Targeting Norovirus
FOOD SAFETY & QUALITY
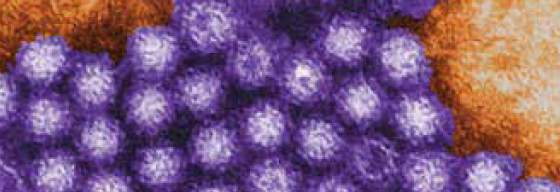
Many people believe that most foodborne illness is caused by bacteria, but the truth is that the most common cause of foodborne-disease outbreaks in the United States is a virus. Human norovirus causes about 21 million illnesses, 70,000 hospitalizations, and 800 deaths each year, according to the Centers for Disease Control and Prevention (CDC) (www.cdc.gov/norovirus).
A Major Challenge
Norovirus is the official genus name for a group of viruses that cause acute gastroenteritis. The most common symptoms are diarrhea, vomiting, nausea, and stomach pain. The illness typically lasts one to two days, is usually not life-threatening, and does not generally cause long-term effects. However, because there are many different types of noroviruses, being infected with one type may not provide protection against other types.
Represented by the prototype strain Norwalk virus, named for Norwalk, Ohio, where the virus was first identified in 1972, the virus is highly contagious. People with the illness can shed billions of virus particles in their feces and vomit, and fewer than 100 virus particles are needed to cause illness in susceptible individuals. It is most contagious during the illness and for three days after recovery. The virus can be present in feces even before a person starts feeling sick and can stay in stool for two or more weeks after the person feels better. It can persist on surfaces for up to two weeks, is resistant to most disinfectants used at manufacturer-recommended concentrations, and may survive temperatures as high as 140°F. There is no drug to treat the illness (antibiotics do not work on viruses), and there is no vaccine to prevent it, although research to develop vaccines is being done or supported by the National Institute of Allergy and Infectious Diseases (www.niaid.nih.gov) and some commercial sources.
People become infected with norovirus by consuming food or liquids that are contaminated with norovirus; touching surfaces contaminated with norovirus, then touching their mouth and subsequently ingesting the virus; or having contact with someone who is infected. The CDC emphasizes that the best way to help prevent norovirus illness is to practice proper hand-washing before eating, preparing, or handling food. Leafy vegetables, fruits, nuts, and molluscan shellfish (oysters, clams, and mussels) are the most common food sources involved in norovirus outbreaks, but any food handled extensively after being cooked is at increased risk of becoming contaminated.
More than 90% of foodborne illness outbreaks on cruise ships are caused by norovirus. Outbreaks can also occur in other settings, such as schools, nursing homes, child care centers, prisons, and military camps, but most of these outbreaks are not associated with food.
Detection Methods Lacking
Genetically, human noroviruses are divided into five genogroups. Genogroups I and II cause virtually all of the human disease, with genogroup II being the most prevalent. Of the many genogroup II strains, genogroup II.4 is responsible for more than 85% of all outbreaks. The viruses are difficult to study because they cannot be cultivated outside of the human body, few commercial diagnostic tests are available in the United States, and only a few scientists are trained specifically in food virology.
Diagnostic methods focus on detecting viral RNA or antigen, according to the CDC. Most public health laboratories test for norovirus using real-time reverse-transcription polymerase chain reaction (RT-qPCR) assays, which can detect as few as 10–100 norovirus genome copies per reaction. The laboratories use different primers to differentiate between genogroup I and genogroup II noroviruses. The assays are also semi-quantitative and can provide estimates of viral load.
Conventional RT-PCR followed by sequence analysis of the RT-PCR products is used for norovirus genotyping. CaliciNet (www.cdc.gov/norovirus/php/reporting.html), a national laboratory surveillance network, was established by the CDC in 2009 to track norovirus outbreaks. Public health and food regulatory laboratories submit norovirus sequences identified from outbreaks to a national database to link norovirus outbreaks that may be caused by common sources (such as specific foods), monitor trends, and identify emerging norovirus strains.
Rapid commercial assays, such as enzyme immunoassays (EIAs), that detect the norovirus antigen have been developed but have poor sensitivity, the CDC said, and are not recommended for diagnosing norovirus infection in sporadic cases of gastroenteritis. Among the tests that are commercially available are the RidaScreen® Norovirus 3rd Generation EIA, the Rida®Gene Norovirus I & II real-time RT-PCR, and the RidaQuick Norovirus qualitative lateral-flow immunochromatographic test for determining genogroup I and II noroviruses from R-Biopharm (www.r-biopharm.com) and the norovirusGI@ceeramTools™ and norovirusGII@ceeramTools™ detection kits for clinical, food, and environmental samples from Ceeram S.A.S. (www.ceeram.com). However, the only test approved for use in the United States is the RidaScreen Norovirus 3rd Generation EIA. The U.S. Food and Drug Administration (www.fda.gov) approved it last year for preliminary identification of genogroups I and II noroviruses when testing multiple specimens during outbreaks but added that samples testing negative should be confirmed by a second technique, such as RT-qPCR (i.e., that EIA kits should not replace molecular methods during outbreak investigations).
--- PAGE BREAK ---
Processing Approaches
In 2011, the National Institute of Food and Agriculture (NIFA) of the U.S. Dept. of Agriculture (USDA) funded a variety of projects as part of NIFA’s Agriculture and Food Research Initiative (AFRI) Food Safety Challenge program (www.csrees.usda.gov/fo/foodsafetyafri.cfm). Among the projects funded were three projects to develop innovative food processing technologies to destroy noroviruses and other foodborne pathogens. These three mega research grants totaled $15 million ($1 million per project per year for five years).
