Bacteriophages: Back to the Future
The scientific community is renewing its attention to these bacteria-killing viruses,which show potential in the reduction of pathogenic contamination of fresh-cut produce and other foods.

He’s not a food scientist, and he was not referencing bacteriophages in his 2006 hit song “What Goes Around,” but Justin Timberlake’s lyrics “what goes around, goes around, goes around, comes back around…” could be applied to the renewed interest in and application of bacteriophages to reduce pathogens in foods. Interest in bacteriophages peaked in the early part of the 20th century and waned in the mid-20th century as antibiotics were developed and disseminated, but was rekindled early in the 21st century as effective alternatives to antibiotics are sought out.
The rise of antibiotic-resistant pathogenic bacteria and interest in natural or “green” antimicrobials over the past 20 years has stimulated interest in the use of bacteriophages as antimicrobials. The development and use of several commercial bacteriophage-based antimicrobials highlights their potential as a valuable intervention against foodborne pathogens when used appropriately.
Bacteriophages are viruses that naturally infect and lyse (kill) bacterial cells, and were widely studied and used before the discovery and dissemination of antibiotics. Since bacteriophages are naturally present in many different environments, including water, soil, and various foods, they are a natural and effective alternative to chemical antimicrobials and antibiotics currently in use (Brussow and Kutter, 2005).
An Historical Perspective
Felix d’Herelle is credited with discovering bacteriophages in 1917 (d’Herelle, 1917), and their existence was confirmed by electron microscopy after a two-decade scientific debate (Sulakveldize, 2011). Bacteriophages (meaning “bacteria eater” from the Greek phagerin) were frequently used as antibacterial agents against a variety of human bacterial infections from the 1920s through the 1940s, and research on them continued in Eastern Europe after World War II. Phages are found in the same varied environments as their bacterial hosts; it is estimated that there are 1032 bacteriophages on earth (Brussow and Kutter, 2005). Bacteriophages have been isolated from a variety of foods (Greer, 2005; Kennedy et al., 1984; Kennedy et al., 1986). Phages are specific for groups of species of bacteria, which allows them to inactivate pathogens while not affecting the remaining overall microbial ecology and balance in a specific animal, environment, or food (e.g., a phage can display lytic specificity against Escherichia coli O157:H7 but not E. coli O104:H4). Bacteriophages are true parasites, with their only hosts being bacterial cells; they have no ability to generate energy or proteins independently and are completely dependent on their host for the cellular machinery for replication (Guttman et al., 2005). They do not have the ability to cause illness in humans or other animals, making them safe for human and animal consumption. Several U.S. regulatory agencies have approved bacteriophages to be used on various foods and food contact surfaces, leading to their potential use as antimicrobialsin foods (Sulakvelidze, 2011).
Biology and Life Cycle
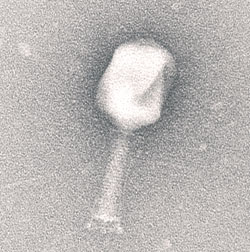
Bacteriophages are classified in one order (Caudovirales), 13 families, and 31 genera (Ackermann, 2005). Over 95% of bacteriophages are tailed phages and belong to one of three families (Podoviridae, Myoviridae, and Siphoviridae) (Guttman et al., 2005). Phages carry their nucleic acid in the capsid, a lipoprotein attached to the tail structure. This whole assembly is referred to as a virion particle.
The tail fibers (components of the tail) of phages play an important role in adsorption, a process that consists of the phage binding to a specific receptor on the bacterial cell surface and irreversibly attaching to the peptidoglycan cell wall) using the tail fibers. Nucleic acids contained in the capsid are then transferred down the tail of the virion particle and into the bacterial cell. Bacteriophage genes are transcribed by the host bacterial cell machinery. Nucleic acids are then packaged into protein structures termed procapsids (Sharma et al., 2011). The last stage in the process is the lysis of the cell, mediated by lysins, enzymes that degrade the peptidoglycan structure, initiating lysis of the peptidoglycan layer and releasing the fully formed bacteriophage particles from the cell, which allows the particles to infect other bacterial hosts. Infection is dependent on the growth temperature of the bacteria, as the bacteria must be metabolically active for the lytic cycle to occur. Some phages are termed lysogenic phages, where their nucleic acids are incorporated into the genome of the bacteria, but cell lysis does not occur until a specific event (e.g., exposure to UV light, chemical exposure) induces the process.
