Dietary Supplements: Nutritional and Legal Considerations
This Scientific Status Summary addresses the role of dietary supplement products in normal health
A PUBLICATION OF THE INSTITUTE OF FOOD TECHNOLOGISTS ’ EXPERT PANEL ON FOOD SAFETY AND NUTRITION
The omnivorous diet of humans can provide the diverse blend of nutrients needed for growth, maintenance, and overall health. For some people, however, food alone may not supply adequate amounts of required nutrients. Nutritional needs change with aging, pregnancy, and lactation and may be altered by acute and chronic diseases or other medical conditions.
During the twentieth century, progress has been made in elucidating the biochemical structures and physiological roles of vitamins and other nutrients. Today, many nutrients can be produced abundantly and inexpensively through laboratory synthesis, microbial fermentation, or other processes. Thus, nutritional imbalances or other special needs for vitamins and minerals, if properly recognized, can be treated with oral dietary supplements or with nutrient solutions administered through other routes if medically warranted.
Once confined mainly to health food stores and pharmacies, dietary supplements now are found in a much broader variety than before in supermarkets and national discount chain stores and are sold though mail order catalogues, television, and the Internet. Although some supplements such as prenatal vitamin/mineral blends require a prescription, most do not and are readily available over-the-counter.
Because dietary supplements are regulated as a special category of foods rather than drugs in the United States, the Institute of Food Technologists is well positioned to address this topic. This Scientific Status Summary specifically addresses dietary supplements—not medical foods nor functional foods—and the role of these products in normal health. The use of dietary supplements for persons diagnosed with cancer, heart disease, HIV, and other chronic illnesses will not be extensively discussed, as these uses would likely fall under drug regulations.
Historical Overview
Until the mid-1990s, the most common dietary supplements were multivitamin/mineral formulations containing varying percentages of the Recommended Dietary Allowances (RDAs) for those nutrients. Publicity surrounding claims made by Nobel-laureate Linus Pauling in the 1970s, that “megadoses”of at least 10 times the RDA of ascorbic acid could prevent or cure the common cold, flu, and cancer (Herbert and Barrett, 1981), may have stimulated public interest in the use of vitamin supplements to enhance health.
Although few of the claims made for supplements in popular books during the 1970s and 1980s were supported by rigorous scientific research, the mainstream scientific community gradually became intrigued by the potential health benefits of dietary supplements. This interest was fueled in part by studies demonstrating that nutrient antioxidants, including vitamins C and E and β-carotene, have a role in protecting cells from oxidative free radical damage. Furthermore, epidemiological studies suggested that a diet rich in fruits and vegetables and abundant in antioxidants, nutrients, and other substances, reduced the risk of coronary heart disease and certain cancers.
By the mid 1990s, “antioxidant” became a household word and antioxidant-fortified supplements and foods appeared on the market. Moreover, as some Americans increasingly became disenchanted with rising health care costs and the perceived impersonal nature of conventional “Western” medicine, there was a dramatic rise in the popularity of various complementary and alternative practices, including Chinese herbal medicine, Ayurvedic medicine (an Indian holistic medical system incorporating foods and herbs), acupuncture, and homeopathy (Angell and Kassirer, 1998; Eisenberg et al., 1998). During the early 1990s, the U.S. Food and Drug Administration (FDA) attempted to increase regulation of herbal products and other dietary supplements (Gilhooley, 1997), creating concern among both consumers and supplement manufacturers.
--- PAGE BREAK ---
Regulatory And Safety Issues
A major change in how supplements are regulated occurred when Congress passed the Dietary Supplement Health and Education Act (DSHEA) of 1994 (Public Law 103-417), which amended the 1958 Food Additive Amendments to the Federal Food Drug and Cosmetic Act. DSHEA defines a dietary supplement as “a product, other than tobacco, intended to supplement the diet that contains at least one or more of the following ingredients: a vitamin, a mineral, an herb or other botanical, an amino acid, or a dietary substance for use to supplement the diet by increasing the total dietary intake; or a concentrate, metabolite, constituent, or extract or combination of any of the previously mentioned ingredients.”
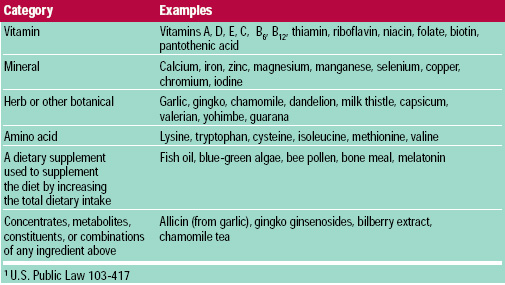 Under the new and broader definition, dietary supplements often contain ingredients not recognized as traditional nutrients. Categories of dietary supplements and examples of materials within each category are listed in Table 1. Prior to DSHEA, the FDA could have challenged the use of non-nutrient ingredients as unapproved food additives.
Under the new and broader definition, dietary supplements often contain ingredients not recognized as traditional nutrients. Categories of dietary supplements and examples of materials within each category are listed in Table 1. Prior to DSHEA, the FDA could have challenged the use of non-nutrient ingredients as unapproved food additives.
DSHEA created the Office of Dietary Supplements (ODS) within the National Institutes of Health to promote and coordinate scientific studies of dietary supplements in relation to health. DSHEA also mandated the formation of the Commission on Dietary Supplement Labels, an independent panel of experts that was appointed to study and make recommendations about regulating and evaluating label claims and other statements for dietary supplements.
Supplements vs. drugs and food additives. How does a dietary supplement differ from a drug, and how does a dietary ingredient in a supplement differ from a food additive in a conventional food? A drug is a product used to “diagnose, cure, mitigate, treat, or prevent diseases,” whereas a dietary supplement is meant to supplement the diet by increasing the total dietary intake of a substance. A food additive is any substance that is either intentionally added (direct additive) to food to improve its shelf-life, texture, nutrition, or other aspect of quality, or that unintentionally contaminates (indirect additive) food, such as packaging materials or machine residues. Dietary ingredients in supplements are exempt from the food additive regulations applicable to conventional foods.
The pre-market approval process for direct food additives and drugs is lengthy and expensive, requiring that manufacturers rigorously test the product’s safety and effectiveness and requiring that this evidence is reviewed by FDA before new products are introduced into the marketplace. Drugs also must be tested for efficacy, which is the desired measure of a product’s effect on a disease condition. Efficacy must be proven through adequate and well-controlled investigations, including human trials, demonstrating that the drug will have the effect claimed on its labeling. Under DSHEA, however, dietary supplement ingredients may be sold without undergoing a formal FDA approval process. Although the supplement manufacturer is not required to provide rigorous scientific evidence of safety or efficacy, the manufacturer should be able to provide information to support any labeling claims.