Hongda Chen, National Program Leader, Bioprocess Engineering and Nanotechnology, USDA/NIFA, said that AFRI invited proposals for projects, and three projects were chosen by a peer-review process. The grants were awarded to Yen-con Hung at the University of Georgia to evaluate the use of advanced processing technologies as multiple hurdles to inactivate Shiga-toxigenic Escherichia coli (STEC) and noroviruses on beef; Haiqiang Chen at the University of Delaware to optimize non-thermal processing technologies to destroy human noroviruses in high-risk foods; and Juming Tang at Washington State University to use microwave technologies to control bacterial and viral pathogens in packaged foods. The grantees must submit an annual progress report to the NIFA Current Research Information System (http://cris.nifa.usda.gov), meet at least once a year, and present their progress at annual grantees meetings, often held in conjunction with food-science professional annual meetings such as the IFT Annual Meeting & Food Expo. The researchers are also encouraged to report on their findings via symposia, conferences, and peer-reviewed journals.
Chen said that the next steps are to ensure that the research, education, and extension activities are on course and that the grantees improve progress and effectiveness through inter-project collaboration; communicate in a timely manner to the broad range of stakeholders; and invite inputs, suggestions, collaborations, and participations.
Broad-Based Collaborative Effort
Also in 2011, USDA/NIFA awarded North Carolina State University (NCSU) a $25 million grant for a five-year Coordinated Agriculture Project to study viruses across the food supply chain with a focus on noroviruses. The grant—one of the largest ever given by the USDA for food safety research—is one of a kind, USDA-NIFA program leader Jeanette Thurston said, in that the project combines research, education, and extension for the ultimate goal of reducing viral foodborne illness throughout the food chain.
The broad-based project, led by Lee-Ann Jaykus, William Neal Reynolds Distinguished Professor in the Dept. of Food, Bioprocessing, and Nutrition Sciences at NCSU, is called the USDA-NIFA Food Virology Collaborative for Outreach, Research & Education, or NoroCORE (http://norocore.ncsu.edu). It involves more than 30 scientists from 18 institutions (see Table 1 on p. 65) and numerous stakeholders in academia, industry, and government, working in an integrated manner to develop improved tools, skills, and capacity to understand and control foodborne virus risks.
 The project has specific objectives organized around six core functions:
The project has specific objectives organized around six core functions:
1. Molecular Virology – develop a fully permissive system to cultivate infectious human norovirus, identify and validate the usefulness of alternative cultivatable surrogates, improve understanding of the role of viruses as a cause of foodborne disease of unknown etiology, and develop models to predict human norovirus evolution and strain emergence.
2. Detection – develop simple, practical, and broadly reactive methods to detect human norovirus in clinical samples and in relevant non-clinical sample matrices (food, water, and environmental); compare the efficacy of methods to discriminate between infectious and non-infectious viruses; validate methods; evaluate candidate biosensor platforms for the detection of human norovirus in clinical samples; and develop microarray-based methods for genotyping of foodborne viruses.
3. Epidemiology and Risk – develop risk assessment models; estimate the economic burden of human norovirus disease; and characterize the burden of endemic and epidemic human norovirus disease (with a focus on foodborne transmission), including epidemiological attribution.
--- PAGE BREAK ---
4. Prevention and Control – evaluate and monitor virus load pre- and post-harvest, develop and/or evaluate methods to prevent virus contamination in foods, and develop and/or evaluate methods to remove and/or inactivate viruses in foods.
5. Extension, Outreach, and Engagement – engage stakeholders; increase appreciation of the role of viruses in foodborne disease, particularly among food safety and public health professionals; evaluate consumer food safety knowledge and refine existing educational resources; field-test food handler user compliance after exposure to an educational intervention; and provide extension and outreach to the molluscan shellfish and fresh produce industries.
6. Capacity Building – create a mechanism to foster information and reagent exchange, expand state and local laboratory capacity in food virology; expand professional capacity with a focus on increasing diversity, and develop a graduate-level interdisciplinary curriculum in food virology.
 At NoroCORE’s first full collaborative meeting in November 2012, each NoroCORE collaborator presented at least one poster on its work so far. Table 2 on p. 65 lists 17 of the 41 papers presented, selected to illustrate the breadth of efforts for each of the six core functions.
At NoroCORE’s first full collaborative meeting in November 2012, each NoroCORE collaborator presented at least one poster on its work so far. Table 2 on p. 65 lists 17 of the 41 papers presented, selected to illustrate the breadth of efforts for each of the six core functions.
Although there are some commercial test kits being marketed for detecting norovirus in foods, Jaykus said, the problem lies in sample preparation. Unlike classical methods for detecting bacteria, norovirus can’t be grown or enriched norovirus in foods, she said. Instead, the virus needs to be concentrated and purified out of the sample matrix and then detected, and that is really complicated because of low levels of contamination, the need to process large sample volumes, and the sensitivity of RT-qPCR to matrix-associated inhibition. There are prototype methods to do that, she said, but nothing has yet been formally approved for routine use with foods and environmental samples. There are no commercial methods to do the concentration step, which would differ by sample matrix, such as food type, water, soil, and so on. The European Union is trying to standardize some methodologies for some matrices, but there are no similar efforts in the United States. The bottom line, she said, is that it’s really hard to detect norovirus contamination in a food sample.
NoroCORE is moving forward in all of the efforts that it committed to do, Jaykus said. The next steps are to increase stakeholder involvement , identify additional stakeholders, and develop a coordinated method to work with them. The ultimate goal, she said, is to at some point see a measurable reduction in foodborne disease caused by norovirus.
 Neil H. Mermelstein, a Fellow of IFT, is Editor Emeritus of Food Technology
Neil H. Mermelstein, a Fellow of IFT, is Editor Emeritus of Food Technology
[email protected]