--- PAGE BREAK ---
Lysis from Without
The concept of “lysis from without” (LO) is critical to understand when discussing the application of bacteriophages in foods. LO is the adsorption of many bacteriophages to a single cell, causing enough damage to the cell wall to cause lysis, without completing the infection cycle (Abedon, 2011). LO is an applicable concept for phage use in foods because at refrigeration temperatures, most pathogenic bacteria will not be metabolically active, and the cycle of phage infection cannot be completed. However, LO can occur as long as adsorption can take place, making it an important mechanism to kill pathogenic bacteria in food.
 Potential Resistance to Bacteriophage Lysis
Potential Resistance to Bacteriophage Lysis
Several bacteriophages specific for foodborne pathogens (Escherichia coli O157:H7, Salmonella spp., and Listeria monocytogenes) have been effective in reducing the pathogen on a number of food commodities. Most studies described here employ a cocktail of multiple bacteriophages specific for a single pathogen in order to minimize the opportunity for development of bacteriophage insensitive mutants (BIM), which are cells resistant to bacteriophage infection. Resistance to lytic infection from multiple phages simultaneously in bacteria in food or food processing environments is unlikely because the conditions (food matrices, storage temperature of foods, temperatures in processing plants) are not permissive for bacterial replication, an essential prelude to the development of bacterial resistance to lytic phage infection. If formulated appropriately, it is unlikely that bacteria will develop resistance to multiple lytic bacteriophages simultaneously because phages in the cocktail should utilize different receptor molecules during the adsorption process.
The multiplicity of infection (MOI), the average number of bacteriophages available to infect a single bacterial cell, is also an important attribute of lytic bacteriophages used to target pathogens. It is important to maintain a sufficiently high MOI to achieve a rapid reduction of bacterial populations, especially under conditions where the predominant lytic mechanisms might be LO or a single bacteriophage replication cycle.
Leafy Greens
Lytic bacteriophages have been effective in reducing populations of several enteric pathogenic bacteria on various produce commodities. Fresh-cut lettuce was inoculated with ca. 2.5 log CFU/cm2 Escherichia coli O157:H7 and sprayed with 6 log PFU/mL of a commercially available (EcoShield™) cocktail, consisting of three bacteriophages, specific for E. coli O157:H7, at an MOI of approximately 1,000. Bacteriophage treatment reduced E. coli O157:H7 populations by 1.9 log CFU/cm2,
occurred within the first 30 min after treatment, and were significantly (P < 0.05) different from untreated controls (Sharma et al., 2009). These results indicate that a bacteriophage mixture can be effective in reducing E. coli O157:H7 populations when sprayed onto fresh-cut lettuce. Spraying the same E. coli O157-specific bacteriophage cocktail on spinach inoculated with ca. 4 log CFU E. coli O157:H7/g resulted in pathogenic reductions of 100% after 24 hr and 120 hr, and 99% by 168 hr when stored at 10°C (Abuladze et al., 2008). Similarly, a mixture of eight lytic bacteriophages (BEC 8) specific for E. coli O157:H7 were, at an MOI of 1, 10, or 100, deposited on fresh-cut baby romaine lettuce or baby spinach leaves (Viazis et al., 2011) and stored at either 4°C, 8°C, 23°C, or 37°C. The results from these experiments also showed that in most cases as the MOI increased, greater population reductions of E. coli O157:H7 occurred, generally within one hour after bacteriophage treatment.