Safety of ingredients. DSHEA allows manufacturers to use ingredients in dietary supplements that have been present in the food supply or that have been marketed as dietary supplement ingredients prior to 1994. A company wishing to market a new dietary supplement ingredient (i.e., an ingredient not marketed prior to October 15, 1994) must submit to FDA a notification including information showing that the ingredient “will reasonably be expected to be safe.” This means that the substance does not present a “significant or unreasonable risk of illness or injury” to most individuals under the typical conditions of use (FDA, 1997a). This notification must be submitted 75 days prior to the expected marketing date. FDA approval, however, is not required before the product is marketed. Although a manufacturer is responsible for ensuring that its dietary supplement products are safe, FDA bears the burden of showing that a supplement is unsafe or mislabeled before it can restrict or ban the product’s use. Thus, it is FDA’s role to address safety problems that arise in the population from the use of dietary supplements.
--- PAGE BREAK ---
Hazardous product reports in the marketplace. Since passage of DSHEA, FDA has identified several problems and manufacturers have conducted recalls to remove potentially dangerous products from the market. State and local governments may set more stringent laws and regulations for the sale of dietary supplements and can prevent the sale of potentially harmful products within their jurisdictions. For example, in the aftermath of a number of deaths attributed to supplements containing the herb ephedra (Ephedra sinica Stapf, FDA, 1997b), Florida, Texas and Ohio moved to limit sales of ephedra and its extracts.
Since 1993, a system has been in place to report to FDA problems related to dietary supplements, infant formulas, and medical foods. Report sources include FDA’s Medwatch program for healthcare providers (1-800-FDA-1088), FDA field offices (telephone blue pages), health agencies, consumers, and health professionals. The FDA Special Nutritionals Adverse Event Monitoring System (SN/AEMS) maintains a searchable database available on the Internet (http://vm.cfsan.fda.gov/~dms/aems.html) where reports can be searched by several categories: product, ingredient, manufacturer, or adverse event as reported. However, because these reports are volunteered, they may not always be accurate.
Classification and Labeling
Dietary supplements are classified as food products, but DSHEA stipulates that such products must be labeled as “dietary supplements” and be sold in the form of pills, capsules, tablets, gelcaps, liquids, powders, or other forms, and not be represented for use as conventional foods. Supplements also cannot be marketed as the only item in a meal or diet.
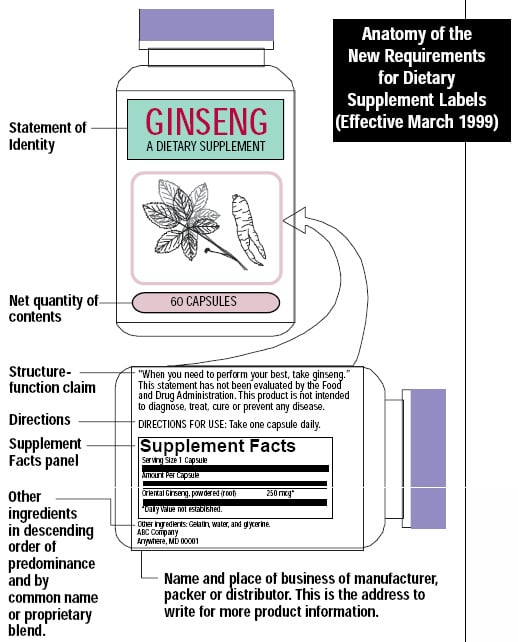 As of March 1999, dietary supplement packages must bear a “Supplement Facts” panel, similar to the “Nutrition Facts” panel mandated for food labels by the Nutrition Labeling and Education Act (NLEA) of 1990. The purpose of this labeling is to provide information about nutrients and other dietary ingredients. Figure 1 shows a typical “supplement facts” panel (Kurtzweil, 1998). The label must list all dietary ingredients and the Daily Values (DV) of the amounts contained in a serving. If no DV has been established for a dietary ingredient, this must be indicated.
As of March 1999, dietary supplement packages must bear a “Supplement Facts” panel, similar to the “Nutrition Facts” panel mandated for food labels by the Nutrition Labeling and Education Act (NLEA) of 1990. The purpose of this labeling is to provide information about nutrients and other dietary ingredients. Figure 1 shows a typical “supplement facts” panel (Kurtzweil, 1998). The label must list all dietary ingredients and the Daily Values (DV) of the amounts contained in a serving. If no DV has been established for a dietary ingredient, this must be indicated.
If a blend of ingredients is proprietary, the total quantity of ingredients per serving must be stated rather than the amount of each individual ingredient in the blend. If an ingredient is an herbal product, the part of the plant (such as the root or leaf) from which the ingredient is derived must be identified. The common name of the botanical as listed in Herbs of Commerce (American Herbal Products Association, Silver Spring, Md.) may be used; if a botanical is not listed in the book, the Latin binomial name (e.g., Echinacea augustifolia DC) must be used. The following information also must appear on the label: statement of identity, which identifies the contents of the product; net quantity of contents; ingredient list (in descending order by weight); and the name and address of the manufacturer, packer, or distributor (FDA, 1997c).
Health claims vs. nutritional support claims. Health claims are specifically defined under NLEA as statements that characterize the relationship between a food substance and a specific disease or health-related condition, and which are based on significant scientific agreement. Health claims on dietary supplement labels must meet the same criteria established under NLEA for conventional food products (Commission on Dietary Supplement Labels, 1997). FDA-authorized health claims include relationships between calcium and osteoporosis, folate and neural tube birth defects, soluble fiber and coronary heart disease, and sugar alcohols and dental caries. Other health claims are approved only for foods but not supplements (Commission on Dietary Supplement Labels, 1997; Storlie et al., 1998).
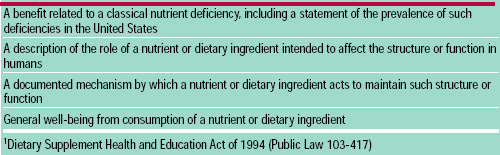 DSHEA permits four types of structure/function claims, formerly referred to as nutritional support statements, to appear on supplement labels (Table 2). Structure/function claims can be distinguished from the more rigorous health claims in that they are not permitted to state or imply a link between a supplement and the treatment, diagnosis, cure or prevention of a disease. DSHEA requires manufacturers of a dietary supplement bearing a structure/function label claim to notify FDA of the claim no later than 30 days after first marketing the product (FDA, 1997d) and to substantiate that the claim is truthful and not misleading. Structure/function claims must be accompanied by the following disclaimer: “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.”
DSHEA permits four types of structure/function claims, formerly referred to as nutritional support statements, to appear on supplement labels (Table 2). Structure/function claims can be distinguished from the more rigorous health claims in that they are not permitted to state or imply a link between a supplement and the treatment, diagnosis, cure or prevention of a disease. DSHEA requires manufacturers of a dietary supplement bearing a structure/function label claim to notify FDA of the claim no later than 30 days after first marketing the product (FDA, 1997d) and to substantiate that the claim is truthful and not misleading. Structure/function claims must be accompanied by the following disclaimer: “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.”
