Confronting Myths About Ozone and Food Safety
PROCESSING
The use of ozone in food processing was affirmed as generally recognized as safe (GRAS) in May 1997 (EPRI, 1997; Graham, 1997) and approved by the U.S. Food and Drug Administration (FDA) as an antimicrobial agent for direct contact with all food types in June 2001 (FDA, 2001). Subsequently, numerous processes have been developed and adopted (EPRI, 2001; Sopher et al., 2002). Risk of recalls due to microbial contamination drives food processors to search for and apply safer and more effective sanitizers. Some antimicrobials are heat, chemical additives, pressure, drying, gases, radiation, ultraviolet light, and ultrasound. Each system has its own special requirements for contact time, temperature, pressure, treatment intensity, and method of application. When properly applied, ozone has the unique ability to kill microorganisms, avoid creation of mutants, and leave no harmful residual chemicals. A clear understanding of the application dynamics is a key factor in the effective use of ozone.
Previous publications have detailed specific uses for ozone (Sopher, 2012). In a quest for better systems, some ozone equipment vendors now offer systems producing much higher levels of ozone in water without emphasis on the actual application process. There is a trend to apply ozone at levels of 8 to 25 parts per million (ppm) and at temperatures above 21°C in water applied at pressures above 25 psig. These practices along with sporadic results from such high-ozone treatment systems have led to an assumption that there is a lack of knowledge concerning accurate answers to the following questions:
• What are the actual dissolved ozone levels of these systems and how were they measured (titration, ozone meter, other)?
• How much ozone is degraded while passing through high pressure nozzles that produce small water droplets?
• Is the ozone wash water temperature low enough to ensure appropriate levels of dissolved ozone in the water?
• Is product pre-cleaning adequate to remove unnecessary ozone-demanding organics before ozone is applied?
• Is the initial ozone demand of fresh wash water known?
• Is the potential for mold and other airborne microbial contaminants in the processing area considered?
Water containing ozone applied at pressures above 20 psi may perform similarly to gaseous rather than aqueous ozone when sprayed on food product surfaces. Ozone’s reactivity in aqueous solution is very high compared to its reactivity in ambient air. Lower temperature increases solubility of oxygen and ozone in water. At 20°C the solubility of oxygen is 0.0439 g/L of fresh water (Handbook of Chemistry and Physics, 1949). Ozone is approximately 13 times more soluble in water than oxygen, depending on pressure (Lenntech, 2013). Ozone in water under high pressure sprayed as small droplets reverts more quickly to oxygen and degases from solution. At elevated temperatures (21°C and above), gaseous ozone is less soluble in water and degrades rapidly to oxygen and other oxygen-containing species, thereby reducing possible benefits from increased oxidative reaction rate. It is critical to ensure that dissolved ozone actually reaches the reaction site to obtain microbial kill. Water containing more than 4 ppm or 5 ppm of dissolved ozone also contains gaseous ozone, air, and/or high purity oxygen, which by mass action at the reaction site on a food product surface may interfere with dissolved ozone access to the target and actually decrease microbial kill (Lenntech, 2013).
These basic facts about ozone indicate that process designs tested recently on food products would be expected to show very limited microbial lethality. Typically, there is about 1 log count reduction in poorly designed application systems. A high pressure water prewash followed by complete immersion of a food product for two or three minutes in cold water providing an applied dose of 3 ppm to 5 ppm of dissolved ozone should have microbial lethality in the range of three- to four-log count reduction. For this reason, thorough prewash of soiled surfaces is strongly recommended. That will remove unnecessary organic load, which has high ozone demand and impedes microbiological lethality of ozone.
--- PAGE BREAK ---
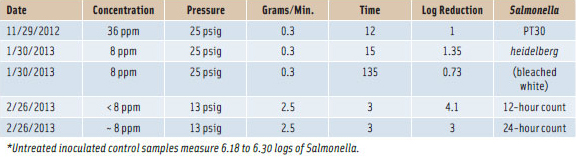
Ozone lethality for microorganisms is measured as the dose of product concentration (C) times the duration of exposure (T), which is parts per million of ozone times minutes of contact time (CT = ozone ppm x minutes of application) (O’Donnell et al., 2012). Kim and Yousef (2000) reported that CT values for Pseudomonas fluorescens, Escherichia coli, Leuconostoc mesenteroides, and Listeria monocytogenes are two or less for five-log count reduction in clean water. In a food system, substances such as protein, fat, and sugar consume ozone and increase the required dose to provide required ozone levels after contact with food products. Thus, it is important to rely on the level of ozone remaining in the stream after contact with food (i.e., applied dose versus absorbed dose). When these application principles are applied correctly, at least a three- to four-log count reduction of Salmonella heidelberg has occurred (see Table 1).