Lytic bacteriophages have also been evaluated under minimal processing and commercial storage conditions used for leafy greens. Immersion of fresh-cut lettuce in solutions of bacteriophages to simulate wash water used for fresh-cut leafy greens did provide protection against cross-contamination with E. coli O157:H7 (e.g, reducing bacterial populations) when fresh-cut lettuce was immersed in solutions of E. coli O157-specific at 9.8 log PFU/mL for 2 min, inoculated with E. coli O157:H7, and stored at 4°C for up to 7 days (Ferguson et al., 2013). Unlike with the spray application of bacteriophages described previously, statistically significant reductions were not observed immediately after phage treatment but only after 24 hr when immersed 9.8 log PFU/mL and reduced population to below detectable limits after 3 days of storage at 4°C. The lack of immediate reduction by immersion is most likely due to the lower number of phages distributed to the surface of lettuce by immersion (compared to spraying), and that phages were sprayed to lettuce before the introduction of the pathogen, limiting the opportunities for bacteriophage-cell interactions. Bacteriophages specific for E. coli O157:H7 were also effective on spinach packaged under modified atmosphere packaging (MAP) and stored at 4°C. MAP is commonly used to preserve the quality of fresh-cut leafy greens sold at retail. Bacteriophage treatment significantly reduced populations by 2.18 log, 3.50 log, and 3.13 log CFU/cm2 after 24 hr on spinach, green leaf, and romaine lettuce, respectively, compared to untreated controls under MAP conditions (Boyaciagolu et al., 2013). Finally, the treatment of fresh-cut lettuce with a combination of E. coli O157-specific bacteriophage (spray treatment) and 50 ppm sodium hypochlorite (immersion treatment) reduced E. coli O157:H7 populations on fresh-cut iceberg lettuce more than either bacteriophage or hypochlorite treatment alone (Ferguson et al., 2013). All of these findings show that bacteriophage treatments could be integrated into current processing and packaging conditions used for leafy greens.
--- PAGE BREAK ---
Tomatoes and Sprouts
Bacteriophages have been effective on fresh and fresh-cut tomatoes as well. A cocktail of five lytic bacteriophages specific for Salmonella Javiana reduced the incidence of internalized S. Javiana, as compared to untreated controls, when 6 log PFU/mL was applied to the blossom end of tomatoes (Ye et al., 2009). Salmonella-specific bacteriophage cocktails, applied at 6 log PFU/mL in combination with the bacterium Enterobacter absuriae, reduced Salmonella spp. populations on mung beans sprouts by 6 log CFU/mL compared to untreated controls (Ye et al., 2010). A synergistic inactivation of S. Javiana on tomatoes was not achieved by the bacteriophage/E. absuriae combination and was equivalent to reductions caused by E. absuriae only (Ye et al., 2009). These results, specifically with Salmonella-specific phages on tomatoes and sprouts, indicate that the effectiveness of bacteriophages to reduce pathogens can be dependent on the produce commodity being treated. Other reports showed that a cocktail of E. coli O157:H7-specific phages reduced initial E. coli O157:H7 populations of 2.81 log CFU/g on cut sliced tomatoes by 99%, 94%, and 96% after 24 hr, 120 hr, and 168 hr, respectively, after being sprayed on cut tomato surfaces (Abuladze et al., 2008). A combination of two bacteriophages, one with specific lytic ability against S. Montevideo, and one with specificity against both S.Typhimurium and S. Enteritidis, applied at a level of 6.7 log PFU/mL, reduced Salmonella counts from an initial population of ca. 7 log CFU/mL in soak water of broccoli seeds by 1.50 log CFU/mL compared to non-bacteriophage treated controls (Pao et al., 2004).
