GRAS Flavoring Substances 19
The 19th publication by the Flavor and Extract Manufacturers’ Association’s Expert Panel on recent progress in the consideration of flavoring ingredients generally recognized as safe under the Food Additives Amendment
As the FEMA Expert Panel enters its fifth decade, it continues to conduct a comprehensive program of safety evaluation of flavoring substances under the authority of Section 201(s) of the Federal Food, Drug, and Cosmetic Act, a provision commonly known as the GRAS (“generally recognized as safe”) provision.
Expert Panel activities primarily involve the evaluation of new flavoring substances and, at regular intervals, the reevaluation of all existing GRAS flavoring substances. The first comprehensive reassessment of GRAS flavoring substances (the GRAS affirmation program, GRASa) was completed in 1985. A second reassessment of the safety of all existing GRAS substances began in 1994 and is scheduled for completion during 2005.
The Panel recognizes that advances in flavor technology will influence the types of substances submitted for GRAS evaluation. The Panel is committed to the comprehensive GRAS evaluations of contemporary products emerging from these new technologies. Flavors are typically mixtures of substances, most of which impart flavor (e.g., menthol and cinnamaldehyde), while others perform non-flavor functions such as preservatives (BHA), solvents (ethyl alcohol), modifiers (neohespiridin dihydrochalcone), and emulsifiers (Table 1). Substances that do not impart flavor may serve several functions and have significant uses in the food supply. In these instances, the Panel evaluates the substance for GRAS status based only on its intended use as a component of a food flavor. This criterion is entirely consistent with Section 201(s), which states that for a substance to be considered GRAS it must be “safe under conditions of intended use.” It is also consistent with past Panel decisions in which substances having non-flavor functions (solvents, modifiers, antioxidants, etc.) have been recognized as GRAS for their intended use in a compounded flavor (Table 1).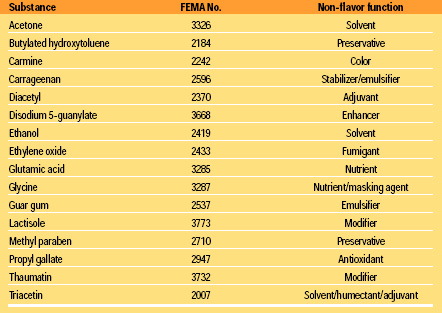
Historically, the vast majority of flavoring substances either occur naturally in food or are chemically close structural relatives of naturally occurring substances. Of the remaining xenobiotic substances, a few either possess structural features that suggest significant toxicity or are expected to be metabolized to substances of significant toxicity. Over the past few years, GRAS applications have been made for a number of chemically designed flavoring substances that contain such structural alerts. Inevitably, extensive and costly studies are required to complete the safety evaluations for these substances. The Panel emphasizes that the manufacturer should seriously consider those factors that may affect the safety assessment during the early stages of design and development of a flavoring substance. Those concerned with the design of novel chemical flavors are urged to avoid, as far as possible, structural features that are associated with potential toxicity. The fact that flavors themselves provide no direct health benefit requires that there be clear evidence of the safety of flavoring substances under conditions of use.