The Food Safety Modernization Act requires prior verification of microbial lethality of process methods. The regulatory trend is to specify demonstration of five-log microbial count reduction for food processes. The scientific basis for this approach is valid in a test laboratory, but the practical interpretation is flawed. To demonstrate a five-log count reduction statistically, a test product is inoculated with a count of six-log or higher. A food product with a microbial count of six-log or higher is unacceptable for commercial processing, and use of inoculated packs for testing in food processing plants is not a safe practice. There is also the question of reliability of inoculated microbial cultures to simulate embedded naturally grown microorganisms.
The generally accepted hurdles of rigorous equipment sanitation, raw product microbial limits, clean handling practices, prewashing, refrigeration, prompt processing, and raw material inspection combine to ensure safe processing. Greater emphasis should be placed on microbial standards for food products to be processed and residual counts after processing rather than exclusive focus on five-log count reduction for process verification. Microbial limits on raw food products have been used effectively for dairy product safety for nearly a century. Air is often overlooked as a significant source of mold and other microbial contamination (EPRI, 1992).
Dee M. Graham, Ph.D., President, R and D Enterprises, 2747 Hutchinson Court, Walnut Creek, CA 94598, ([email protected]). Charles D. Sopher, Ph.D., President, C&S AgriSystems, PO Box 1479, Washington, NC, 27889, ([email protected]). Rip G. Rice, Ph.D., President, Rice International Consulting Enterprises, 1710 Hickory Knoll Rd, Sandy Springs, MD, 20860, ([email protected]). Ahmed E. Yousef, Professor, Depts. of Food Science & Technology and Microbiology, The Ohio State University, 110 Parker Food Science Building, 2015 Fyffe Rd., Columbus, OH 23210-1007, ([email protected])
References
EPRI (Electric Power Research Institute). 1992. Ozonation of cooling tower water in food processing. EPRI Technical Assessment vol. 4, no. 4.
EPRI (Electric Power Research Institute). 1997. Prepared by: R and D Enterprises, Walnut Creek, CA, Expert Panel Report: Evaluation of the History and Safety of Ozone in Processing Foods for Human Consumption. EPRI Technical Report 108026 Vols. 1, 2, and 3.
EPRI (Electric Power Research Institute). 2001. The use of ozone as an antimicrobial agent: agricultural and food processing technical assessment. EPRI TR-1005962.
FDA (U.S. Food and Drug Administration). 2001. Secondary direct food additives permitted in food for human consumption, Fed. Reg. 66(123): 33829–33830.
Graham, Dee M. 1997. Use of ozone in food processing. Food Technol. 51(6): 72–75.
Handbook of Chemistry and Physics. 1949. 31st ed. Chemical Rubber Publishing Company, Cleveland, Ohio.
Kim, J.G. and Yousef, A.E. 2000. Inactivation kinetics of foodborne spoilage and pathogenic bacteria by ozone, in Journal of Food Science, Vol. 65, No. 3, p. 521-528.
Lenntech. 2013. Ozone generation (website). Available at: http://www.lenntech.com/library/ozone/transfer/ozone-transfermechanisms.htm. Accessed June 25, 2013.
O’Donnell, C., Tiwari, B.K., P.J. Cullen, and R.G. Rice, editors. 2012. Ozone in Food Processing. Blackwell Publishing, West Sussex, UK, pp. 277–278.
Sopher, C.D. 2012. Ozone technologies in agri-food (presentation). Int’l Ozone Association, Pan American Group Conference & Exposition, Milwaukee, Wisconsin.
Sopher, C.D., Graham, D.M., Rice, R.G., and Strasser, J. 2002. Studies on the use of ozone in production agriculture and food processing (presentation). Int’l Ozone Association, Pan American Group Conference & Exposition, Raleigh-Durham, NC.