Melons
 Bacteriophages specific for E. coli O157:H7, Salmonella spp., and Listeria monocytogenes reduced these pathogens on fresh-cut cantaloupe and honeydew melons in several studies. A cocktail of four lytic bacteriophages (SCPLX-1), specific for Salmonella Enteritidis, was applied to inoculated fresh-cut honeydew melons through a spot treatment and reduced Salmonella by approximately 3.5 log CFU when stored at 5°C and 10°C after 3 days when compared to untreated controls (Leverentz et al., 2001). In this study, bacteriophages were more effective in reducing Salmonella populations at 5°C and 10°C than at 20°C. Similar to the spray application of bacteriophages to fresh-cut lettuce, this work showed that bacteriophage activity can be enhanced at lower temperatures, and the antibacterial activity of bacteriophages occurs almost immediately once applied to inoculated fresh-cut melons. A combination of 6 (LMP-102) and 14 (LMP-103) lytic bacteriophages specific for L. monocytogenes were applied to cut honeydew melons by either spot or spray application (Leverentz et al., 2003). Treatments of LMP103/LMP102 were significantly (P < 0.05) more effective in reducing L. monocytogenes on honeydew squares stored at 10°C for up to 7 days compared to treatments with water or the bacteriocin nisin alone (Leverentz et al., 2003). The spot application of a cocktail of three E. coli O157:H7-specific lytic bacteriophages to fresh-cut cantaloupes inoculated with E. coli O157:H7 and stored at 4°C for 7 days reduced the pathogen by 2.5 log CFU/mL compared to untreated control, and bacteriophage treatments were more effective at 4°C as compared to 20°C (Sharma et al., 2009). These studies indicated that bacteriophages can be effective in reducing pathogenic bacterial counts in freshcut melon tissues when combined with temperatures less than 10°C. The lytic activity of bacteriophages against bacterial foodborne pathogens on fresh-cut melons seems to be enhanced at low storage temperatures. Cocktails of Salmonella specific bacteriophages were unable to reduce S. Enteritidis populations on apple slices stored at 10°C for 7 days (Leverentz et al., 2001), but lytic bacteriophages specific for L.monocytogenes (LMP103/LMP 102) reduced bacterial populations of the pathogen on apple slices compared to slices that did not receive a phage treatment (Leverentz et al., 2001). Lytic phages specific for L. monocytogenes were not as affected by the low pH on apple slices as lytic phages specific for S. Enteritidis. These findings indicate that some phages may be more affected by extrinsic factors than others, and each phage cocktail/pathogen combination should be evaluated for each commodity.
Bacteriophages specific for E. coli O157:H7, Salmonella spp., and Listeria monocytogenes reduced these pathogens on fresh-cut cantaloupe and honeydew melons in several studies. A cocktail of four lytic bacteriophages (SCPLX-1), specific for Salmonella Enteritidis, was applied to inoculated fresh-cut honeydew melons through a spot treatment and reduced Salmonella by approximately 3.5 log CFU when stored at 5°C and 10°C after 3 days when compared to untreated controls (Leverentz et al., 2001). In this study, bacteriophages were more effective in reducing Salmonella populations at 5°C and 10°C than at 20°C. Similar to the spray application of bacteriophages to fresh-cut lettuce, this work showed that bacteriophage activity can be enhanced at lower temperatures, and the antibacterial activity of bacteriophages occurs almost immediately once applied to inoculated fresh-cut melons. A combination of 6 (LMP-102) and 14 (LMP-103) lytic bacteriophages specific for L. monocytogenes were applied to cut honeydew melons by either spot or spray application (Leverentz et al., 2003). Treatments of LMP103/LMP102 were significantly (P < 0.05) more effective in reducing L. monocytogenes on honeydew squares stored at 10°C for up to 7 days compared to treatments with water or the bacteriocin nisin alone (Leverentz et al., 2003). The spot application of a cocktail of three E. coli O157:H7-specific lytic bacteriophages to fresh-cut cantaloupes inoculated with E. coli O157:H7 and stored at 4°C for 7 days reduced the pathogen by 2.5 log CFU/mL compared to untreated control, and bacteriophage treatments were more effective at 4°C as compared to 20°C (Sharma et al., 2009). These studies indicated that bacteriophages can be effective in reducing pathogenic bacterial counts in freshcut melon tissues when combined with temperatures less than 10°C. The lytic activity of bacteriophages against bacterial foodborne pathogens on fresh-cut melons seems to be enhanced at low storage temperatures. Cocktails of Salmonella specific bacteriophages were unable to reduce S. Enteritidis populations on apple slices stored at 10°C for 7 days (Leverentz et al., 2001), but lytic bacteriophages specific for L.monocytogenes (LMP103/LMP 102) reduced bacterial populations of the pathogen on apple slices compared to slices that did not receive a phage treatment (Leverentz et al., 2001). Lytic phages specific for L. monocytogenes were not as affected by the low pH on apple slices as lytic phages specific for S. Enteritidis. These findings indicate that some phages may be more affected by extrinsic factors than others, and each phage cocktail/pathogen combination should be evaluated for each commodity.
