
Lots to Learn About Safety and Quality at IFT19
FOOD SAFETY AND QUALITY PREVIEW
Article Content
The Scientific Program at IFT19 in New Orleans, June 2–5, 2019, will feature more than 100 oral sessions, 605 printed poster papers, and 118 e-poster presentations. The following are brief descriptions of sessions related to food safety and quality.
Food Safety
In session 107, “Biosensing Technology for Practical Applications in Food Production,” on Monday, June 3, from 10:30 a.m. to noon, Sundaram Gunasekaran will describe an immuno-sensor that uses gold nanoparticles to provide rapid and highly sensitive visible detection of bacteria in real matrices without requiring a readout device; Sam R. Nugen will describe a bacteriophage-based membrane filtration assay that rapidly and quantitatively detects Escherichia coli in water; Silvana Andreescu will describe portable, reagent-less, nano-biosensors for monitoring food freshness, safety, and quality and their possible use in smart packaging; and Eric S. McLamore will describe nano-biosensors for rapid detection of bacteria in water and on fresh vegetables.
In session 118, “Product Recalls: What Have We Learned and How Can We Prevent the Next One From Occurring?” on Monday, June 3, from 3:30 p.m. to 5 p.m., Suresh de Costa will discuss how best agricultural practices, environmental monitoring, and sanitary design in packing houses mitigate food safety risk; Jitu R. Patel will discuss current efforts by industry and regulatory agencies to control bacterial pathogens in fresh produce; and Harshavardhan Thippareddi will discuss mitigation of the risk of contamination by Listeria monocytogenes in the production and processing of ready-to-eat meat and poultry products.
In session 119, “Safety Assessment: Confidence in Your Ingredients From Nature to Table,” on Monday, June 3, from 3:30 p.m. to 5 p.m., Debby Newslow will describe the requirements of the Preventive Controls for Human and Animal Food regulations, Foreign Supplier Verification Programs regulation, Mitigation Strategies to Protect Food Against Intentional Adulteration, and the Pasteurized Milk Ordinance; Michael Dziewatkoski will discuss how analytical testing helps in the development of quality specifications and how the specifications determine consumer acceptance of ingredients; and Ray A. Matulka will discuss the parallels between a safety assessment and components of the Food Safety Modernization Act (FSMA) objectives and techniques to determine the safety of ingredients and final products.
In session TRC201, “Food Safety: Integrating Traceability, Blockchain Technology, and FSMA,” on Tuesday, June 4, from 11:45 a.m. to 1:15 p.m., Maria Velissariou will describe how the IFT Global Food Traceability Center is developing capabilities and tools focusing on interoperability standards for efficient global food traceability across the entire supply chain; Purnendu C. Vasavada will discuss developing and implementing an effective food safety plan for FSMA compliance, specifically with respect to validation and verification; and Emily Lyons will describe blockchain technology and how it can be adopted by the food industry to increase traceability and food safety.
In session 323, “Pet Food Safety: Full Circle,” on Wednesday, June 5, from 10:30 a.m. to noon, Greg Aldrich will discuss how processes affect pet food safety, nutrient utilization, and shelf life and will describe pet food safety research being conducted in academia; Michele Sayles will discuss applying microbial ecology research methods to enhance the overall safety of pet food products; and Amanda Ungs will discuss how the rendering industry focuses on the safety of products that end up in pet food.
In session 324, “Enhancing Food Safety Education in Food Science and Engineering Courses Using Simulation,” on Wednesday, June 5, from 10:30 a.m. to noon, educators will describe simulation-based education modules for use in food science lectures and laboratory courses: Ahmed E. Yousef will discuss the microbial inactivation module; Ashim K. Datta will discuss the food safety module; Gail M. Bornhorst will discuss challenges in the use of the modules in the food science classroom from an instructor’s viewpoint; Ruth Oni will discuss the microbial inactivation module and the risk assessment module; Nitin Nitin will discuss a biofilm sanitation module; and Mayuri S. Ukidwe, Rosana Moreira, and Kanishka Bhunia will discuss a module demonstrating the effect of temperature and water activity on microbial inactivation.