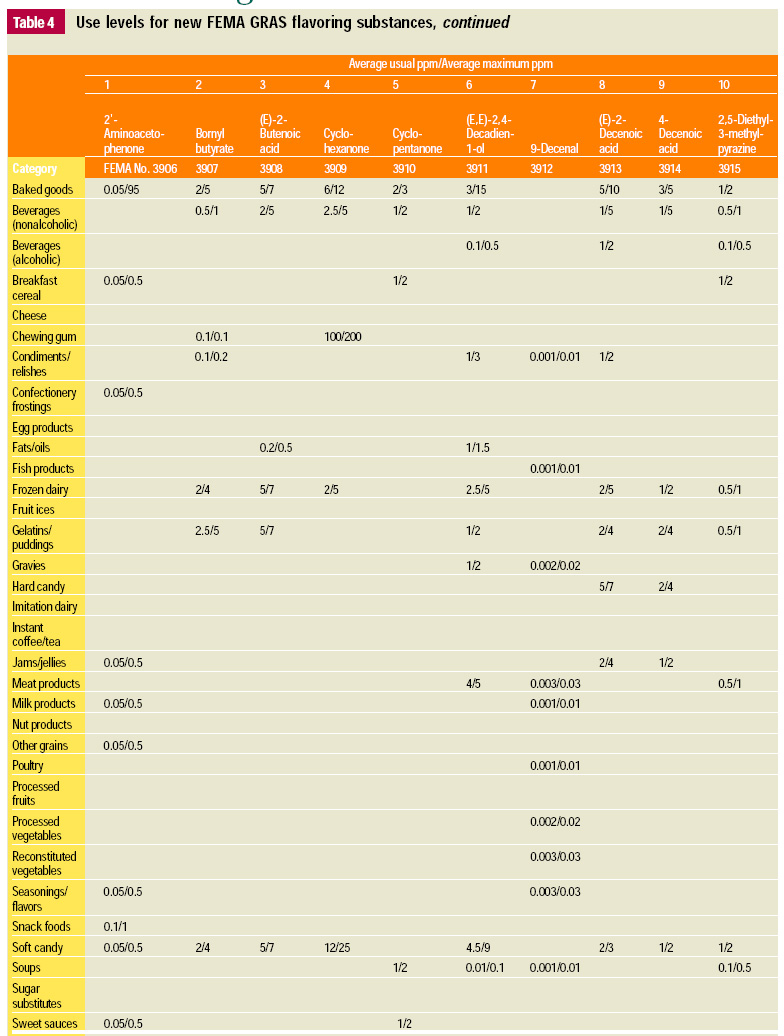
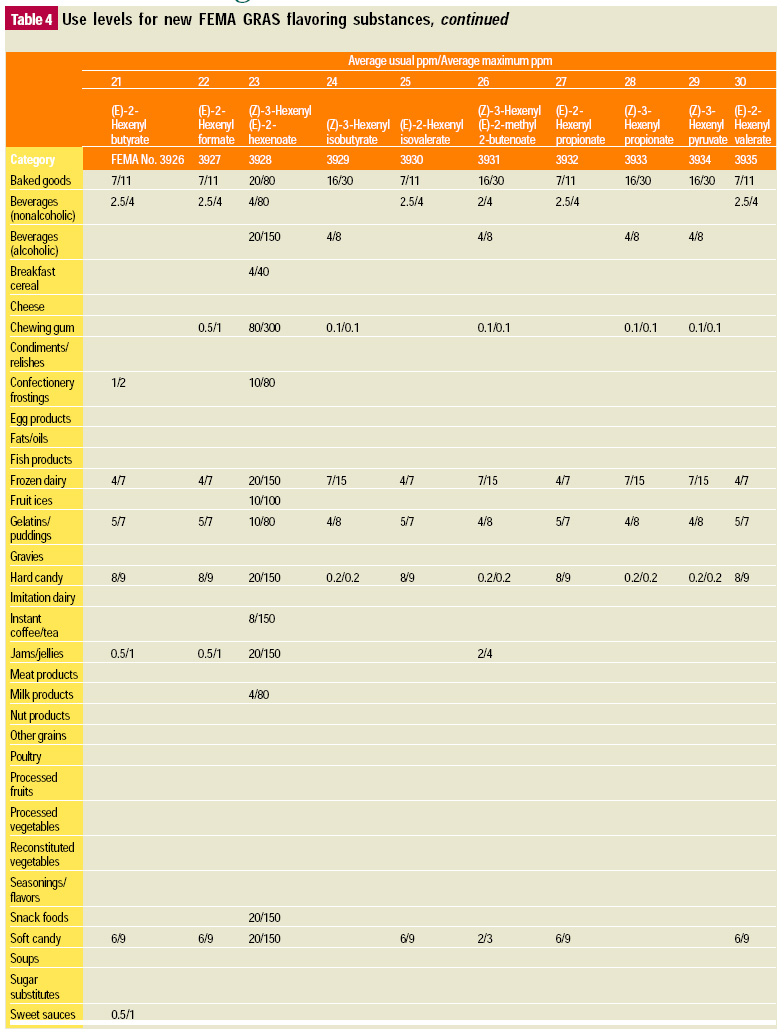
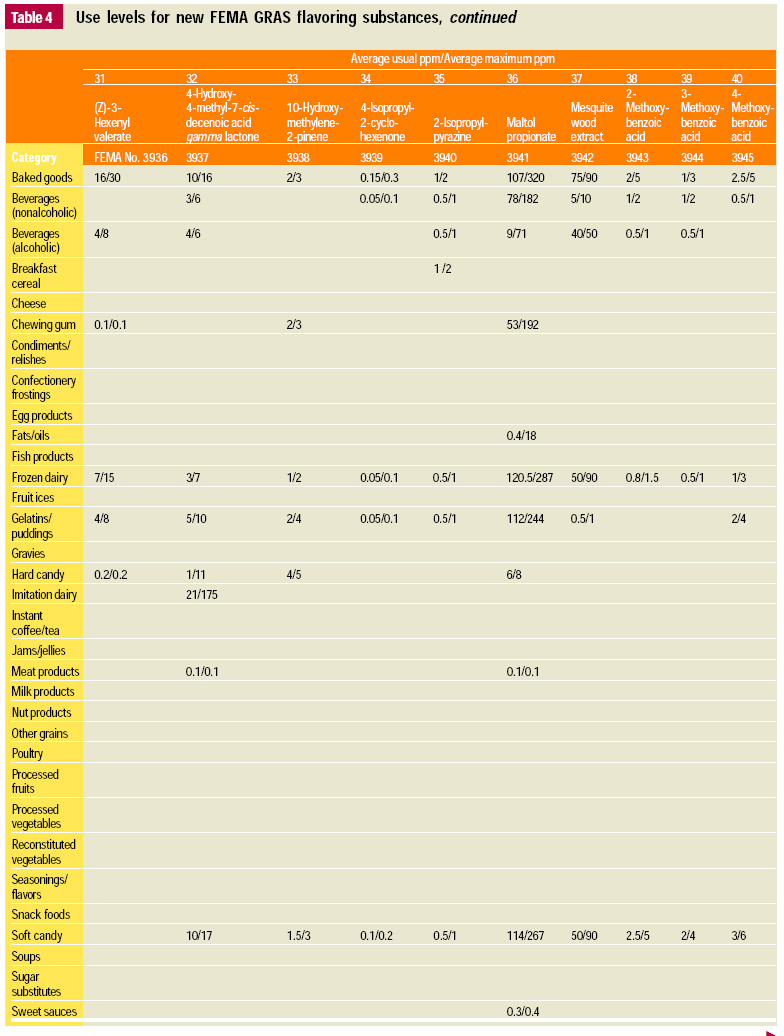
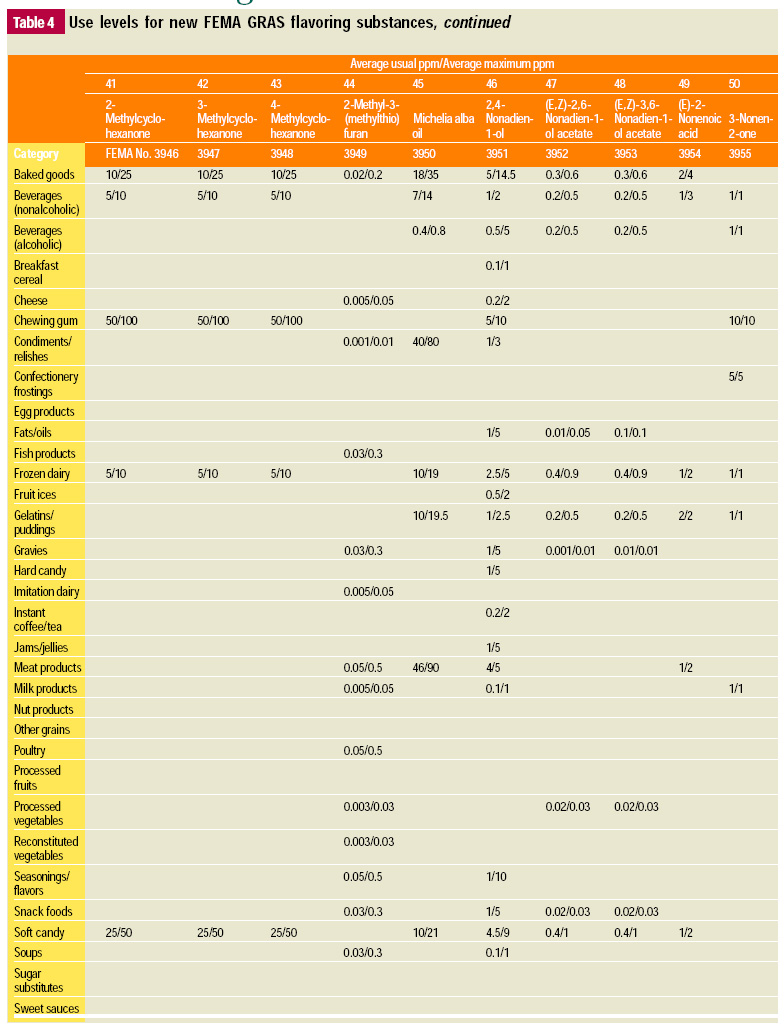