--- PAGE BREAK ---
In November 1998, the Federal Trade Commission (FTC, 1998) issued for the industry an advertising guide on dietary supplements that clarified truthful claims with respect to advertising. The guide states that dietary supplement manufacturers must back up explicit claims and implied benefits made for their products and that: “FDA has primary responsibility for claims on product labeling, including packaging, inserts, and other promotional materials distributed at the point of sale. The FTC has primary responsibility for claims in advertising, including print and broadcast ads, infomercials, catalogs, and similar direct marketing materials. Marketing on the Internet is subject to regulation in the same fashion as promotions through any other media.”
The functions, benefits, and safety of dietary supplements are discussed below, by supplement category.
Vitamins
Functions. Vitamins are essential organic compounds that perform numerous and diverse metabolic functions, often serving as enzymatic cofactors. With some exceptions, vitamins or their precursors must be obtained from food or supplements. The main exceptions are vitamin D, a hormone-like vitamin that can be synthesized in the skin upon exposure to sunlight and other forms of ultraviolet radiation, and therefore is not absolutely required in the diet, and vitamin K which can be synthesized by intestinal microflora. Some vitamins have multiple chemical forms or isomers that vary in biological activity. For example, four forms of tocopherol occur in food, with α-tocopherol having the greatest vitamin E activity. Numerous carotenoids occur in nature and in food, but only a handful have vitamin A activity. Some carotenoids, such as lycopene, do not possess vitamin A activity but, nonetheless, have important health benefits.
The popular literature has emphasized that natural vitamins are superior to synthetic ones, even though the active ingredient is often chemically identical in both forms. However, added folic acid in dietary supplements or fortified foods is better absorbed than naturally occurring folate in foods. Pregnant women administered synthetic folic acid (pteroylglutamic acid) had higher serum folate levels than women given the natural conjugated form of the vitamin (Neuhouser et al., 1998).
Benefits. The mechanisms by which vitamins prevent illnesses are not well understood, and the amounts needed to lower risks for certain disease conditions may be higher than the current recommended levels for preventing nutritional deficiencies. For example, the Institute of Medicine (IOM, 1998a) recommends that to prevent neural tube birth defects, women of child-bearing age should consume 400 μg of folic acid per day (but not more than 1,000 μg/day) from fortified foods and/or dietary supplements in addition to folate obtained from a varied diet. Ascorbic acid intakes of 80-200 mg daily (8 - 20 times the amounts needed to prevent scurvy) may be necessary to enhance certain physiological functions and minimize specific disease risks (Weber et al., 1996).
Certain vitamins act as antioxidants, protecting cells from free radical-induced damage. The minerals selenium and manganese, required constituents of glutathione peroxidase and superoxide dismutase, respectively, also may serve as antioxidants. Products labeled “high in antioxidants” or having similar content claims must contain at least 20% of the Reference Daily Intake (RDI) of ascorbic acid, α-tocopherol, or β-carotene, per reference amount customarily consumed (FDA, 1997e). However, the actual amounts of antioxidants that confer protection are not known and probably vary among individuals, since persons exposed to increased oxidative stress may have elevated antioxidant requirements (Block, 1992; Halliwell, 1997). Moreover, ascorbic acid, α-tocopherol and β-carotene interact and complement each other. Similarly, polyphenolic compounds also protect these vitamins by supporting their antioxidant functions (Jacob, 1995).
Safety. Foods typically contain safe levels of vitamins, but dietary supplements that contain high levels of vitamins could be hazardous. Therefore, in formulating supplements, manufacturers should keep the per serving amounts well below levels known to result in adverse effects. The Institute of Medicine (IOM)/Food and Nutrition Board (FNB) is establishing tolerable upper intake levels (UL) for nutrients (IOM, 1998a, b). The UL is the maximum level of daily nutrient intake that is unlikely to pose adverse health risks to almost all of the individuals in the group for whom it is designed. Although excess water-soluble vitamins are usually excreted in urine, thus minimizing toxicity, adverse symptoms from consuming high doses of water-soluble nutrient supplements have been reported, including diarrhea from vitamin C (Levine et al., 1995) and peripheral neuropathy from vitamin B6 (Snodgrass, 1992).
--- PAGE BREAK ---
The RDA for niacin, a vitamin that is also used as a drug to lower blood cholesterol levels, is 16 mg for adult men (IOM, 1998a), a level that maintains normal metabolism and prevents the deficiency disease pellagra. The UL for niacin is 35 mg/day (IOM, 1998a). The pharmacological dose to reduce serum lipids is close to 3 g of nicotinic acid per day, an amount virtually impossible to obtain from food. Physicians who prescribe nicotinic acid must carefully monitor a patient’s response and adjust the dose accordingly, to minimize unpleasant and potentially hazardous side effects such as skin flushing, pruritis, hyperglycemia, duodenal ulcers, and liver toxicities. 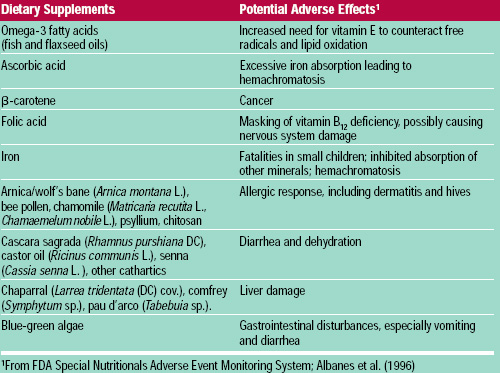
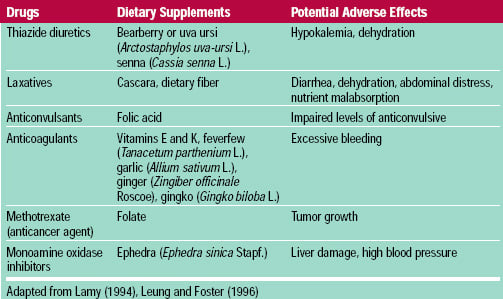 Fat-soluble vitamins are more readily stored in the body than water-soluble ones, and therefore have the potential to be more dangerous. Vitamins E and K are considered relatively non-toxic; acute or chronic over-consumption of vitamins A and D, however, is hazardous. Vitamin A can cause spontaneous abortions and birth defects, especially at levels above 20,000 IU/day (NRC, 1989). A high vitamin D intake can cause hypercalcemia, leading to the deposition of calcium in soft tissues and serious damage to the heart and kidneys. Other potential adverse effects of vitamins are shown in Table 3. Vitamins and other dietary supplements also may interact with prescription medications (Table 4), although relatively little is known about such interactions.
Fat-soluble vitamins are more readily stored in the body than water-soluble ones, and therefore have the potential to be more dangerous. Vitamins E and K are considered relatively non-toxic; acute or chronic over-consumption of vitamins A and D, however, is hazardous. Vitamin A can cause spontaneous abortions and birth defects, especially at levels above 20,000 IU/day (NRC, 1989). A high vitamin D intake can cause hypercalcemia, leading to the deposition of calcium in soft tissues and serious damage to the heart and kidneys. Other potential adverse effects of vitamins are shown in Table 3. Vitamins and other dietary supplements also may interact with prescription medications (Table 4), although relatively little is known about such interactions.
