New Insights on Food Nanoscience
IFT’s sixth International Food Nanoscience Conference explored research and emerging applications in process monitoring, food safety, packaging, and encapsulation, regulatory activities, and consumer perception.
Presenters at IFT’s sixth International Food Nanoscience Conference, which took place July 12–13, 2013 in Chicago, IL, provided a wealth of insights into the burgeoning area of nanotechnology. The participants described nanotechnology applications in the food system and other sectors, research into potential applications, research on safety, international developments, U.S. regulatory framework, and consumer perception toward the technology. The conference proceedings are published in the March 2014 issue of Comprehensive Reviews in Food Science and Food Safety, and are accessible at http://onlinelibrary.wiley.com/doi/10.1111/1541-4337.12055/abstract. This synopsis provides an overview of the presentations relating to the food system.
Food System Applications
Frans Kampers, Coordinator of Innovative Technologies at Wageningen University and Research Centre, and Rickey Yada, Canada Research Chair in Food Protein Structure at the University of Guelph and the Advanced Foods and Materials Canada Network, addressed current, emerging, and potential applications in Europe, Asia, and North America. Timothy Duncan, Research Chemist at the U.S. Food and Drug Administration (FDA)/Center for Food Safety and Applied Nutrition (CFSAN) and the Institute of Food Safety and Health, reviewed considerations for nanocomposites in food contact material applications. Gail Barnes, Partner of Personify L.L.C., described pursuit of turbulent flow UV-C for liquid dairy products.
Frans Kampers categorized food system applications into six areas: process monitoring via biosensors; food quality and safety; packaging; process innovations; structure modifications and texture engineering; and encapsulation and delivery. He mentioned instrumentation developed by InnoSieve Diagnostics (Wageningen, The Netherlands) that detects, counts, and differentiates live from dead bacteria in fluid samples. Also, Wageningen UR developed a system to detect specific bacteria via a nitrocellulose membrane with a two-sided antibody tag system using colloidal carbon nanoparticles coated with a specific antibody and a protein-binding tag. A nanostructure comprised of a cascade of sieves of different shapes and sizes, built on a silicon wafer using the same technology used for a microprocessor, can be used to filter microorganisms or separate out components from liquids (e.g., fat globules in milk).
Timothy Duncan explained that in packaging applications nanomaterials can increase polymer strength, provide flame resistance, attenuate polymer permeability (e.g., to moisture or gas), increase barrier properties, enhance biodegradability, absorb or block ultraviolet light, and provide antimicrobial, sensing, and anti-counterfeiting properties. The largest category of potential applications, he said, is high-barrier plastics. He mentioned that there are hundreds of examples of clay/polymer nanocomposites in the literature addressing a breadth of materials (e.g., ethylene-vinyl alcohol, poly(lactic acid), poly(propylene), and low-density polyethylene). Duncan also detailed a number of applications-related considerations (e.g., polymerization methods).
Gail Barnes said a turbulent flow UV-C pre-treatment combined with pasteurization can achieve a level of microbial reduction similar to ultra-pasteurization, and has other benefits such as improved energy efficiency, additional shelf life extension, no destruction of heat-sensitive enzymes (e.g., amylase, lactase, lipase), and higher quality product.
--- PAGE BREAK ---
John DiLoreto, founder of NanoReg and Executive Director of the Society of Chemical Manufacturers and Affiliates Nanotechnology Coalition, and Shaun Clancy, Director of Product Regulatory Services, Evonik Corp., also discussed food system applications (e.g., nanoclays used in beer bottle packaging to retard gas transfer and improve beverage stability with the added benefits of decreased bottle weight and reduced transportation costs; and a silicon carbide and silicon dioxide composite that can facilitate heat transfer for cooling (Johnson 2010)).
Ongoing Research and Future Applications
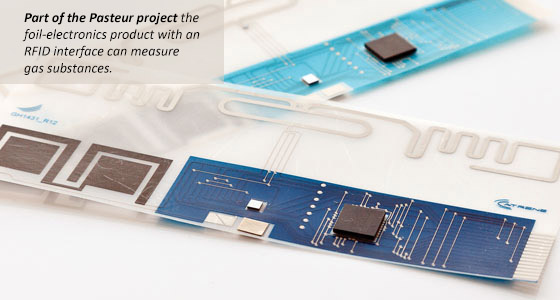
Frans Kampers described research activities in Europe, and commented that nanotechnology is extremely important for the entire European research community. The Pasteur Project is pursuing process monitoring of the quality and lifetime of perishables throughout the food chain using smart RFID tracking on packages, with the meat chain as a case study. The project led to the development of a foil-electronics product (Figure 1) with an RFID interface that can measure gas substances. QualityNano is developing safety assessment methods, involving synthesis, interlaboratory characterization of engineered nanomaterials, and collaborative methods development. NanoLyse is pursuing the detection, quantification, and tracking of nanoparticles in complex matrices. NanoNextNL, the most important program in The Netherlands, he said, has four food-related programs: food process monitoring and product quality assessment, molecular structure of food, novel food products and process innovation, and microdevices for structuring and isolation.