Meat and Poultry
Bacteriophages have been shown to reduce bacterial pathogens on various meat commodities. O’Flynn showed that a cocktail of three phages specific for E. coli O157:H7 was applied to beefsteaks at an MOI of 106 and reduced populations by 4–5 log CFU compared to controls (O’Flynn et al., 2004). Goode and co-workers (2003) showed that a single phage treatment of Salmonella Enteritidis-contaminated chicken skin, applied at an MOI of 1, was able to reduce bacterial populations by 1 log CFU/cm2 over 48 hr compared to untreated controls (Goode et al., 2003). A single bacteriophage specific for Campylobacter jejuni was used to significantly (P < 0.05) reduce pathogen populations by 2.2 log CFU in 24 hr compared to untreated controls (Goode et al., 2003). Combining phage treatment and freezing also inactivated C. jejuni on chicken breasts (Atterbury et al., 2003).
In some cases, as with produce, the meat matrix to which bacteriophages are being applied can affect their lytic ability against the target pathogen. A single phage (LH7) specific for L. monocytogenes reduced the pathogen in broth when used in combination with the bacteriocin nisin, but this combination was not effective in reducing L. monocytogenes on raw ground beef samples (Dykes and Moorhead, 2002). Similarly, in vivo feeding studies with Salmonella-specific bacteriophage in poultry broilers required a higher MOI (108-9) than the MOI level used in in vitro studies (106) to reduce Salmonella spp. in animals. The lower titers of bacteriophage may have been ineffective in reducing Salmonella spp. populations in broilers for several reasons: 1) there were not enough phages present to replicate in host Salmonella cells in broilers, leading to a decline in the persistence of the phages in the birds; 2) other bacteria in the ceca and intestinal environment may have interfered with the bacteriophage-Salmonella cell interactions. Bacteriophages specific for E. coli O157:H7 were more effective at reducing the pathogen in sheep when applied at a MOI of 1 (compared to MOI of 10 or 100), and were significantly more effective (P < 0.05) in reducing E. coli O157:H7 populations in sheep compared to untreated controls (Callaway et al., 2008). Non-oral applications of bacteriophages to cattle (steers) significantly reduced E. coli O157:H7 levels compared to levels in untreated animals, but did not completely eliminate the pathogen from steers (Sheng et al., 2006).
--- PAGE BREAK ---
 Regulatory Status of Bacteriophage Products
Regulatory Status of Bacteriophage Products
Several U.S. federal agencies have issued various degrees of approval for the use of lytic bacteriophages for specific and distinct purposes. The U.S. Dept. of Agriculture (USDA) issued two no objection letters for the use of bacteriophages targeting E. coli O157:H7 (Finalyse) and Salmonella spp. (Armament) developed by Omnilytics™, Salt Lake City, Utah, for use as hide sprays on cattle prior to slaughter (USDA/FSIS, 2013; Omnilytics, 2007). The U.S. Food and Drug Administration (FDA), and subsequently the U.S. Dept. of Agriculture’s Food Safety and Inspection Service (FSIS), approved the use of a mixture of six bacteriophages (ListShield™, manufactured by Intralytix Inc.) specifically for the bacterial foodborne pathogen L.monocytogenes on ready-to-eat meat and poultry products (Anonymous, 2006a). This product was approved as a direct food additive, similar to chemical ingredients, as the FDA made its own determination that ListShield™ does not currently pose a human health risk when used in the manner described in the regulations. Another lytic bacteriophage product, Listex P100 (Micreos Food Safety, formerly EBI Food Safety), is a single bacteriophage which the FDA declared as GRAS (Generally Recognized as Safe), suggesting that it has no current scientific objection to the use of the product (Anonymous, 2006b). Specifically, it was to be used to inhibit the growth of L. monocytogenes in Brie, cheddar, and Swiss cheeses (Anonymous, 2006b). Its GRAS status was expanded for use in other ready-to-eat foods as well (Anonymous, 2007).