Minerals
Functions. There are at least 21 essential mineral elements, inorganic compounds that are involved in such metabolic processes as muscle contraction, transmission of nerve impulses, maintenance of water and acid-base balance, and catalysis of numerous other biological reactions. Iron is an essential constituent of hemoglobin. Copper, magnesium, and zinc are co-factors for a variety of enzymes. As previously mentioned, manganese and selenium can function as antioxidants and contribute to endothelial integrity.
Calcium and phosphorus are major components of bone. A dietary deficiency of calcium is associated with osteoporosis. For many individuals, calcium supplementation may be a more realistic option for obtaining the adult Adequate Intake (AI) of 1,000-1,200 mg calcium/day than relying upon calcium rich foods. Calcium supplements reduced bone turnover during weight loss (Ricci et al., 1998) and maintained calcium absorption and bone density in winter when sunlight exposure, necessary for vitamin D activation, is diminished (Storm et al., 1998). Although calcium supplementation helps prevent loss of bone density, its effect is weaker than treatment with estrogen, calcitonin, or bisphosphonates, a reflection of the complex etiology of osteoporosis (Riggs et al., 1998).
Trivalent chromium is a cofactor for insulin and thus plays an important role in glucose metabolism, although there are no convincing data suggesting that chromium supplements are useful for preventing or treating diabetes. In recent years, chromium picolinate has been promoted in the popular literature as an aid for losing weight and increasing strength and lean tissue, but there is no convincing evidence to support these claims. Although chromium picolinate supplements in conjunction with an exercise program reduced total cholesterol, LDL-cholesterol, and insulin levels in a group of young adult males compared to exercise alone, the subjects in this study showed no differences in strength or lean body mass (Boyd et al., 1998), and supplementation alone was not evaluated.
Safety. It is unlikely for one to consume toxic levels of minerals just from food, although selenium toxicity has been reported in China and other areas where high concentrations of selenium exist in soil and accumulate in plants. The human intestine has limited capacity to absorb mineral cations, thus the excessive consumption of one mineral can lead to impaired absorption of others. For example, a high iron intake inhibits the absorption of zinc, while an excessive intake of zinc hinders copper absorption.
--- PAGE BREAK ---
Iron deficiency anemia remains a global public health problem typically associated with poverty and is more common in women and children. However, iron also acts as a pro-oxidant inducing free radical damage, and an excessive iron intake may promote lipid oxidation, damaging mitochondria and DNA. Furthermore, a substantial proportion of the population has genetic traits for hemachromatosis and tends to accumulate toxic levels of iron in the liver (Bothwell, 1995; Lynch, 1995). Cellbased theories on how iron overload promotes hepatic, cardiac, and pancreatic disease have been reviewed by Britton et al. (1994). Opposition to widespread iron fortification of foods to prevent anemia in women and children may reduce the number of iron fortified products available, creating a need for these sensitive populations to rely on supplements. Men and post-menopausal women have lower iron needs and thus do not require iron supplementation in most cases. FDA requires child-resistant closures on prenatal iron supplements to prevent accidental poisoning of young children, for whom the lethal dose is about 3 g (NRC, 1989).
Commercial calcium supplements, notably dolomite and bonemeal, may be contaminated with lead at levels exceeding FDA tolerable daily intake limits (Bourgoin et al., 1993). However, mineral, as well as most vitamin supplements are generally manufactured according to United States Pharmacopeia (USP) guidelines prohibiting such contamination. The USP is a nongovernmental, voluntary organization that establishes guidelines for vitamin and mineral supplement strength, purity, and quality.
Amino Acids
Functions. A balanced diet provides the nine essential amino acids that humans cannot synthesize; hence, protein deficiency is rare in the United States. Although solutions of free amino acids may be appropriate for total parenteral nutrition of critically ill patients, there is no evidence that amino acid supplements are beneficial for healthy persons.
Safety. Unlike vitamins and minerals, there are no Dietary Reference Intakes (DRIs), dietary reference values for the intake of nutrients and food components by Americans and Canadians, for amino acids. Excess amino acids must be deaminated before being used as an energy source, and high intakes may be injurious to persons with impaired kidney function. The excessive consumption of protein or amino acids may lower calcium stores by increasing the excretion of urinary calcium, which is needed to buffer the excreted protein waste. Although there is no clear association between high protein intake and the risk for osteoporosis, susceptible individuals should refrain from consuming a high protein diet or taking amino acid supplements (NRC, 1989).
Supplements of L-tryptophan, a precursor of the brain neurotransmitter serotonin, became popular as a natural sleep aid in the late 1980s. However, a large 1989 epidemic of eosinophilia-myalgia syndrome (EMS), a rare and sometimes fatal systemic illness characterized by elevations of certain white blood cells and severe muscle pain, was associated with the use of tryptophan supplements. Therefore, FDA requested that manufacturers recall supplements containing synthetic tryptophan (Glickstein et al., 1990; Kamb et al., 1992). It is not clear if the adverse effects were due to the amino acid, a contaminant, or to their interaction. A contaminating dimer of tryptophan was found in supplements produced by one manufacturer that supplied most of the tryptophan used in the United States, so most of the EMS cases were related to this source. However, this contaminant may not be the sole cause of EMS (Roufs, 1992). Furthermore, cases of EMS possibly related to mixtures of amino acids have been reported since the original outbreak, which involved supplements containing only L-tryptophan. The finding in 1998 of impurities in some dietary supplement products containing 5-hydroxy-L-tryptophan, which is synthesized from L-tryptophan in the body, suggests that continued caution may be warranted with the use of tryptophan supplements (FDA, 1998).
Botanicals
Functions. This category of dietary supplements is controversial because many botanical supplement ingredients are not derived from plants normally used for food, and safety and efficacy often have not been established with randomized, placebo-controlled clinical experiments. Plant products and their extracts are sources of traditional nutrients as well as biologically-active non-nutrient phytochemicals. For example, the large family of phenolic compounds, which includes anthocyanins and other flavonoids, function as antioxidants and thus may protect cells from free radical damage.
--- PAGE BREAK ---
The concentration and distribution of phytochemicals in medicinal vascular plants varies in the roots, stems, leaves, flowers, and fruits, and also fluctuates during the growth cycle or season. Moreover, plants or related species similar in appearance may have vastly different chemical compositions. For example, the composition of ginseng grown in Korea (Panax ginseng C.A. Mey) is different from that of the native American plant (Panax quinquefolius L.). This may create difficulties in formulating botanical supplements with consistent ingredients and potencies and presents challenges for inexperienced plant collectors.
Conventional medical journals have begun to publish more controlled clinical studies using botanicals. A review of 18 randomized controlled trials concluded that saw palmetto (Serenoa repens [Bartr.] Small) extracts improve male urological symptoms with less likelihood of adverse events compared with the prescription drug finasteride, but the authors cautioned that long-term effectiveness against benign prostatic hyperplasia had not been documented (Wilt et al., 1998). Although Chinese herbal medicine improved the symptoms of patients with irritable bowel syndrome compared with those given placebos, only those patients who had received individualized herb therapy maintained improvement 14 weeks after cessation of therapy (Bensoussan et al., 1998). A special article, reviewing 12 popular herbs, was published in the Archives of Family Medicine to increase physician awareness of the history, safety and efficacy of these products (O’Hara et al., 1998).