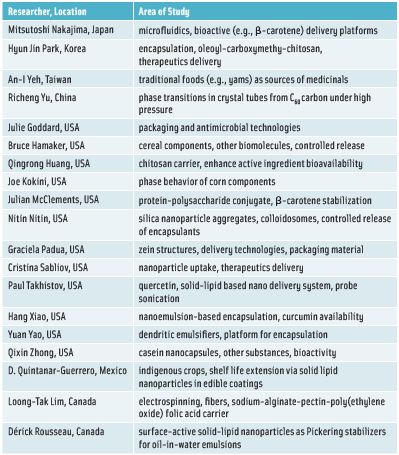
Rickey Yada briefly described as representative of ongoing research the potential applications that four individuals in Asia and 15 in North America are pursuing. Much of the work focuses on bioactives, he said, with some people pursuing encapsulation systems to enhance delivery. Some research reflects interest in whether certain ingredients can survive processing conditions, and other research reflects interest in antioxidant components of teas. The specific research pursuits of the individuals that Yada mentioned are listed in Table 1.
NanoRelease Project and NanoCharacter Initiative
Richard Canady, Director of the Center for Risk Science and Application at the International Life Sciences Institute (ILSI), provided an overview of two ILSI-led projects: NanoRelease and NanoCharacter. The NanoRelease Project, he said, was initiated to identify, evaluate, and develop methods needed to confidently detect, characterize, and evaluate intentionally-produced nanoparticles released from food along the alimentary tract. The project involves more than 70 experts in five task groups. Steve Roberts, Director of the Center for Environmental and Human Toxicology at the University of Florida, explained that an important aspect is distinguishing what we need to know from what would be nice to know, given the extraordinary challenge of understanding nanomaterial behavior in the complex, dynamic gastrointestinal (GI) tract. Rickey Yada, Susann Bellmann, Study Director at the Dutch Institute of Applied Scientific Research (TNO), and Timothy Duncan described the activities of four of the five task groups, which are focused on material characteristics, alimentary canal environments, alimentary canal models, measurement methods, and risk management.
The NanoCharacter Initiative is a collaboration to improve the comparability of nanomaterial characterization by creating a framework and roadmap to implement reporting of comparable nanomaterial characterization data across environment, health, and safety studies.
Dynamic In Vitro Gastrointestinal Model
Susann Bellmann and colleagues Susann Allelein and Tessa ten Cate have been studying the behavior of nanosized layered double hydroxide clay materials as model nanocompounds for food and pharmaceutical applications using a dynamic in vitro gastrointestinal digestion model called Tiny-TIM. She said the model mimics body temperature; peristaltic mixing; controlled gastric emptying; secretion of gastric acid and digestive enzymes, bile, and bicarbonate; dynamically and site-dependent changing pH values; and removal of digested products. They found that a small amount of particles remain intact or are persistent in the system, and that the percentage remaining was slightly greater for the heat-treated particles (higher stability during gastrointestinal conditions) than non-heat-treated particles. Bellmann said they concluded that nanomaterials are exposed to dynamically changing conditions during their transit along the GI tract which influence their fate (e.g., agglomeration, aggregation, or disintegration).
--- PAGE BREAK ---
Nanodelivery of α-Tocopherol
Lacey Simon, graduate student in the Biological and Agricultural Engineering Department at Louisiana State University (LSU), described her study with Rhett Stout and Cristina Sabliov at LSU of the ability of a nanodelivery system to improve bioavailability of bioactives. She described use of poly(lactide-co-glycolide) and PLGA-chitosan nanoparticles for delivery of α-tocopherol, and indicated that they found that both systems improved bioavailability of α-tocopherol.
Developments in Nanomaterial Synthesis
Rajender Varma, Senior Scientist at the U.S. Environmental Protection Agency, addressed sustainable synthesis of nanomaterials based on green chemistry principles such as those relating to nanocatalysis in water and protocols which use alternative energy sources such as microwave heating, ultrasound, and photocatalysis. He said synthetic approaches to nanomaterials include a top-down approach involving grinding and chemical and laser abrasion of bulk materials and another technique, called a bottom-up approach, which involves the reduction of a metal salt to reveal the nanoform metal which is then capped and dispersed. The greener strategies use vitamins or antioxidants (e.g., phenols from coffee or tea) as reducing agents for salts in aqueous medium.