EcoShield™ (Intralytix Inc.) has also been cleared by the FDA through a “Food Contact Notice” to be used, without labeling, on red meat parts and trim intended to be ground (Anonymous, 2011). The same approval has been granted by FSIS (USDA/FSIS, 2013). Furthermore, it has also received a temporary exemption for two years from the U.S. Environmental Protection Agency, stating that its use does not require the establishment of a tolerance of lytic bacteriophages when used on food contact surfaces in food processing plants (Anonymous, 2011). Outside the United States, the aforementioned Listex P100 has been approved for use as a “processing aid” for meat, seafood, cheese and ready-to-eat foods by Food Standards Australia and New Zealand (FSANZ, 2012). Most recently, FDA approved SalmoFresh—a Salmonellaspecific cocktail of bacteriophages—as GRAS, specifically for the use in red meat and poultry (Anonymous, 2013).
These regulatory approvals by federal agencies indicate that the use of lytic bacteriophages in foods poses a minimal risk to human health. Although they are not anticipated to affect the sensory, nutritional, or phytochemical status of foods to which they are applied, more studies are needed in order to evaluate the effect of bacteriophages on compositional and sensory aspects of produce commodities.
We’ll close this article with lyrics from Bob Dylan’s “The Times, They Are A-Changing.” According to the song, “The slow one now will be later fast.” Once an area of research that was considered “slow,” applying bacteriophages to foods has become “fast” in the past 15 years, with more data and well-conducted studies to inform food safety professionals about the potential role of bacteriophages in reducing foodborne pathogens. Bacteriophages provide food safety professionals another tool to consider in reducing levels of foodborne pathogens.
--- PAGE BREAK ---
Desirable Properties of Bacteriophages for Use Against Pathogenic Bacteria
Several authors (Hagen and Loessner, 2010; Goodridge and Bisha, 2011) have listed desirable properties of bacteriophages used for biocontrol of bacterial pathogens in foods or feeds, which can be applied to foods or food processing environments. The properties that the experts list include the following.
1) The phage should have a broad host range capable of infecting several strains of target species and/or genus.
2) The phage should be lytic.
3) It should be able to be propagated on a nonpathogenic host.
4) The complete genome sequence should be known.
5) Phages should not transduce bacterial DNA.
6) There should be an absence of any bacterial pathogenic genes or allergenic proteins.
7) Oral feeding trials should have no adverse effect on animals or humans.
8) Phage products should be amendable for scale-up and large production as well.
Manan Sharma, Ph.D., a Member of IFT, is a Research Microbiologist in the Environmental Microbial and Food Safety Laboratory, U.S. Dept. of Agriculture Agricultural Research Service, Henry A Wallace Beltsville Area Research Center, 10300 Baltimore Ave., Beltsville MD 20705 ([email protected]).
Larry Goodridge, Ph.D., a Member of IFT, is an Associate Dean of the Graduate School and an Associate Professor of Food Microbiology in the Dept. of Animal Sciences at Colorado State University, 350 West Pitkin St., Fort Collins, CO 80523 ([email protected])
References
Abedon, S. 2011. Lysis from without. Bacteriophage 1: 46-49.
Abuladze, T., Li, M., Menetrez, M.Y., Dean, T., Senecal, A. and Sulakvelidze, A. 2008. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef with Escherichia coli O157:H7. Appl. Environ. Microbiol. 74: 6230-6238.
