Removing and Preventing Biofilms
FOOD SAFETY AND QUALITY
Some species of bacteria, including pathogens such as Listeria monocytogenes, Escherichia coli, Campylobacter jejuni, and Salmonella, are capable of forming biofilms on surfaces of foods and food processing equipment. They pose a hazard to the food industry because they are difficult to remove and highly resistant to conventional cleaning and sanitizing agents. Biofilms can be harder to remove than planktonic cells (individual cells suspended in media) and may therefore pose a more significant food safety risk.
A biofilm is a thin layer of densely packed microbial cells encapsulated within a polymer matrix of proteins, nucleic acids, and polysaccharides. It can be composed of cells of a single species or multiple species. It is formed in five stages: 1) bacterial cells attach reversibly to a surface via van der Waals forces, hydrophobic interactions, or electrostatic interactions; 2) they excrete an extracellular polymeric substance that increases the adhesion and makes the attachment irreversible; 3) microcolonies begin to form and the biofilm begins to mature; 4) the biofilm continues to mature and becomes a three-dimensional structure with channels running between clusters of cells for delivery of water and nutrients and removal of waste; and 5) cells break off from the mature structure to form new biofilms.
Biofilms offer benefits to bacteria, protecting them from the effects of antimicrobial compounds, chemical stresses, and physical stresses; decreasing the potential for dehydration; and facilitating the exchange of nutrients, metabolites, and genetic material. The cells communicate with each other by a signaling process known as quorum sensing, which allows the bacteria to function for the benefit of all the cells within the biofilm. Research is being conducted to find methods to disrupt quorum sensing and thus the formation of biofilms, such as enzymatically degrading the signaling molecules, hindering the generation of signals, blocking the receipt of signals, and developing compounds that inhibit quorum sensing.
Research is also being conducted on such approaches as introducing inhibitors that interfere with microbial cell-to-cell signaling into foods to prevent microbial persistence and proliferation or be used in cleaning solutions to prevent biofilm formation on food processing equipment. A number of other approaches besides quorum sensing to prevent or remove biofilms have been researched. They include use of lactic acid bacteria, plant proteases such as ficin, bacteriophages, nanotechnology, and probiotics.
Meeting Challenges
Lynne McLandsborough, professor of food microbiology, University of Massachusetts, Amherst, said that the heightened concern about biofilms in the processing environment has increased the emphasis on hygienic design of food processing equipment, specifically prevention of rough places for bacteria to accumulate and ease of breakdown for cleaning. The biggest challenge, she said, is to develop a food safety culture that includes diligent environmental monitoring. This means that food companies must do more than swab the same areas every time, she added, but instead take time to actively look for potential harboring sites for bacteria.
Scott Burnett, RD&E director of food safety and quality, Ecolab Food & Beverage, said that significant progress has been made in understanding the microbiology of biofilms comprising thermotolerant and thermophilic spore-formers within food processing equipment. While typically not pathogenic, these organisms can contribute to quality defects in finished products, thereby limiting their market value. Research groups from industry and academia have invested in research to better understand the ecology of these microbial communities. For example, he said, these biofilms reside within niche sites in equipment that are not commonly accessible for direct visual inspection or for use of microbial indicator detection technologies.
The industry is challenged, he said, with maximizing the output of finished products while meeting market-driven specification expectations. Generally, without rigorous attention to biofilm control within systems, production runs are limited before microbial counts become elevated, indicating that biofilm communities within niche points have begun to grow and shed bacteria into the product stream. At this point, production may be halted for sanitation, limiting the efficiency of operations. In addition to gaining an understanding of the microbiology, research teams are also focused on methods of biofilm control, and several creative approaches have been offered.
The first line of defense, Burnett said, is aggressive focus on sanitary design of equipment followed by a robust preventive maintenance program designed to minimize niche points within processing equipment where spore-formers may find initial harborage and begin the steps in biofilm formation. The second is focus on cleaning programs designed for biofilm removal from surfaces. Ecolab has developed a method of rigorous cleaning called surface energy enhanced cleaning that disrupts complex polysaccharide matrices from within, providing a superior precleaning step prior to sanitation.This approach, he said, works where traditional cleaning practices do not (i.e., down to the niche points harboring microbial biofilms) and allows for longer production runs of high-quality finished products.
Katie Moore, director of field sales, Sterilex, said that biofilms typically grow in difficult-to-clean areas where they can be undisturbed, and the biggest challenge is access. If food processors could reach and hand-scrub every square inch of their facilities and processing equipment on a regular basis, biofilm growth would be very unlikely, she said. Many plants do not have access to all parts of their processing equipment, they may have very limited time for sanitation, or it may be a dry processing environment that doesn’t allow for breakdown and wet cleaning/scrubbing. The food processing industry is working to address this in many ways, she said, including better equipment and plant design, allowing for proper breakdown and cleaning; a bigger focus on sanitation and adequate time, tools, and personnel; and new product and sanitation chemical innovations that provide a way to remove biofilms.