In session 333, “Food Safety and Regulation of Insect-Based Food,” on Wednesday, June 5, from 1:15 p.m. to 2:45 p.m., Jakub Dzamba will discuss enabling companies to enter the insect-based foods business; Jodi P. Williams will discuss U.S. Department of Agriculture (USDA) research funding opportunities and previously funded projects on edible insects; and Ravi Jadeja will discuss regulatory and third-party audit compliance challenges regarding using insects as ingredients.
Regulatory
In session 102, “California Prop 65: Everything You Need to Know Before You’re Asked About It,” on Monday, June 3, from 7:45 a.m. to 8:45 a.m., Martin J. Hahn will describe the requirements of California’s Proposition 65, which lists chemicals known to cause cancer, birth defects, or other reproductive harm, and the challenges associated with a food or beverage product that contains a listed or proposed ingredient; Gina M. Reo will describe how Proposition 65 differs from FSMA and will provide recommendations for developing a food safety plan; and Sylvester Mosley will present approaches for communicating Proposition 65 safety issues to company management and the public.
In session 126, “Natural Colors: Opportunities for Innovation,” on Monday, June 3, from 3:30 p.m. to 5 p.m., John Cox will discuss global regulations regarding natural colors and the process for achieving new approvals; Diego Darquea will discuss technical and regulatory challenges, including lack of global harmonization, differing definitions of natural, and labeling regulations; and Deepti Dabas will discuss the properties of red pigments from natural sources, their performance in food applications, and methods to stabilize them.
In session 218, “Functional Food Regulations in the United States, Europe, and the Asian Subcontinents,” on Tuesday, June 4, from 12:30 p.m. to 2 p.m., Jerzy Zawistowski will discuss the nutraceutical regulations of Asian countries; Ashish Talati will discuss the regulations in the United States and Canada; Asim K. Duttaroy will discuss the regulations in Europe; Claudia A. Lewis will discuss U.S. regulation of medical foods; and Andrew Shao will discuss global market entry regulations for nutraceuticals, functional foods, and supplements.
In session 234, “Hazard Assessment and Standard Development for Colors From Natural Sources,” on Tuesday, June 4, from 2:15 p.m. to 3:45 p.m., Bhakti Petigara Harp will describe differences among the regulations of the United States, European Union, and other countries in how color additives are approved and which colors are allowed; Mark A. Goldschmidt will discuss how manufacturers have the responsibility to certify the quality and safety of colors and will describe a case study of conducting a hazard analysis under FSMA rules; Roger A. Clemens will discuss potential safety challenges; and Zhuohong Xie will discuss Food Chemicals Codex standards for food colors from natural sources and projects to develop more modern standards to mitigate the risk of potential adulteration.
In session 304, “The Labeling of Bioengineered (BE) Foods: Consumer Perception and Industry Impact,” on Wednesday, June 5, from 8:30 a.m. to 10 a.m., Martin J. Hahn will discuss the USDA’s proposed National Bioengineered Food Disclosure Standard and how industry can mitigate the risk of litigation regarding labeling of foods as non-GMO or non-BE; Kathy Musa-Veloso will discuss consumer perception of BE foods and how labeling influences purchase intent; and Erin Healy will focus on regulations pertaining to BE foods.
In session 302, “Update on European and U.S. Regulatory Developments in Nutrition and Health,” on Wednesday, June 5, from 8:30 a.m. to 10 a.m., Robert C. Post will discuss the new Nutrition Facts labeling rules, which will be implemented in January 2020, and the related U.S. Food and Drug Administration initiatives to define “healthy”; Dominique A. Taeymans will provide an update on European regulatory developments regarding nutrition labeling and nutrition and health claims; Luke Murphy will discuss how European Union states are using taxation to stimulate healthier consumption of foods and beverages and encourage companies to reformulate products; and Raymond Ellard will discuss how the European Food Safety Authority’s risk assessments are evolving in the direction of nutrition and health.