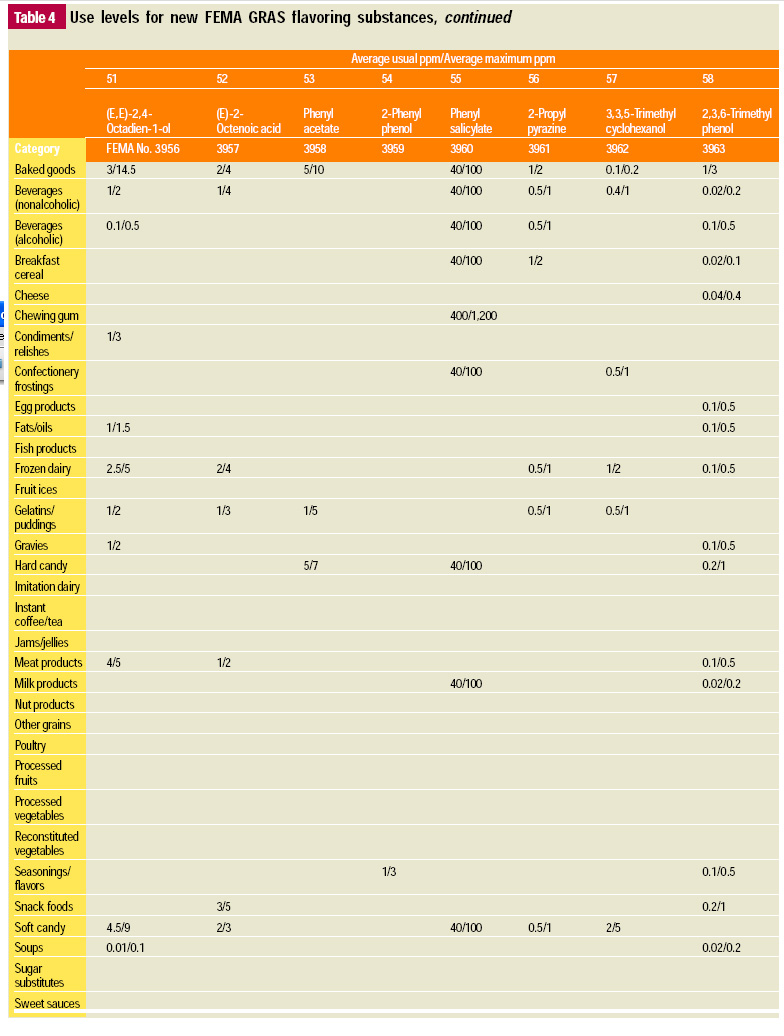
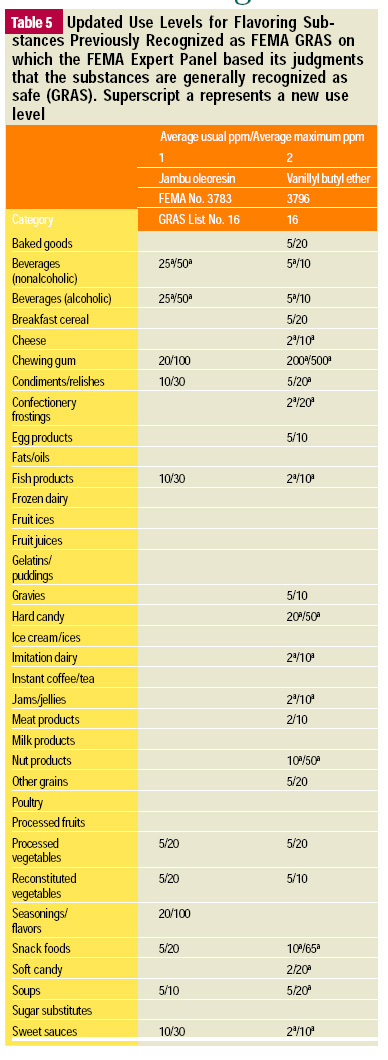
In this, the 19th GRAS publication, 58 new GRAS flavoring substances are identified together with new use levels and food categories for two existing GRAS substances. These are shown in the tables on pages 76–84.