Ackermann, H.–W. 2005. Bacteriophage classification. In Bacteriophages: Biology and applications, eds. E. Kutter and A. Sulakvelidze, 67-90. CRC Press, Washington, D.C.
Anonymous. 2006a. Listeria-specific bacteriophage preparation. Food additives permitted for direct addition to food for human consumption. 21 CFR Part 172.785. Fed. Regist. 71:47729–47732.
Anonymous. 2006b. Agency response letter, GRAS notice no. GRN 000198. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, Silver Spring, Md.
Anonymous. 2007. Agency response letter, GRAS Notice No. GRN 000218. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, Silver Spring, Md.
Anonymous. 2011. Escherichia coli O157:H7 specific bacteriophages; temporary exemption for the requirement of a tolerance. Fed. Regist. 76: 71 (April 13, 2011) p. 20542. http://www.gpo.gov/fdsys/pkg/FR-2011-04-13/pdf/2011-8712.pdf . Accessed July 5, 2011.
Anonymous. 2013. Agency Response Letter GRAS Notice No. GRN 000435. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, Silver Spring, Md. http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm345473.htm. Accessed April 13, 2013.
Atterbury, R.J., Connerton, P.L., Dodd, C.E.R., Rees, C.E.D. and Connerton, I.F. 2003. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 69: 6302–6306.
Atterbury, R.J., Van Bergen, M.A.P., Ortiz, F., Lovell, M.A., Harris, J.A., De Boer, A., Wagenaar, J.A., Allen, V.M., and Barrow, P.A. 2007. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 73: 4543-4549.
Boyacioglu, O. Sharma, M., Sulakvelidze, A. and Goktepe, I. 2013. Biocontrol of Escherichia coli O157:H7 on fresh-cut leafy greens: using a bacteriophage
cocktail in combination with modified atmosphere packaging. Bacteriophage. Accepted for publication. http://www.landesbioscience.com/journals/bacteriophage/article/24620/?show_full_text=true.
Brussow, H. and Kutter, E. 2005. Genomics and evolution of tailed phages. In Bacteriophages: Biology and Applications, eds. E. Kutter and A. Sulakvelidze, 91-128. CRC Press, Washington D.C.
Callaway, T.R., Edington, T.S., Brabban, A.D., Anderson, R.C., Rossman, M.L., Englier, M.J., Carr, M.A., Genovese, K.J., Keen, J.E., Looper, M.L., Kutter, E.M., Nisbet, D.J. 2008.
Bacteriophages isolated from feedlot cattle can reduce Escherichia coli O157:H7 populations ruminant gastrointestinal tracts. Food Path. Dis. 5: 183-191.
d’Herelle, M.F. 1917. On an invisible microbe antagonistic to dysentery bacilli. Note by M.F. d’Herelle, presented by M. Roux. Comptes Rednus Academie des Sciences 1917. 165: 373-75.
Dykes, G.A., and Moorhead, S.M. 2002. Combined antimicrobial effect of nisin and listeriophage against Listeria monocytogenes in broth but not in buffer or on raw beef. Int. J. Food Microbiol. 73: 71–81.
Ferguson, S. Roberts, C., Handy, E., and Sharma, M. 2013. Lytic bacteriophages reduce Escherichia coli O157:H7 on fresh-cut lettuce introduced through cross-contamination. Bacteriophage. Accepted for publication. http://dx.doi.org/10.4161/bact.24323.
FSANZ. 2012. Approval report for Application A1045: Bacteriophage preparation P100 as a processing aid. http://www.foodstandards.gov.au/_srcfiles/A1045%20Bacteriophage%20as%20a%20PA%20AR%20FINAL.pdf. Accessed April 12, 2013.
Goode, D., Allen, V.M. and Barrow, P.A. 2003. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69: 5032–5036.
Goodridge, L.D., and Bisha, B. 2011. Phage-based biocontrol strategies to reduce foodborne pathogens in foods. Bacteriophage 1: 130-37.