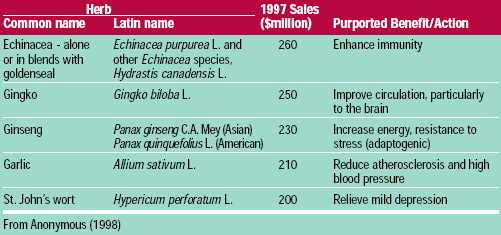 The five most popular botanicals in the United States are listed in Table 5. These materials are sold singly and in combination with other dietary supplement ingredients. These herbs are also added to some products such as juices and water. Tyler (1993) considers these particular botanicals to be safe and effective in healthy individuals.
The five most popular botanicals in the United States are listed in Table 5. These materials are sold singly and in combination with other dietary supplement ingredients. These herbs are also added to some products such as juices and water. Tyler (1993) considers these particular botanicals to be safe and effective in healthy individuals.
Safety. Many botanical supplements are consumed with no adverse effects, yet some products with known hazards are present in the marketplace (Tables 3 and 4). The most serious problems have resulted from ingesting the herb ephedra (Ephedra sp.), also known as ma huang. Ephedrine and related alkaloids in this product are potent central nervous system stimulants. Dietary supplements containing ephedra are promoted for increasing energy and aiding in weight loss, but may be accompanied by such adverse effects as anxiety, heart attack, stroke, and seizures, sometimes resulting in death. A number of deaths caused by cardiac arrest have been attributed to ephedra. FDA has proposed to limit the ephedrine alkaloid content in supplements to 8 mg per serving, with a limit of 3 servings/day, and to prohibit the incorporation of other stimulants such as caffeine or yohimbine (an alkaloid in Pausinystalia yohimba Pierre) that can augment the effects of alkaloids (FDA, 1997b).
Herbal teas are popular among consumers trying to avoid caffeine, but herbal tea supplements may contain guarana (Paullinia cupana H.B.K.), maté (Ilex paraguariensis St. Hil.), or cola nut (Cola nitida Schott & Endl.), ingredients that are rich sources of caffeine. Since caffeine disclosure is not required for dietary supplements, consumers who take certain herbal supplements may inadvertantly be exposed to undesirable or high levels of caffeine. Commercial herbal tea products may also contain potentially dangerous compounds such as hepatotoxic pyrrolizidine alkaloids and various tannins, allergens, and psychoactive compounds (Manteiga et al., 1997).
Other Dietary Supplement Products
Hormones are not typically considered to be part of the diet, yet hormonal products represent an important segment of the dietary supplement market. Hormone supplements are promoted for maintaining or restoring physiological levels of hormones that decline with aging. Some preparations contain meat animal by-products such as ovaries, uteri, and testes. Plant-derived ingredients include phytoestrogens and other compounds that mimic mammalian hormones. Melatonin supplements, that contain the synthetic form of a hormone produced by the brain’s pineal gland, have been promoted to aid sleep, counter jet lag, and slow aging, but there are concerns about potential side effects (Grafias, 1996).
Dehydroepiandrosterone (DHEA) is an anabolic steroid that is promoted for enhancing physical strength and immunity, which diminish during aging. The International Olympic Committee considers DHEA an illegal steroid and has banned its use. A recent analysis of sixteen commercial DHEA products revealed that only half the products contained the actual amount of DHEA stated on the product label, with actual levels varying between 0-150% of the label content (Parasrampuria et al., 1998). A variety of adverse side effects has been associated with DHEA and reported to the FDA SN/AEMS. Another steroidal hormone, androstenedione, became popular in 1998 amid publicity that it was used by baseball slugger Mark McGwire (Schrof, 1998). However, a recent study indicated that oral supplements of androstenedione did not enhance muscle strength in men and was associated with adverse effects including a reduction of serum HDL-cholesterol and an elevation of serum estradiol levels (King et al., 1999).
--- PAGE BREAK ---
Oral creatine supplementation is popular among athletes, but many studies have failed to show an ergogenic benefit. Furthermore, numerous side effects including renal abnormalities have been reported for creatine (Pritchard and Kaira, 1998), and there is growing concern about potential longterm adverse effects on various organ systems (Juhn, 1999).
Recently, FDA issued warnings for products chemically related to ɣ-butyrolactone (GBL) and ɣ-hydroxybutyric acid (GHB), which are marketed as sleep aids. GBL and GHB have been associated with at least three deaths and more than 100 adverse reactions, including coma and breathing difficulty (FDA, 1999). Some of the products contain 1,4 butanediol, which is converted to GHB when ingested.
Occurrence of Nutritional Deficiencies in the United States
Classical nutritional deficiency diseases, caused by inadequate intakes of specific vitamins and minerals, are rare in the United States today. Scurvy, caused by a vitamin C deficiency, now occurs mainly in infants who are exclusively fed cow’s milk or among elderly individuals habitually consuming severely restricted diets. Beriberi, caused by a thiamin deficiency, is seen mostly in alcoholics who have an increased requirement and often an inadequate intake of thiamin. Rickets declined after milk began to be routinely fortified with vitamin D about sixty years ago, replacing cod liver oil as the chief source of this vitamin for children. Although seldom seen today, rickets may occur if the conversion of vitamin D to its biologically active form is impaired due to kidney failure or other diseases. Similarly, pellagra, which was a widespread problem in the southern United States during the early part of the twentieth century, has virtually disappeared as both niacin and tryptophan—which can be converted to niacin—are prevalent in the American diet.
Public health measures such as the fortification of breads and cereals with iron and some B vitamins, the widespread use of iodized salt, and the fortification of milk with vitamin D have markedly reduced the incidence of deficiency diseases that were once common. Furthermore, the year-round availability of fruits and vegetables, and the diversity and affordability of the food supply in general, have helped eliminate nutrient deficiencies. Today, nutrition-related diseases in the United States are typically chronic in nature and are more likely associated with an excessive intake of fats, sweets, and calories from all sources, rather than from dietary inadequacies.
Certain segments of the population, however, are susceptible to nutritional deficiencies because of elevated requirements arising from medical or environmental conditions or inborn errors of metabolism (NRC, 1989). For example, a thiamin deficiency may exist in renal patients undergoing chronic dialysis or in patients on long term intravenous feeding. Smokers may require more vitamin C than nonsmokers to counteract the increased metabolic turnover of the vitamin associated with smoking. A vitamin E deficiency is sometimes seen in premature, low birth weight babies, or in individuals with abnormal fat absorption. Persons with other malabsorption conditions such as atrophic gastritis, or those suffering from cancer or other serious illnesses, also may have increased requirements for certain nutrients. A dietary deficiency of vitamin B12 is rare, even among vegans, and more than 95% of vitamin B12 deficiencies in the United States are due to inadequate absorption of this nutrient.
