made from an organic solvent containing a fluorescently tagged polymer and a water phase containing a surfactant. After a microfluidization or sonication step to reduce the size of the emulsion droplets, the organic solvent was evaporated and the particles freeze-dried. The fluorescently tagged nanoparticles were fed to rats to determine where the particles ended up in their bodies and whether there was any toxicity associated with ingested nanoparticles.
Results showed that the particles were found to reach various organs in the body, especially the spleen, liver, and kidney, as shown by confocal microscopy and fluorescence measurement. Sabilov said that the study showed no apparent adverse effects of polymeric nanoparticles on organ tissues, as assessed by her collaborator, Timothy W. Morgan, Associate Professor in the College of Veterinary Medicine at Mississippi State University, but added that further histological studies need to be conducted to fully assess the nanoparticle safety under both acute and chronic conditions.
Prabir K. Dutta, Distinguished University Professor in the Chemistry Dept. at Ohio State University (OSU), said that he and his colleague, W. James Waldman, Associate Professor in the Pathology Dept. at OSU, have been examining how the physicochemical properties of commercially available bulk food additives affect intestinal epithelial cells in vitro. The additives—zinc oxide, silica, titanium dioxide, and silver—are widely used in food and food packaging, Dutta said, but as sold in bulk are not all of nanoparticle size (less than 100 nm).
The researchers added the particles to human intestinal epithelial cells in a stomach model and a stomach & intestines model and measured cell uptake, cell death, cell proliferation, and mitochondrial activity and determined whether any inflammation markers were released. The results, Dutta said, have shown that zinc oxide nanoparticles dissolve in the acidic medium in the stomach and show modest toxicity while titanium dioxide and silica have almost no cell toxicity; silver is definitely toxic.
--- PAGE BREAK ---
The next phase, he said, is to conduct in vivo studies with hairless mice to determine whether the ingested nanoparticles cross the intestinal epithelium, enter the circulation, and accumulate in other organs such as the liver, kidneys, brain, and lungs. They will use oxide nanoparticles (zinc, silicon, and titanium) with entrapped fluorescent dyes to visualize the biodistribution of the particles in the body. The nanoparticles are prepared by sol-gel chemistry, taking care that the surface properties of the particles remain similar to those of unmodified commercial particles.
Mengshi Lin, Associate Professor of Food Science at the University of Missouri, described the studies he and Associate Professor Azlin Mustapha conducted on developing new methodologies and strategies for extraction, detection, and characterization of nanoparticles in food matrices and the toxicity of the nanoparticles. They spiked engineered nanoparticles—silver, zinc oxide, and titanium dioxide nanoparticles—from commercial sources into various food samples such as wheat flour, yam & corn starch, and pears and used a combination of techniques, including scanning electron microscopy, transmission electron microscopy, energy dispersive spectrometry, and inductively coupled plasma optical emission spectrometry to detect the nanoparticles. The results, Lin said, showed that contamination of engineered nanoparticles in foods can be detected and quantified by the combination of techniques.
The researchers also evaluated the safety and toxicity of nanoparticles by exposing natural gut bacteria and human intestinal epithelial cells (Caco-2 cells) to different concentrations of zinc oxide and silver nanoparticles. First, they exposed Escherichia coli, Lactobacillus acidophilus, and Bifidobacterium animalis to various concentrations of zinc oxide and silver nanoparticles at 37°C for 24 hours in a shaking incubator and then enumerated them at different lengths of time. The results showed that the nanoparticles have some antibacterial properties that inhibit growth of bacteria. Zinc oxide, in various concentrations, slowed the growth of the bacteria for up to 10 hours but did not significantly affect their numbers at the end of 24 hours and were relatively mild, Lin said, not as toxic as silver. Next, the researchers exposed Caco-2 cells to different concentrations of zinc oxide and silver nanoparticles and subjected them to the MTT Cell Proliferation Assay to determine effects of nanoparticles on cell viability. The results, Lin said, demonstrated that higher concentrations of nanoparticles do have toxic effects on Caco-2 cells.
Challenges Ahead
 Now that researchers have the ability to study nanoparticles and develop new nanomaterials with new properties, Chen said, researchers must determine whether these materials will be beneficial and safe. Nanotechnology is a very exciting area, he said, with a lot of potential benefits, but addressing safety is critical. Communication of the benefits and risks is also important, so people can have a better understanding of and confidence in nanotechnology. There is also a need for more collaboration among industry, government, and academia in nanotechnology research. There is much to learn, he said, but resources are limited.
Now that researchers have the ability to study nanoparticles and develop new nanomaterials with new properties, Chen said, researchers must determine whether these materials will be beneficial and safe. Nanotechnology is a very exciting area, he said, with a lot of potential benefits, but addressing safety is critical. Communication of the benefits and risks is also important, so people can have a better understanding of and confidence in nanotechnology. There is also a need for more collaboration among industry, government, and academia in nanotechnology research. There is much to learn, he said, but resources are limited.
Sabliov said we have to learn more about engineered particles to take advantage of them in foods. All nanoparticles are different and need to be organized into classes and characterized. Questions must be answered: How do the nanoparticles change and interact with the food matrix? What happens to the
particles in the GI tract? Are the nanoparticles absorbed? Are they still in the nanometer range when absorbed? What’s the extent of absorption? If absorbed, where do nanoparticles go in the body, and are they safe?
Dutta said that there is a need for increased collaboration with food companies to answer questions such as the following: Are food companies using nanoparticles? What fraction of a product is nano? What is the state of these particles? Have they been modified in any way to enhance their property? How are they released in the body? Where are they released, and how much is in the form of nanoparticles? After researchers know that the food is being eaten and actually releasing nanoparticles in the stomach, he said, then they can use models on how nanoparticles move through the gut and across the gut. Just because a nanoparticle is in the food doesn’t mean that it’s in the liver. Particle size, composition, and surfaces can be readily modified. If nanoparticles aggregate, their dynamics as they go through the GI tract are different. And each food matrix is different. Lin said that there is insufficient data about the fate and behavior of engineered nanoparticles in food matrices and in digestion through the GI tract, and little is known about how nanoparticles translocate across the cell membrane. A major issue, he said, is the need for more research funding in this area.
Upcoming Conferences on Nanotechnology
Nanotech Conference & Expo 2013, Washington, D.C., May 12–16, 2013 (www.techconnectworld.com/Nanotech2013), will include the NNI signature initiative symposium “Nanotechnology for Sensors and Sensors for Nanotechnology: Improving and Protecting Health, Safety, and the Environment.”
IFT International Food Nanoscience Conference, Chicago, Ill., July 12–13, 2013 (www.ift.org), will include sessions on current and emerging applications, evaluating safety, international perspectives, consumer perceptions and education. A primer/refresher workshop on the morning of July 12 prior to the conference will allow participants to build or refresh their understanding of nanoscience.
IFT Annual Meeting & Food Expo, Chicago, Ill., July 13–16, 2013 (www.ift.org), will include the symposia “Applications of Nanotechnology Advances in Food Engineering and Processing” and “Applied Use of Nanotechnology in Detecting Foodborne Pathogens in Meat Processing.”
International Symposium on Nanotechnology, Occupational and Environmental Health, Nagoya, Japan, October 28–31, 2013 (http://square.umin.ac.jp/nanoeh6/index.html), will include presentations of the latest knowledge to reduce potential nanotechnology-related risks.
 Neil H. Mermelstein, a Fellow of IFT, is Editor
Neil H. Mermelstein, a Fellow of IFT, is Editor
Emeritus of Food Technology
[email protected]