Consistent with other GRAS publications (Oser et al., 1985; Burdock et al., 1990; Smith and Ford, 1993, 1996, 1997; Newberne et al., 1998), GRAS 19 contains the safety evaluation of flavoring substances for which significant relevant scientific data have become available (process flavors and cinnamyl anthranilate). It also clarifies the official name of one FEMA GRAS flavoring substance (FEMA No. 2804).
Recent Developments in the Flavor Industry
In the decade of the 1990s, the flavor industry became increasingly global. It has committed to an international program in which all flavoring substances in the global marketplace will be recognized as safe for their intended use in food. The globalization of the flavor industry has had a significant impact on the activities of the FEMA Expert Panel and international committees responsible for the safety evaluation of flavoring substances (e.g., the Joint Expert Committee on Food Additives, the Food and Agriculture Organization, and the Scientific Committee for Foods). In response, the Expert Panel has engaged in an extensive review of all scientific data relevant to the safety evaluation of existing FEMA GRAS substances. Since 1994, the Expert Panel has reevaluated and reaffirmed the GRAS status (GRASr) of almost 1,000 flavoring substances (Fig. 1). When necessary, the Panel has requested that additional studies be performed to complete the GRASr process.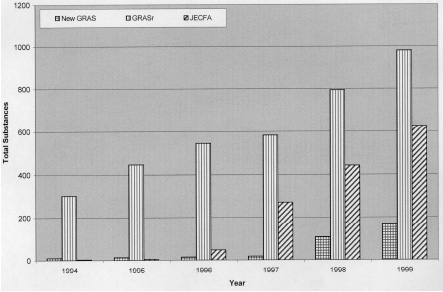
--- PAGE BREAK ---
The Panel recognizes that the GRASr program must be transparent worldwide. Therefore, it has initiated a program to publish in the peer-reviewed literature, scientific reviews of the data that, in part, provide the scientific basis for the Panel’s GRAS decisions (Adams et al., 1996, 1997, 1998; Newberne et al., 1999).
In the past three years, the Panel has also experienced a dramatic increase in the number of GRAS applications for new flavoring substances. The majority of the new GRAS substances have been in use worldwide, but have not been recognized as GRAS for use in the United States. By comparison, the number of substances recognized as GRAS between 1997 and 1999 exceeded the total number of substances recognized as GRAS in the prior two decades.
International programs on flavor safety evaluation have also felt the impact of globalization. Since 1996, FAO/WHO’s Joint Expert Committee on Food Additives has evaluated the safety of more than 600 flavoring substances (JECFA, 1996, 1998, 1999), as shown in Fig. 1. Globalization of the flavor industry has created a multifaceted international program of safety evaluation of flavoring substances that will eventually provide a global list of flavoring substances recognized as safe for their intended use in food (Munro et al., 1998).
Process Flavors
Process flavors (PFs) are formed from the thermal reactions of low-molecular-weight substances (e.g., amino acids, peptides, monosaccharides, and fatty acids) that are components of proteins, carbohydrates, and fats. As a result of cooking, low levels of PFs are produced that enhance the taste and aroma of food. For decades, manufactured PFs have been added to food to mimic the chemical reactions that result from cooking.
These reactions often involve the formation of two types of amines—monocyclic heteroaromatic amines (MHAs) and polycyclic heteroaromatic amines (PHAs). Low levels (parts per million, ppm) of MHAs (e.g., pyrazines and thiazoles) are mainly responsible for the desirable flavor and aroma of cooked food. Lower levels (parts per billion, ppb) of PHAs (e.g., imidazo-quinolines and imidazo-quinoxalines), which form upon prolonged cooking at elevated temperatures, do not significantly contribute to the flavor and may be considered side-products. However, high levels of PHAs have been shown to be mutagenic in standardized test assays and carcinogenic in rodent studies (Munro et al., 1993). Therefore, the formation of PHAs may potentially affect the safety of PFs.