Food Fraud
In session TRC101, “Can We Win the Fight Against Food Fraud?” on Monday, June 3, from 10:15 a.m. to 11:45 a.m., Chris Elliot, Bertrand E. Emond, and John Spink will discuss how industry and regulators can work together to win the fight against food fraud.
In session 210, “Food Fraud Prevention: How Food Standards Complement Information Databases and Vulnerability Assessments,” on Tuesday, June 4, from 10:30 a.m. to noon, Karen Everstine will discuss how a food fraud database can be used to develop standards and protection efforts and will describe the foods most prone to fraud, the most common adulterants and methods of adulteration, and analytical methods targeting fraud; Zhuohong Xie will discuss how food standards and reference materials can be used to verify the identity, quality, and purity of food ingredients and help ensure the safety and integrity of the food ingredient supply chain; and Sneh Bhandari will describe techniques for determining the authenticity of food ingredients and reducing the risk of economically motivated adulteration.
In session 321, “Best Practices for Fraud Prevention in the Global Organic Supply Chain,” on Wednesday, June 5, from 10:30 a.m. to noon, Kim Dietz and Gwendolyn Wyard will describe the Organic Trade Association’s new Organic Fraud Prevention Solutions program; and Erin Heitkamp will describe the organic supply chain vulnerabilities that pose the highest risk of fraud and a system for achieving organic supply-chain integrity.
Sensory
In session 110, “Designing Food and Beverages for Niche Populations,” on Monday, June 3, from 10:30 a.m. to noon, Carolyn Ross will discuss food research with children with developmental delays and how food preferences of young children with Down syndrome can be applied by food product developers; Dolores Oreskovich and Allison Baker will discuss working with populations with swallowing challenges and development of appropriate food products with high nutritional value; and Wendy Wismer will discuss sensory and consumer science tools and approaches that are most appropriate for product development for cancer patients.
In session 208, “Sensory/Consumer Data and Unmet Expectations: The Mother of All Frustration,” on Tuesday, June 4, from 10:30 a.m. to noon, Karen Graves and other speakers will discuss situations where sensory and consumer testing results did not come out as expected and affected business decisions, describing what occurred, what could have been done differently, and how the situation was managed; Sola (Olusola) Ojeh will discuss discrimination testing situations; Sharon L. Bender will discuss descriptive testing situations; and Ratapol Teratanavat will discuss consumer testing situations.
In session 232, “Consumer Choice, Not a New Nicholas Sparks Novel; Rather, How We Observe and Measure Matters,” on Tuesday, June 4, from 2:15 p.m. to 3:45 p.m., John C. Castura will discuss whether the number of available choices creates an overload effect in consumers; Ann Colonna will argue that the difficulties associated with making choices might be more related to the complexity of the choice task and the cognitive decision-making factors than simply to the number of choices; and Amy Bowen will discuss the determinants of choice behavior and how artificial intelligence technology that recommends options based on previous purchases will continue to learn from consumer habits and choices.
In session 331, “Building Behavioral KPIs Into Food-Product Decision Making,” on Wednesday, June 5, from 1:15 p.m. to 2:45 p.m., David Lundahl will describe how implicit, explicit, and prospective modes of human thinking can be used to help with study design; Paul Conner will describe implicit and explicit behavioral techniques and how they can be incorporated into behavioral key performance indicators; and Christopher Simmons will discuss how context and priming influence product testing.
Food Technology Articles