Conveying the breadth of materials that can be used to create nanoparticles very efficiently upon exposure of metal salts, he mentioned lemon balm extract used with Fe(NO3)3 for in situ formation of nano iron in soils, naturally occurring biodegradable plant surfactants to synthesize gold nanostructures, a variety of sugars used with metal salts (e.g., gold), carboxymethyl cellulose, and others. Cyano salts can be hydrolyzed with water alone to generate shape-selective nanostructures. Agricultural residues, beet juice, and waste such as red grape-pomace from wine-making, and a number of other byproducts—namely glycerol from biodiesel production—can be used to generate nanoparticles in a sustainable manner.
Addressing Risks
Kampers said there are four basic nanoparticle parameters—size, solubility, biocompatibility, and zeta potential—that influence toxicological hazard. He described an ILSI project to address the parameters in the context of food manufacturing applications, which he said led to the development of a five-step approach to safety assessment: “Practical Guidance for the Safety Assessment of ENMS in Food” (Cockburn et al., 2012).
--- PAGE BREAK ---
International Challenges and Collaborations
Shaun Clancy said terminology/definitions, safety, regulation, and international cooperation are four challenges in the field of nanotechnology that are faced around the world. He mentioned, for example, that a single product may be regulated under three, four, or five different definitions and under some definitions may be a nanomaterial but not under a different statute.
Health Canada’s view is more focused on manufactured nanomaterials than incidental or naturally-occurring nanomaterials, and those that are nanoscale in any dimension. The European Union’s recommendation includes natural and incidental as well as manufactured and is not based on mass, as many definitions are, but on size and particle number, a very important distinction with regulatory implications, Clancy commented. The International Organization for Standardization’s Technical Committee 229, American Society of Testing and Materials, and other groups are making progress on terms and definitions, but a great deal of terminology still needs to be finalized or accepted, he commented.
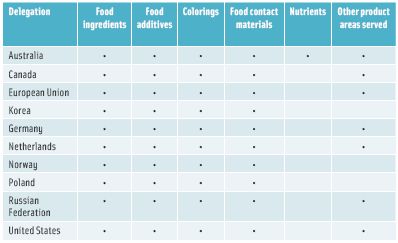
The OECD Working Party on Manufactured Nanomaterials is looking to share information among countries about regulatory actions, voluntary programs, and data, he said. Some of their major contributions to date are guidance documents on how they view consideration of nanomaterials, and preparation of nanomaterials in testing and sample preparation. The organization also created a research program, and identified a list of 60 endpoints, which include physical chemistry, ecological toxicology, mammalian toxicology, and environmental fate, that are pertinent to preparation of dossiers. The OECD has a Working Party on Nanotechnology that has a number of projects, one of which led to a recent publication reporting the results of a survey of food nanotechnology regulatory frameworks (OECD 2013) (Table 2). The International Organization for Standardization has a Task Group on Consumer and Societal Dimensions that is evaluating whether there is enough opportunity for people to engage with and provide input into technical committees.
Regulatory Perspectives and Initiatives
Clancy said regulatory agencies have begun to hold workshops, including one scheduled for the United States in 2014, to gain common understanding in different areas (e.g., measurement, linking of nanoproperties and physicochemical properties to biological properties). He mentioned that many countries have been considering appropriate oversight for the development of the technology without hindering its viability. He mentioned that France is developing a nanomaterial registry; and Belgium, Denmark, and Italy are considering registries. At the EU level, the EC has considered whether to take action to address nanomaterials, and a number of nongovernment organizations believe that REACH (Registration, Evaluation, Authorisation, and Restriction of Chemicals) should be reopened and text created to address nanomaterials. Environment Canada, Health Canada, and the U.S. EPA, he said, are assessing nanomaterials in commerce, for industrial purposes, and evaluating substance or pre-manufacture notifications. The U.S. and Canada have a number of Regulatory Cooperation Council projects focused mainly on industrial chemicals, he said, to better harmonize regulatory approaches.
Michael Adams, Deputy Director of the Office of Food Additive Safety at the U.S. FDA/CFSAN described the FDA’s regulatory framework for the food additives program and its policy guidance documents. He said the agency takes a risk-based and product-specific approach to nanotechnology within the regulatory framework. Where the statue is vague or ambiguous, FDA encourages premarket consultation. He pointed out that the industry has the statutory requirement to market safe products that are properly labeled and not misbranded under the law. Additionally, he said the industry is obligated to approach the agency about any negative developments regarding their materials, so the agency can conduct an evaluation.