At the request of the Expert Panel, FEMA sponsored a comprehensive study to identify and quantitate PHAs present in a wide variety of PFs and to determine the intake of PHAs that are added to or formed naturally in cooked food. In the study completed in 1999, 102 PFs were analyzed for the presence of 5 PHAs:
2-amino-3-methylimidazo[4,5-f]-quinoline (IQ)
2-amino-3,4-dimethyl-3H-imidazo[4,5-f]quinoline (MeIQ)
2-amino-3,8-dimethylimidazo[4,5-f]-quinoxaline (MeIQx)
2-amino-2,4,8(or 3,7,8)-trimethylimidazo[4,5-f]quinoxaline (diMeIQx)
2-amino-N-methyl-5-phenylimidazo pyridine (PhIP)
With a detection limit of 50 ppb (Gross, 1990; Knize and Salmon, 1998), 10 of 90 samples contained one or two PHAs at levels <50 ppb, while 2 samples contained PHAs barely above the 50-ppb limit (67 ppb IQ and 59 ppb MeIQx). There were no detectible levels of PHAs in 78 samples.
Using the 67 ppb IQ and 59 ppb MeIQx as a “worst-case scenario” for exposure to PHAs through PF consumption, the total daily per capita intake of IQ and MeIQx from consumption of PFs added to food was estimated to be 1.62 × 10–3 and 1.43 ×10–3 ng/day, respectively. Based on levels of IQ and MeIQx that form naturally in cooked meats (Skog, 1993; Jackson et al., 1994), it was concluded that the intake of PFs added to food is negligible compared to the intake from cooked foods. In addition, the intake levels of PHAs that resulted in toxicity in animal studies are at least 100,000 times the total intake of PHAs from consumption of cooked meats and that added to food. On the basis of these observations, the Panel judged that process flavors do not present a safety concern under current conditions of use.
--- PAGE BREAK ---
Safety Evaluation of Cinnamyl Anthranilate
Cinnamyl anthranilate (CA)—FEMA No. 2295—was reported to be used as a flavoring substance beginning in the 1940s (Opdyke, 1975). In 1980, the results of a two-year National Toxicology Program (NTP) bioassay study were released (NCI, 1980), concluding that CA was carcinogenic for male and female B6C3F1 mice and male F344/N rats. Under pressure from public interest groups, the Food and Drug Administration evaluated these results and, in 1985, concluded that CA should be prohibited from use in food. In compliance with FDA’s decision, flavor industry members voluntarily agreed not to use CA as a flavoring substance.
In national surveys (NAS, 1972, 1975) prior to 1982, the use of CA was less than 300 kg/year. The estimated daily per capita intake (“eaters only”) was <1 μg/person/day from use of CA as a flavoring substance. The intake calculation used was: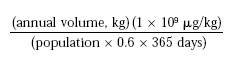
where population = 215 × 106 for the U.S. in 1975 and 0.6 represents the assumption that only 60% of the flavor volume was reported in the surveys. In three surveys after 1982 (NAS, 1982, 1987; FEMA, 1999), there was no reported usage of CA as a flavoring substance.
Additional scientific studies on CA and significant advancements in interpretation of the results of rodent bioassays have provided the basis for reevaluation of the safety of CA for use as a flavoring substance. Following is the Expert Panel’s reevaluation of the safety of CA for use as a flavoring substance.
Metabolism. A two-year bioassay (NCI, 1980) was performed with CA (see Toxicological Studies section below). Results of the bioassay stimulated numerous metabolic studies which are described here.
At low dose levels in rodents, CA is hydrolyzed to cinnamyl alcohol and anthranilic acid. However, at high oral dose levels (>750 mg/kg body weight/day) in mice, ester hydrolysis is incomplete, resulting in the in-vivo presence of the intact ester (Keyhanfar and Caldwell, 1996). Saturation of the hydrolysis pathway has been observed only at high dose levels in mice (Keyhanfar and Caldwell, 1996; Caldwell and Viswalingam, 1989).
Keyhanfar and Caldwell (1996) conducted three studies:
1. A single dose of 250 mg/kg was administered by intraperitoneal (i.p.) injection to both F344/N rats and CD-1 mice, and urine was collected for 24 hr. In the rat, 95% of the dose was recovered as hippuric acid and 4% as benzoic acid; no unchanged CA was recovered. In mice, 77% of the dose was recovered as hippuric acid, 19% as benzoic acid, and 2% unchanged.