Simply killing free-swimming and biofilm-related microorganisms without removing the biofilm structure from a surface can result in rapid recolonization of organisms within the remaining biofilm matrix. She said that Sterilex has spent the last 20 years developing solutions based on its proprietary PerQuat technology, a chemistry specifically formulated to penetrate biofilm; kill microorganisms such as Listeria, E. coli, Salmonella, Campylobacter, and Pseudomonas within biofilm; and remove biofilm from surfaces, preventing biofilm recurrence and microbial recontamination. This innovative technology is based on the generation of a unique salt that forms when combining an alkaline peroxygen compound and a positively charged phase-transfer catalyst along with other formulation components to provide cleaning and biofilm removal as well as control of pathogenic microorganisms.
She said that Sterilex’s products, which are registered and approved by the U.S. Environmental Protection Agency (EPA), can be foamed, sprayed, or used in soak applications to reach inaccessible areas where biofilms grow, such as in drains. She added that the company is continually developing additional product innovations that provide enhanced sanitation on surfaces where biofilms are likely to be found and recently introduced Sterilex Ultra Step, a sanitizer that can be applied in solid form on floors and in drains found in low-moisture processing environments.
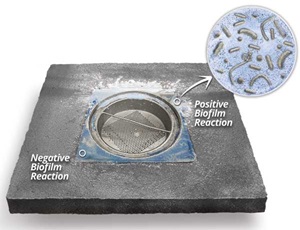 Moore said that one of the most important recent achievements is rapid biofilm diagnostics, which is the ability to rapidly identify biofilm harborage niches on food processing equipment in a cost-effective manner. Biofilms are found most often on non-visible, difficult-to-clean surfaces such as in hollow rollers, weld cracks, and screw holes and can take root on scratched or damaged surfaces. While routine environmental swabbing and adenosine triphosphate testing are valuable data points, she said, they only sample areas that the swab can reach. Sterilex’s new Indicon Gel is meant to seep into cracks, crevices, and other difficult-to-reach areas and provide nearly instantaneous visual indication of a potential biofilm harborage niche on a surface. Designed to rapidly react with a byproduct naturally produced by microorganisms within biofilm, the product is sprayed onto surfaces, and when the blue gel contacts biofilm, it produces a white foam of microbubbles within two minutes, providing a visual indication of potential harborage niches. This provides quality and operations personnel with a clear guide as to where they may be at risk for biofilm formation and spread and enables them to develop ongoing preventive maintenance programs to address this risk.
Moore said that one of the most important recent achievements is rapid biofilm diagnostics, which is the ability to rapidly identify biofilm harborage niches on food processing equipment in a cost-effective manner. Biofilms are found most often on non-visible, difficult-to-clean surfaces such as in hollow rollers, weld cracks, and screw holes and can take root on scratched or damaged surfaces. While routine environmental swabbing and adenosine triphosphate testing are valuable data points, she said, they only sample areas that the swab can reach. Sterilex’s new Indicon Gel is meant to seep into cracks, crevices, and other difficult-to-reach areas and provide nearly instantaneous visual indication of a potential biofilm harborage niche on a surface. Designed to rapidly react with a byproduct naturally produced by microorganisms within biofilm, the product is sprayed onto surfaces, and when the blue gel contacts biofilm, it produces a white foam of microbubbles within two minutes, providing a visual indication of potential harborage niches. This provides quality and operations personnel with a clear guide as to where they may be at risk for biofilm formation and spread and enables them to develop ongoing preventive maintenance programs to address this risk.