The agency’s draft guidance issued in 2011 identifies basic questions that manufacturers should ask themselves: Does an engineered material or end product have at least one dimension in the nanoscale range (about 1–100 nm)? Does an engineered material or end product exhibit properties or phenomena, including physical or chemical properties or biological effects, which are attributable to its dimension? He said the guidance speaks to the importance of physical properties manifested by the product and the perspective that the nanoscale range may not cover all of the things that the agency is interested in from a safety point of view. Adams said the considerations apply to new products, but may also apply when manufacturing changes alter the dimensions, properties, or effects of an FDA-regulated product or any of its components. The draft guidance issued in 2012 that applies to food and food packaging materials conveys that in the area of nanotechnology manufacturers should ask themselves the same questions that they would normally ask with respect to functionality and safety, and that they need to be related to the size of the particle and whether the size has any particular, unique effect; if so, he said that needs to be described to the agency when approaching the agency with a safety dossier. Adams also mentioned chemistry-focused guidance that was issued amongst a series of guidances between 2007 and 2009 that the agency updated to address nanotechnology, with particle size-related language.
--- PAGE BREAK ---
Consumer Perception
Mitchell Cheeseman, Managing Director of Environmental and Life Sciences at Steptoe & Johnson LLP, said that there is hope that if people are provided accurate and complete information by sources they perceive as credible they can be educated that the benefits of nanotechnology in food and food packaging are larger than the risks and that the technology needs to move forward. He said there are a number of surveys of consumer attitudes on nanotechnology, some of which address food-related nanotechnology. Depending on the survey, between 40–80% of consumers know little or nothing about nanotechnology, and generally less than half think they understand it. He cautioned that some surveys have also shown that when consumers learn more about nanotechnology and its risks, their attitudes become more negative. Surveys have also shown that consumers are less positive about nanotechnology in food and food packaging than they are about the technology overall, and they are more willing to accept nanotechnology in food packaging than food products. Thus, addressing benefits is key to acceptance, he said, and the positive story about the potential benefits of nanotechnology in food, food processing, and food packaging needs to be told more often.
Rosetta Newsome, Ph.D., is Director, Science and Policy Initiatives,
Institute of Food Technologists, Chicago, IL ([email protected]).
Acknowledgements
IFT greatly appreciates the speakers’ participation in the conference and the invaluable involvement and input of the moderators—Richard Canady, Jozef Kokini of Purdue University, Rickey Yada, and Corey Wright of the U.S. Dept. of Commerce. IFT also appreciates the invaluable efforts and guidance of the 2013 International Food Nanoscience Conference Planning Committee members—Vijay Arora of Kraft Foods Inc., Betty Bugusu of Purdue Univ., Richard Canady, Henry Chin, retired from Coca-Cola Co., Renata Clarke and Vittorio Fattori of the Food and Agriculture Organization of the United Nations, John Floros of Kansas State Univ., Gustavo Gutierrez of the National School of Biological Sciences of IPN, Jozef Kokini, Cristina Sabliov of Louisiana State Univ., Jordi Serratosa of the European Food Safety Authority, Paul Takhistov of Rutgers Univ., Corey Wright, Yuan Yao of Purdue Univ.; and Will Fisher, Jennifer London, George Miller, Rosie Newsome, and Aimee Pagano of IFT. IFT also extends its gratitude to Wiley (Ames, Iowa) and the Netherlands Office for Science & Technology of the Royal Netherlands Embassy (Washington, DC) for sponsoring the conference and to Katherine Wilkes, a student at Purdue Univ., for her diligent transcription of the audio recording of the conference, which contributed immensely to the preparation of the proceedings and this synopsis.
References
Cockburn, A., Bradford, R., Buck, N., Constable, A., Edwards, G., Haber, B., Hepburn, P., Howlett, J., Kampers, F., Klein, C., Radomski, M., Stamm, H., Winjnhoven, S., and Wildemann, T. 2012. Approaches to the safety assessment of engineered nanomaterials (ENM) in food. Food Chem. Toxicol. 50: 2224-42.
Johnson, D. 2010. Necessity is the mother of invention in nanotech. IEEE Spectrum. [Internet]. New York, N.Y. IEEE; C _ 2013. Available at http://spectrum.ieee.org/nanoclast/semiconductors/nanotechnology/necessity-is-the-mother-of-invention-in-nanotech. Accessed January 30, 2014.
OECD. 2013. Regulatory Frameworks for Nanotechnology in Foods and Medical Products: summary Results of a Survey Activity. OECD Science, Technology and Industry Policy Papers, No. 4. Organisation for Economic Co-operation and Development. OECD Publishing. Available at http://dx.doi.org/10.1787/5k47w4vsb4s4-en. Accessed August 27, 2013.