2. In a multiple dose study, male CD-1 mice received i.p. injections of 5, 10, 20, 50, 100, or 250 mg/kg bw. Over all dose levels, the relative amounts of hippuric acid and benzoic acid present in the urine as metabolites were essentially unchanged. However, at dose levels ≥10 mg/kg bw, unhydrolyzed CA was detected in the urine. The relative amount of CA increased with increasing dose levels of >10 mg/kg bw.
3. The third study (Keyhanfar and Caldwell, 1996) investigated the effects of dietary concentrations of 0, 100, 1,000, 5,000, 15,000, or 30,000 ppm of CA administered in feed to male and female B6C3F1 mice for 21 days. These levels are calculated to provide an average daily intake of ca. 0, 15, 150, 750, 2,250, or 4,500 mg/kg, respectively (FDA, 1993). The two highest concentrations correspond to the same dose levels used in the two-year NTP bioassay (NCI, 1980). In both the male and female mice, unchanged CA was detected in the urine at dietary levels of ≥5,000 ppm (ca. 750 mg/kg bw/day). There was no evidence of unhydrolyzed ester in the urine of humans administered a single oral dose of 250 mg CA.
--- PAGE BREAK ---
Large doses of CA administered to mice, resulting in saturation of the hydrolysis pathway, have also been associated with hepatic enzyme induction (Caldwell, 1992). The enzymatic basis for the species differences in metabolism has been studied in hepatic microsomes of rats, mice, and humans. The results show that while CA is hydrolyzed relatively slowly by hepatic microsomes of rats and humans, the ester is essentially unreactive in mice liver microsomes, with <10% hydrolysis occurring over a 24-hr period (Caldwell, 1992). In mice, CA was shown to cause a pattern of enzyme induction that is characteristic of peroxisome proliferation, including increases in cytochrome P450, lauric acid omega-hydroxylation, and peroxisomal fatty-acid oxidation (Viswalingam et al., 1988). Peroxisome proliferation would not be expected in humans (Keyhanfar and Caldwell, 1996).
Toxicological Studies. Groups of 50 F344/N rats or 50 B6C3F1 mice of each sex were fed in diets containing 0, 15,000 or 30,000 ppm for 103 weeks and then observed for an additional 2–3 weeks. The dietary levels of 15,000 and 30,000 ppm are calculated to provide an average daily intake of 2,250 and 4,500 mg/kg bw/day, respectively (FDA, 1993). Control groups consisted of 50 untreated rats and 50 untreated mice of each sex. All surviving animals were terminated and necropsied at 105–107 weeks. Dose-related reductions in mean body weight gain occurred in all groups of dosed male and female rats and mice. Mean body weight gains for high-dose groups of both sexes of mice were as much as 30% lower than those for respective control groups (NCI, 1980).
The pathological findings were as follows:
1. Renal Non-Neoplastic and Neoplastic Lesions. Chronic renal inflammation was observed in control (35/ 48), low-dose (47/50), and high-dose (44/49) groups of male rats, but was less in female rats (controls, 9/48; low-dose 9/50; high-dose, 16/50). An increased incidence of renal mineralization in the low-dose (17/50) and high-dose groups (30/49) was observed in male rats compared to controls (0/48), but no significant increase (controls, 2/48; low-dose 0/50; high-dose, 3/50) was observed in treated female rats compared to controls. The lower incidence of chronic inflammation and lack of renal mineralization in all groups of female rats suggest that renal toxicity is less pronounced in the female rat than in the male rat. No increased incidences of renal toxicity or renal neoplasms were reported for dosed groups of male or female mice.
Tubule adenomas (2/50) and adenocarcinomas (2/50) of the renal cortex were reported in the high-dose group of male rats, but their incidences were not statistically significantly increased compared to controls (0/48). No renal tumors were observed in control or low-dose groups of male rats or in any group of female rats or mice. Based on the historical incidence among male controls at the laboratory (0/634) and the incidence in all laboratories in the NTP Testing Program (8/1,538, 0.37%), the NTP report concluded that “Under the conditions of these 2-year dietary studies, there was evidence of carcinogenicity of cinnamyl anthranilate in male F344/N rats based on the increased incidence of renal tubule adenomas and adenocarcinomas” (NCI, 1980).