Greer, G.G. 2005. Bacteriophage control of foodborne bacteria. J. Food Prot. 68: 1102-1111.
Guttman, B., Raya, R. and Kutter, E. 2005. Basic phage biology in Bacteriophages: biology and applications, eds. E. Kutter, and A. Sulakvelidze, 29-66. CRC Press, Washington, D.C.
Hagens, S., and Loessner, M.J. 2010. Bacteriophage for biocontrol of foodborne pathogens: calculations and considerations. Curr. Pharm. Biotechnol. 11: 58-68.
Kennedy, J.E., Oblinger, J.L., and Bitton, G. 1984. Recovery of coliphages from chicken, pork sausage and deli meats. J. Food Prot. 47: 623-626.
Kennedy, J.E., Wei, C.I. and Oblinger, J.L. 1986. Characterization of coliphages recovered from foods according to temperature of infectivity. J. Food Prot. 49: 952-954.
Leverentz, B., Conway, W.S., Alavidze, Z., Janisiewicz, W.J., Fuchs, Y., Camp, M.J., Chighladze, E. and Sulakvelidze, A. 2001. Examination of bacteriophage as a biocontrol method of Salmonella on fresh cut fruit: a model study. J. Food Prot. 64: 1116-1121.
Leverentz B., Conway, W.S., Camp, M.J., Janisiewicz, W.J., Abuladze, T., Yang, M., Saftner, R. and Sulakvelidze, A. 2003. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 69: 4519-4526.
O’Flynn, G., Ross, R.P., Fitzgerald, G.F., and Coffey, A. 2004. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 70:3417-3421. Omnilytics. 2007.
OmniLytics announces USDA/FSIS allowance for bacteriophage treatment of salmonella on livestock. http://www.omnilytics.com/news/news019.html. Accessed on April 12,2013.
Pao, S., Randolph, S.P., Westbrook, E.W. and Shen, H. 2004. Use of bacteriophages to control Salmonella in experimentally contaminated sprout seeds. J. Food. Sci. 69: M127-M129.
Sharma, M., Patel, J.R., Conway, W.S., Ferguson, S., and Sulakvelidze, A. 2009. Effectiveness of bacteriophages in reducing Escherichia coli O157:H7 on fresh-cut cantaloupes and lettuce. J. Food Prot. 72: 1481-1485.
Sharma, M., and Sharma, G.C. 2011. Viruses in Decontamination of Fresh and Minimally Processed Produce, ed. V.M. Gomez-Lopez, 285-295. Wiley Blackwell, Hoboken, N.J.
Sheng, H., Knecht, H.J., Kudva, I.T., Hovde, C.J. 2006. Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Appl. Environ. Microbiol. 72: 5359-5366.
Sulakvelidze, A. 2011. Safety by nature: potential bacteriophage applications. Microbe 6: 122-126.
USDA/FSIS. 2013. Food Safety and Inspection Service new technology information table. http://www.fsis.usda.gov/regulations/New_Technology_Table_Feb_06/. Accessed April 12, 2013.
Viazis, S., Akhtar, M., Feritag, J., and Diez-Gonzalez, F. 2011. Reduction of Escherichia coli O157:H7 viability on leafy green vegetables by treatment with a bacteriophage mixture and transcinnamaldehyde. Food Microbiol. 28:149-157.
Ye, J., Kostrzynska, M., Dunfield, K. and Warriner, K. 2009. Evaluation of a biocontrol preparation consisting of Enterobacter asburiae JX1 and a lytic bacteriophage cocktail to suppress the growth of Salmonella Javiana associated with tomatoes. J. Food Prot. 72: 2284-2292.
Ye, J., Kostrzynska, M., Dunfield, K. and Warriner, K. 2010. Control of Salmonella on sprouting mung bean and alfalfa seeds by using a biocontrol preparation based on antagonistic bacteria and lytic bacteriophages. J. Food Prot. 73: 9-17.