It is more common for Americans to report consuming dietary intakes that fall below the RDA for certain nutrients than to exhibit overt deficiency symptoms. According to the U.S. Department of Agriculture’s (USDA) 1994-96 Continuing Survey of Food Intakes by Individuals (CSFII), the nutrients most likely to be consumed below RDA levels were iron, calcium, zinc, and magnesium, and women were more likely than men to fall short of the recommendations. For example, females 12-19 years of age had the lowest calcium intake of any age/sex group, meeting only 64% of the RDA for calcium, while women greater than 20 years of age consumed 77% of the RDA. On the other hand, men 20-69 years of age consumed at least 99% of the RDA for calcium.
--- PAGE BREAK ---
Failure to meet the RDA intakes does not necessarily mean that an individual is not meeting nutrient requirements, since RDAs represent population averages with margins of safety that account for presumed variability in requirements among individuals. Also, dietary intake data do not correlate precisely with disease risk. For example, although about 10 million Americans have osteoporosis, it is not possible to attribute the disease solely to a lack of dietary calcium, since genetic predisposition, gender, estrogen, vitamin D intake, and lifestyle factors such as amount of physical activity and exposure to sunlight also are risk factors (NIH, 1998).
When are nutrient supplements warranted for the general population? Many health professionals advise the routine use of calcium and/or vitamin D supplements to help prevent osteoporosis, especially for high-risk or elderly individuals. Iron is routinely given during pregnancy, and folic acid is recommended for all women of child-bearing age. Recent studies suggest that dietary supplements of folic acid, vitamin B6, and vitamin B12 may have broad public health benefits, as these nutrients are associated with lowering blood homocysteine levels, which may reduce the risk of coronary heart disease (Welch and Loscalzo, 1998). There is concern among some professionals that the American population is consuming an imbalance of polyunsaturated fatty acids, with the ratio of omega-6 to omega-3 fatty acids being larger than optimal. Since few commonly consumed foods are good sources of omega-3 fatty acids, supplements may be more widely recommended in the future.
Using relative risk estimates, Bendich et. al. (1997) estimated that almost $20 billion in hospital expenses might be avoided if women of child-bearing age took folic acid and multivitamins with zinc daily, and if older adults consumed vitamin E supplements daily. The Council for Responsible Nutrition (Washington, D.C.), a dietary supplement trade association, recently summarized the benefits of supplement use for protecting against diseases or conditions such as osteoporosis, heart disease, some birth defects, infectious disease in the elderly, cataracts and macular degeneration, and some cancers and reducing health care costs (Dickinson, 1998).
Supplement Use
Data from nutritional surveys. National nutrition surveys have focused on the use of traditional vitamin and mineral supplements. About 25% of 11,643 adults who took part in the 1992 National Health Interview Survey took supplements every day (Slesinski et al., 1996). Individuals taking vitamins had significantly lower fat diets, with more fiber and vitamins (calcium for women), suggesting that supplement users have generally healthy lifestyles. Users of dietary supplements tend to be female, college educated, and have incomes over $50,000 (Eisenberg et al., 1998). In one survey, elderly Georgia residents who used dietary supplements were more likely to be physically active, and to have more arthritis or stomach problems than their non-user neighbors (Houston et al., 1997). Calcium, vitamin E, and vitamin C were the most popular supplements used within this group, and the researchers concluded that supplement use was part of a cluster of health behaviors practiced by this group.
Of more than 2,000 Americans questioned in a recent telephone survey of alternative medical practices, 12.1% reported using herbal products and 5.5% reported using megavitamins (Eisenberg et al.,1998). Herbs were identified as one of the most commonly used treatments for allergies, insomnia, and digestive disorders; vitamins were used for high blood pressure, but other uses were not explored in the study. These products were not fully reimbursable by insurance companies. Concurrent use of herbal products and/or high doses of vitamins was reported by almost 1/5 of the 44% of persons taking prescription medications.
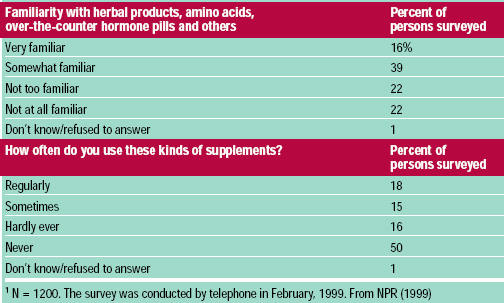 In February 1999, National Public Radio, Kaiser Family Foundation, and Harvard University Kennedy School of Government conducted a telephone survey of 1200 American adults, of whom 412 reported using supplements regularly or sometimes. Although 55% of the individuals surveyed were very or some what familiar with supplements other than vitamins or minerals, only 33% reported using these products regularly or sometimes (Table 6). Supplement users were more likely to believe that taking supplements is beneficial and that these products are safe. Regular supplement users were not different from non-users in their attitudes about government regulation of dietary supplements.
In February 1999, National Public Radio, Kaiser Family Foundation, and Harvard University Kennedy School of Government conducted a telephone survey of 1200 American adults, of whom 412 reported using supplements regularly or sometimes. Although 55% of the individuals surveyed were very or some what familiar with supplements other than vitamins or minerals, only 33% reported using these products regularly or sometimes (Table 6). Supplement users were more likely to believe that taking supplements is beneficial and that these products are safe. Regular supplement users were not different from non-users in their attitudes about government regulation of dietary supplements.
--- PAGE BREAK ---
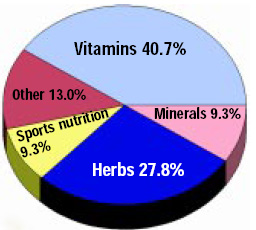 Dietary supplement industry sales data. Consumers spent an estimated $6.5 billion in 1996, double the expenditures in 1990-91 (Kurtzweil, 1998). The U.S. dietary supplement market was $12.8 billion in 1997, with sales projected to continue to increase, especially through Internet marketing (Anonymous, 1998). Vitamins still dominate the U.S. dietary supplement market, accounting for nearly half the sales in 1997 (Figure 2), followed by herbs and botanicals. These sales figures do not include over-the-counter medications that provide calcium or other minerals.
Dietary supplement industry sales data. Consumers spent an estimated $6.5 billion in 1996, double the expenditures in 1990-91 (Kurtzweil, 1998). The U.S. dietary supplement market was $12.8 billion in 1997, with sales projected to continue to increase, especially through Internet marketing (Anonymous, 1998). Vitamins still dominate the U.S. dietary supplement market, accounting for nearly half the sales in 1997 (Figure 2), followed by herbs and botanicals. These sales figures do not include over-the-counter medications that provide calcium or other minerals.