Better Juice and Ingredion Collaborate and More Ingredient News
News about food industry suppliers

How to Formulate for Food Intolerances
In this column, the author describes the global prevalence of food intolerances and provides insight into state-of-science ingredient replacement and removal methods when formulating gluten-free and lactose-free foods.

Top 10 Functional Food Trends: Reinventing Wellness
Consumer health challenges, mounting interest in food as medicine, and the blurring line between foods and supplements will spawn functional food and beverage opportunities.

Battling Biofilms
In this column, the author describes the stages of biofilm development in food processing plants, methods of removal, and best practices for prevention.

Natural Product Expo West Attention-Getters: Highlights From the Event
Food Technology Contributing Editor Linda Milo Ohr reports on trends she tracked at Natural Products Expo West 2024.
IFT Podcasts

Episode 29: All About Food Safety Culture
In this podcast, we discuss food safety culture, including how food safety culture is established, measured, and how they are expected to change in light of ongoing advancements in food science and policy. Our guests include Hugo Gutierrez, Global Food Safety and Quality Officer for Kerry, and Bob Gravani, Professor Emeritus of Food Science and Director Emeritus of the National Good Agricultural Practices (GAPs) Program at Cornell University.

Episode 28: Spicing Up the Future of Food
In this podcast we’ll focus on some of nature’s herbs, spices and the extracts that offer potential health benefits. We'll also talk with the article's author, Linda Ohr, about what the research tells us, as well as some entrepreneurial companies who have tapped these spices' hidden potential for their current and future product innovation.

 A flash point tester that checks compliance. The MiniFlash FP Vision Flashpoint tester (right) by Grabner Instruments allows users to determine the flash point of liquids and solids while checking compliance with transportation regulations for pr…
A flash point tester that checks compliance. The MiniFlash FP Vision Flashpoint tester (right) by Grabner Instruments allows users to determine the flash point of liquids and solids while checking compliance with transportation regulations for pr…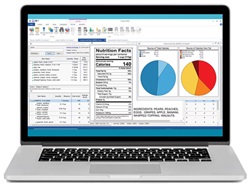 A suite of software products analyzes nutrition and makes labels. The Genesis R&D Food Formulation and Labeling Software (below) simplifies the process of developing a food product from conceptualization to regulatory-compliant labeling. Integrated with ESHA Research’s database of raw ingredients, additives, and recipes, the software provides lab-accurate analysis of a product’s nutritional content and generates a Nutrition Facts label. A similar software program now exists for the supplement industry. ESHA Research, Booth 3635
A suite of software products analyzes nutrition and makes labels. The Genesis R&D Food Formulation and Labeling Software (below) simplifies the process of developing a food product from conceptualization to regulatory-compliant labeling. Integrated with ESHA Research’s database of raw ingredients, additives, and recipes, the software provides lab-accurate analysis of a product’s nutritional content and generates a Nutrition Facts label. A similar software program now exists for the supplement industry. ESHA Research, Booth 3635 Increase productivity with quick analysis. LECO Corporation’s TGM800 Thermogravimetric Moisture Determinator (above)measures moisture content with a primary loss-on-drying method. The instrument offers time-saving features such as the ability to measure 16 samples at a time and intuitive software. LECO also offers the Pegasus BT 4D, a tool that utilizes high-performance thermal modulation to identify unknown analytes in food samples. LECO Corp., Booth 625
Increase productivity with quick analysis. LECO Corporation’s TGM800 Thermogravimetric Moisture Determinator (above)measures moisture content with a primary loss-on-drying method. The instrument offers time-saving features such as the ability to measure 16 samples at a time and intuitive software. LECO also offers the Pegasus BT 4D, a tool that utilizes high-performance thermal modulation to identify unknown analytes in food samples. LECO Corp., Booth 625 Conduct food analyses inside and outside the laboratory. The Epsilon 1 (left), a small benchtop energy-dispersive spectrometer, analyzes elemental concentrations of elements and impurities with x-ray fluorescence. Compact and cost-effective, the Epsilon 1 has a bright touchscreen, a high-power x-ray tube, and a built-in computer, all of which make for a robust design that provides compliant results without recalibrating. Malvern Panalytical, Booth 1352
Conduct food analyses inside and outside the laboratory. The Epsilon 1 (left), a small benchtop energy-dispersive spectrometer, analyzes elemental concentrations of elements and impurities with x-ray fluorescence. Compact and cost-effective, the Epsilon 1 has a bright touchscreen, a high-power x-ray tube, and a built-in computer, all of which make for a robust design that provides compliant results without recalibrating. Malvern Panalytical, Booth 1352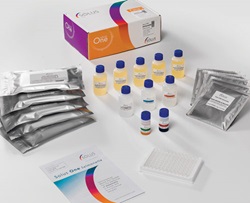 Pathogen testing can be rapid and efficient. Solus One is a collection of highly efficient assays for next-day detection of various foodborne pathogens. There are Solus One kits for next-day detection of Salmonella(right) in food samples and Listeria spp. in environmental samples. The kits are said to be reliable, robust, and validated by independent laboratories. Solus Scientific, Booth 4724
Pathogen testing can be rapid and efficient. Solus One is a collection of highly efficient assays for next-day detection of various foodborne pathogens. There are Solus One kits for next-day detection of Salmonella(right) in food samples and Listeria spp. in environmental samples. The kits are said to be reliable, robust, and validated by independent laboratories. Solus Scientific, Booth 4724