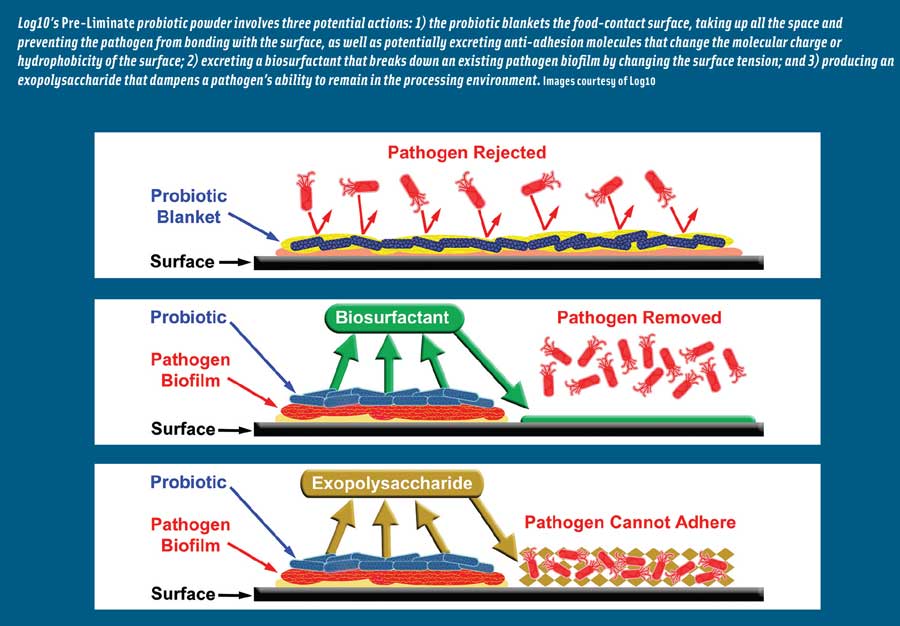
Siobhan Reilly, chief executive officer, Log10, said that her company has developed a combination of beneficial probiotic microorganisms that produce biosurfactants and extracellular polysaccharides. The biosurfactants help break down pathogenic biofilm, and the extracellular polysaccharides allow for a new nonpathogenic biofilm to be established. In short, she said, the company is using good bacteria to prevent and/or eliminate bad bacterial biofilms from food processing equipment and simultaneously serve as a nonpathogenic replacement to prevent the recurrence of any unwanted biofilms. The company has worked with university researchers to identify the genes in probiotic bacteria that produce biosurfactants, allowing the company to effectively screen and select the best biosurfactant-producing microorganisms. By identifying the gene that produces biosurfactants, she said, Log10 researchers can know how to express this gene at all times.
Biofilms consisting of multiple species rather than a single species present a more difficult hurdle for food processing plants, she said. Listeria monocytogenes, Salmonella species, and Bacillus species can exist in the same biofilm, and organisms that have the ability to attach and form extra-tough biofilms may host deadly, drug-resistant organisms that may not have the ability to form biofilms at all. Close attention should be paid to understanding how spore formers such as Bacillus and Clostridium may replicate and sporulate within the same biofilm, she stressed. A combination of a protective biofilm and sporulation ensures that an organism is even more resistant to heat, sanitizers, or any other treatment than ever before, and multiple-species biofilms can quickly obtain new organisms with multiple drug resistances. The ability to transfer or pick up genes that allow for drug resistances (e.g., methicillin-resistant Staphylococcus aureus) represent a more stable, more resistant biological hazard.
She added that the use of new/different materials for food equipment and food-contact surfaces could help combat the formation of pathogenic biofilms. Biofilms form differently on different metals, plastics, and composites, she said, and knowing which material should be used for food processing equipment could have a profound impact on biofilm formation. Materials made of copper, for example, have some antimicrobial properties. Even the pore size of the metal making up the food-contact surface can play a role in biofilm formation and adhesion. These developments, however, are slow to appear in or on new processing equipment that’s affordable.
Reilly said that Log10’s Pre-Liminate is a dry probiotic powder consisting of a combination of strains of Lactobacillus and Bifidobacterium that are effective against Salmonella, Listeria, and Clostridium with other ingredients that are generally recognized as safe. Water, as in a water-based sanitizer, cannot be introduced into some processing equipment, so the company developed a powder that flows better than water. The organisms are aerosolized and can be taken up easily by air currents into places that a liquid could never get to, she said. The powders can be dispensed from any suitable aerosol container or by hand. The company has screened thousands of Lactobacillus and Bifidobacterium strains to find ones that are most effective. It has a huge culture bank, so the formulation of the powders can be customized to fit a user’s specific needs.
Darla Goeres, associate research professor and director, Standardized Biofilm Methods Laboratory (SBML), Center for Biofilm Engineering (CBE), Montana State University, and her colleagues Paul Sturman, industrial coordinator, and Diane Walker, research engineer, said that the food industry has begun to recognize that illness can be attributed to bacteria that exist in biofilms. These surface-attached, matrix-encapsulated microorganisms can be substantially tougher to eradicate, either because of the protection afforded by living in a slimy community or by forming in hard-to-reach places.
Educating industry about biofilm is a challenge. As with the recreational water and spa industry, there have been some in industry who are not comfortable even acknowledging that a biofilm issue exists; this is understandable, Goeres said, since their products would have to be reevaluated. To try to engage those in the food and beverage industry, the CBE included a session on food biofilms at its recent biannual Montana Biofilm Science and Technology Meeting.
Methods development for recovering biofilm is another area that could use some expansion, Goeres said. While many methods exist for free-living bacteria, biofilm methods are still limited. The SBML’s mission is the development and validation of quantitative standard methods for growing, treating, sampling, and analyzing biofilm bacteria. Goeres and her team are working with the EPA on the development and validation of an efficacy test for hard-surface-biofilm efficacy claims. Some of the CBE’s Industrial Associate companies are, with food safety in mind, developing and testing chemistries specifically for use against biofilm. Some of their challenges lie in creating fast-acting formulas that are both effective against biofilm and not at levels of environmental concern.
 Neil H. Mermelstein,
Neil H. Mermelstein,
IFT Fellow, Editor Emeritus of Food Technology
[email protected]