Conclusion
Although nearly half of the adults in the United States regularly consume dietary supplements, relatively little data exist on the physiological effects of many available products. Future nutrition surveys must carefully document the extent of supplement use, which will be a challenge given the large number of products now available. Because the nature of dietary supplements available in the marketplace is diverse, it is difficult to provide general recommendations regarding use. Fortunately, the growing popularity of dietary supplements should spur additional, much-needed research on optimal dosages, interactions with drugs and food components, and the relationships between supplements on health maintenance and chronic disease prevention in persons of different genders and ages.
The creation of the International Bibliographic Information on Dietary Supplements (IBIDS) database (http://odp.od.nih.gov/ods/databases/ibids.html), a joint project of the ODS and the USDA Food and Nutrition Information Center, early in 1999 should provide scientists and the public with easier access to publications on supplements. A forthcoming report on the value of vitamin and mineral supplements for improving the nutritional status of low income Americans is expected to be issued late in 1999 by the USDA and the Life Sciences Research Office of the American Society for Nutritional Sciences (Bethesda, Md.).
The passage of DSHEA has created an economic and regulatory environment favorable to the expanded marketing, sales, and distribution of dietary supplements. Opportunities for consumers to purchase dietary supplements in a free market economy are vastly increased, but expectations remain that government agencies provide protection from unsafe or mislabeled products. One of the future challenges with respect to dietary supplements will be to reconcile these apparently opposing forces. Furthermore, better education of consumers would aid their critical evaluation of advertising and label claims and enable them to reach informed decisions regarding which, if any, dietary supplements to take.
INSTITUTE OF FOOD TECHNOLOGISTS
The Society for Food Science and Technology
221 N. LaSalle St., Ste. 300, Chicago, IL 60601-1291 USA
Tel. 312-782-8424 • Fax: 312-782-8348
E-mail: [email protected] • URL: http://www.ift.org
This and other Scientific Status Summaries are published by the Institute of Food Technologists’ Expert Panel on Food Safety and Nutrition in Food Technology. Scientific Status Summaries, which are not necessarily written by the Expert Panel, are rigorously peer-reviewed by the Expert Panel as well as by individuals outside the panel who have specific expertise in the subject. IFT’s Expert Panel on Food Safety and Nutrition, which studies significant food-related issues and oversees timely production of Scientific Status Summaries, comprises academicians representing expertise in one or more areas of food science/technology and nutrition.
The Scientific Status Summaries may be reprinted or photocopied without permission, provided that suitable credit is given.
MARY ELLEN CAMIRE AND MARK A. KANTOR
Author Camire is Associate Professor, Dept. of Food Science and Human Nutrition, University of Maine, 5736 Holmes Hall, Orono, ME 04469-5736; author Kantor is Associate Professor and Extension Specialist, Dept. of Nutrition and Food Science, University of Maryland, 3304 Marie Mount Hall, College Park, MD 20742-7521
References
Albanes, D., Heinonen, O.P., Taylor, P.R., Virtamo, J., Edwards, B.K., Rautalahti, M., Hartman, A.M., Palmgren, J., Freedman, L.S., Haapakoski, J., Barrett, M.J., Pietinen, P., Malila, N., Tala, E., Liippo, K., Salomaa, E.R., Tangrea, J.A., Teppo, L., Askin, F.B., Taskinen, E., Erozan, Y., Greenwald, P., and Huttunen, J.K. 1996. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: Effects of base-line characteristics and study compliance. J. Natl. Cancer Inst. 88: 1560-1570.
Angell, M. and Kassirer, J.P. 1998. Alternative medicine—the risks of untested and unregulated remedies (editorial). New Engl. J. Med. 339(12): 839-841.
Anonymous. 1998. Nutrition industry braces for a competitive future. Nutr. Business J. 3: 1-5, 13, 18.
Bendich, A., Mallick, R., and Leader, S. 1997. Potential health economic benefits of vitamin supplementation. West. J. Med. 166: 306-312.
Bensoussan, A., Talley, N.J., Hing, M., Menzies, R., Guo, A., and Ngu, M. 1998. Treatment of irritable bowel syndrome with Chinese herbal medicine. J. Am. Med. Assn. 280: 1585-1589.
Block, G. 1992. The data support a role for antioxidants in reducing cancer risk. Nutr. Rev. 50(7): 207-213.
Bourgoin, B.P., Evans, D.R., Cornett, J.R., Lingard, S.M., and Quattrone, A.J. 1993. Lead content in 70 brands of dietary calcium supplements. Am. J. Public Health 83: 1155-1160.
Bothwell, T.H. 1995. Overview and mechanisms of iron regulation. Nutr. Rev. 53: 237-245.
Boyd, S.G., Boone, B.E., Smith, A.R., Conners, J., and Dohm, G.L. 1998. Combined dietary chromium picolinate supplementation and an exercise program leads to a reduction of serum cholesterol and insulin in college-aged subjects. J. Nutr. Biochem. 9: 471-475.
Britton, R.S., Ramm, G.A., Olynyk, J., Singh, R., O’Neill, R., and Bacon, B.R. 1994. Pathophysiology of iron toxicity. Adv. Exp. Med. Biol. 356: 239-253.
Commission on Dietary Supplement Labels. 1997. Report to the President, Congress, and the Secretary of the Department of Health and Human Services. Commission on Dietary Supplement Labels/Office of Disease Prevention and Health Promotion, U.S. Govt. Printing Office, Washington D.C.
Dickinson, A. 1998. Optimal Nutrition for Good Health: The Benefits of Nutritional Supplements. Council for Responsible Nutrition, Washington, D.C.
Eisenberg, D.M., Davis, R.B., Ettner, S.L., Appel, S., Wilkey, S., Van Rmpay, M., and Kessler, R.C. 1998. Trends in alternative medicine use in the United States, 1990-1997. J. Am. Med. Assn. 280: 1569-1575.
FDA. 1999. FDA warns about GBL-related products. FDA Talk Paper. T99-21. May 11. http://www.fda.gov/bbs/topics/ANSWERS/ANS00953.html.
FDA. 1998. Impurities confirmed in dietary supplement 5-hydroxy-L-tryptophan. FDA Talk Paper. T98-48. Aug. 31. http://www.fda.gov/bbs/topics/ANSWERS/ANS00891.html.
FDA. 1997a. Premarket notification for a new dietary ingredient, final rule. Food and Drug Administration. Fed. Reg. 62: 49886-49892.
FDA. 1997b. Dietary supplements containing ephedrine alkaloids; proposed rule. Food and Drug Administration. Fed. Reg. 62: 30678-30724.
FDA. 1997c. Statement of identity, nutrition labeling and ingredient labeling of dietary supplements, Final rule. Food and Drug Administration. Fed. Reg. 62: 49826-49858.
FDA. 1997d. Requirements for nutrient content claims, health claims, and statements of nutritional support, Final rule. Food and Drug Administration. Fed. Reg. 62: 49859-49868.
FDA. 1997e. Food labeling; nutrient content claims: Definition for “high potency” and definition of “antioxidant” for use in nutrient content claims for dietary supplements and conventional foods, final rule. Food and Drug Administration. Fed. Reg. 62: 49868-49881.
FTC. 1998. Dietary supplements: An advertising guide for industry. Federal Trade Commission, Washington, D.C.
Gilhooley, M. 1997. Herbal remedies and dietary supplements: The boundaries of drug claims and freedom of choice. Fl. Law Rev. 49: 665-722.
Glickstein, S.L., Gertner, E., Smith, S.A., Roelfos, R.I., Hathaway, D.E., Schlesinger, P.A., Schned, E.S. 1990. Eosinophilia-myalgia syndrome associated with L-tryptophan use. J. Rheumatol. 17: 1534-1543.
Grafias S. 1996. Melatonin: A trusty travel companion? Physician and Sportsmedicine. 24: 19-20.
Halliwell, B. 1997. Antioxidants and human disease: A general introduction. Nutr. Rev. 55 (I, Part II) S44-S51.
Herbert, V. and Barrett, S. 1981. Vitamins and “Health” Foods: The Great American Hustle, 189 pp. George F. Stickley Co., Philadelphia, Pa.
Houston, D.K., Johnson, M.A., Daniel, T.D., and Poon, L.W. 1997. Health and dietary characteristics of supplement users in an elderly population. Intl. J. Vit. Nutr. Res. 67: 183-191.
IOM. 1998a. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Food and Nutrition Board/Institute of Medicine, 425 pp. National Academy Press, Washington, D.C.
IOM. 1998b. Dietary Reference Intakes: A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients. Subcommittee on Upper Reference Levels of Nutrients, Food and Nutrition Board/Institute of Medicine, 71 pp. National Academy Press, Washington, D.C.
Jacob, R.A. 1995. The integrated antioxidant system. Nutr. Res. 15: 755-766.
Juhn, M.S. 1999. Oral creatine supplementation. Separating fact from hype. Physician and Sportsmedicine. 27(5): 47-61, 89.
Kamb, M.L., Murphy, J.J., Jones, J.L., Caston, J.C., Nederlof, K., Horney, L.F., Swygert, L.A., Falk, H., and Kilbourne, E.M. 1992. Eosinophilia-myalgia syndrome on L-tryptophan-exposed patients. J. Am. Med. Assn. 267: 77-82.
King, D.S., Sharp, R.L., Vukovich, M.D., Brown, G.A., Reifenrath, T.A., Uhl, N.L., Parsons, K.A. 1999. Effect of oral androstenedione on serum testosterone and adaptations to resistance training in young men. J. Am. Med. Assn. 281: 2020-2028.
Kurtzweil, P. 1998. An FDA guide to dietary supplements. FDA Consumer 32(5): 28-35.
Lamy, P.P. 1994. Drug-nutrient interactions in the aged. In “Handbook of Nutrition in the Aged,” ed. R.R. Watson., pp. 165-200. CRC Press, Boca Raton, Fla.
Leung, A.Y., and Foster, S. 1996. Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics. 649 pp. John Wiley & Sons, New York.
Levine, M., Dhariwal, K.P., Welch, R.W., Wang, Y., and Park, J.B. 1995. Determination of optimal vitamin C requirements in humans. Am. J. Clin. Nutr. 62 (suppl.): 1347S-1356S.
Lynch, S.R. 1995. Iron overload: Prevalence and impact on health. Nutr. Rev. 53: 255-260.
Manteiga, R., Park, D.L., and Ali, S.S. 1997. Risks associated with consumption of herbal teas. Rev. Environ. Contam. Toxicol. 150: 1-30.
National Institutes of Health/Osteoporosis and Related Bone Diseases-National Resource Center. 1998. Osteoporosis overview.
NPR. 1999. National Public Radio/Kaiser Family Foundation/Kennedy School of Government Survey on Americans and Dietary Supplements. http://www.npr.org/programs/specials/survey/front.html.
NRC. 1989. Recommended Dietary Allowances, 10th ed. Food and Nutrition Board/National Research Council, 285 pp. National Academy Press, Washington, D.C.
Neuhouser, M.L., Beresford, S.A., Hickok, D.E., and Monsen, E.R. 1998. Absorption of dietary and supplemental folate in women with prior pregnancies with neural tube defects and controls. J. Am. Coll. Nutr. 17: 625-630.
O’Hara, M., Kiefer, D., Farrell, K., and Kemper, K. 1998. A review of 12 commonly used medicinal herbs. Arch. Family Med. 7: 523-536.
Parasrampuria, J., Schwartz, K., and Petesch, R. 1998. Quality control of dehydroepiandrosterone dietary supplement products. J. Am. Med. Assn. 280: 1565.
Pritchard, N.R., and Kaira, P.A. 1998. Renal dysfunction accompanying oral creatine supplements. Lancet 351: 1252-1253.
Ricci, T.A., Chowdhury, H.A., Heymsfield, S.B., Stahl, T., Pierson, R.N., and Shapses, S.A. 1998. Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J. Bone Miner. Res. 13: 1045-1050.
Riggs, B.L., O’Fallon, W.M., Muhs, J., O’Connor, M.K., Kumar, R., and Melton, L.J. 1998. Long-term effects of calcium supplementation on serum parathyroid hormone level, bone turnover, and bone loss in elderly women. J. Bone Miner. Res. 13: 168-174.
Roufs, J.B. 1992. Review of L-tryptophan and eosinophilia-myalgia syndrome. J. Am. Diet. Assn. 92: 844-850.
Schrof, J.M. 1998. McGwire hits the pills. U.S. News & World Report. Sept. 7, pp. 53-54.
Slesinski, M.J., Subar, A.F., and Kahle, L.L. 1996. Dietary intake of fat, fiber and other nutrients is related to the use of vitamin and mineral supplements in the United States: The 1992 National Health Interview Survey. J. Nutr. 126: 3001-3008.
Snodgrass, S.R. 1992. Vitamin neurotoxicity. Molec. Neurobiol. 6(1): 41-73.
Storlie, J., O’Flaherty, M.J., and Hare, K. 1998. Food or supplement? Choosing the appropriate regulatory path. Food Technol. 52(12): 62-69.
Storm, D., Eslin, R., Porter, E.S., Musgrave, K., Vereault, D., Patton, C., Kessenich, C., Mohan, S., Chen, T., Holick, M.F., and Rosen, C.J. 1998. Calcium supplementation prevents seasonal bone loss and changes in biochemical markers of bone turnover in elderly New England women: A randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 83: 3817-3825.
Tyler, V.E. 1993. The Honest Herbal. 375 pp. Pharmaceutical Products Press, New York.
Weber, P., Bendich, A., and Schalch, W. 1996. Vitamin C and human health—A review of recent data relevant to human requirements. Intl. J. Vit. Nutr. Res. 66: 19-30.
Welch, G.N. and Loscalzo, J. 1998. Homocysteine and atherothrombosis. New Engl. J. Med. 338: 1042-1050.
Wilt, T.J., Ishani, A., Stark, G., MacDonald, R., Lau, J., and Mulrow, C. 1998. Saw palmetto extracts for treatment of benign prostatic hyperplasia. A systemic review. J. Am. Med. Assn. 280: 1604-1609.