Chronic renal nephropathy (i.e., inflammation and mineralization) and renal tubule neoplasms were reported when CA was administered to male rats in the diet for two years. The increase in the incidence of renal neoplasms in male rats was not statistically significant compared to controls. Although treated female rats also exhibited a slight increase in the incidence of renal inflammation, they did not show any renal tubule neoplasms. Also, no renal neoplasms were reported in control or treated B6C3F1 mice. The data clearly indicate that renal toxicity and subsequent neoplasms are sex and species specific effects and occur only at chronic high levels of intake (>2,000 mg/kg bw/day). Also, at these high dose levels, unhydrolyzed intact ester was present in vivo.
The sensitivity of the male rat to these types of kidney changes is related to spontaneous nephropathy during aging, which may be exacerbated by administration of high dose levels of test material (Boorman et al., 1990). Similar findings have been observed at high intake levels of other substances (NTP, 1992, 1993a, b). In addition, dosed male rats showed significantly lower growth rates (30% lower). It would appear that the renal neoplasms were unrelated to the administration of CA in the diet. It is evident that the renal effects in the male rat are species- and sex-specific phenomena that reflect the sensitivity of the male rat kidney to chronic progressive nephropathy, focal hyperplasia, and specific tumorigenic responses (Adams et al., 1996, 1998; Boorman et al., 1990).
--- PAGE BREAK ---
2. Pancreatic Acinar-Cell Neoplasms in Male Rats. The incidence of pancreatic acinar-cell adenomas (2/45) and carcinomas (1/45) was increased in the high-dose males (3/45; 7%) compared to controls (0/42). No pancreatic neoplasms were observed in low- and high-dose female rats. Although the increased incidence of pancreatic neoplasms was not statistically significant compared to control rats in the study, according to NTP the historical incidence of this type of neoplasm in aging F344/N control rats is extremely low—the historical incidence for controls in participating NTP laboratories is 6/1,538 (0.28%). Therefore, NTP considered occurrence of these neoplasms to be related to administration of the test material.
Since completion of the two-year bioassay with CA, results of other carcinogenicity studies have identified two factors which are strongly associated with the appearance of pancreatic acinar-cell neoplasms in the male F344/N rat. The incidence of these lesions in the male F344/N rat is clearly related to the use of corn oil as a gavage vehicle (NTP, 1994). Groups of male rats were given 0, 2.5, 5.0, or 10 mL/kg corn oil by gavage daily, 5 days a week, for two years. A statistically significant and dose-related increase in the incidence of pancreatic acinar-cell hyperplasia (controls, 8/50; 2.5 mL/kg, 28/47: 5.0 mL/kg, 28/50; 10 mL/kg, 35/50) and adenomas (controls, 0/50; 2.5 mL/kg, 8/47: 5.0 mL/kg, 10/50; 10 mL/kg, 23/50) was observed. One carcinoma was reported in one male rat at the 5 mL/kg level. NTP concluded that “use of corn oil as a gavage vehicle may have a compounding effect on the interpretation of chemically-induced proliferative lesions of the exocrine pancreas in male F344/N rats” (NTP, 1994).
The appearance of these neoplasms of the exocrine pancreas has also been related to hepatic peroxisome proliferation in male F344/N rats. The sex-specific phenomenon also has been observed when F344/N male rats were exposed to high dose levels of other peroxisome proliferators (e.g., butyl benzyl phthalate and hypolipidemic drugs, clofibrate and nafenopin) (Malley et al., 1995; NTP, 1997a; Reddy and Qureshi, 1979; Svoboda and Azarnoff, 1979). The appearance of tumors in male rats is consistent with studies showing that testosterone stimulates, and estrogen inhibits, the growth of pancreatic acinar-cell neoplasms in rats (Lhoste et al. 1987 a, b; Sumi et al. 1989; Longnecker and Sumi et al., 1990).
The impact of diet on the occurrence of these neoplasms in male rats has been recently studied (NTP, 1997b). The incidence of pancreatic acinar-cell neoplasms was 10/50 and 0/50, respectively, for male rats fed either ad libitum or placed on a restricted feed protocol containing 12,000 ppm butyl benzyl phthalate for two years. Therefore, the appearance of these neoplasms is sex-, species-, dose-, and diet-dependent among rodents. Several human studies of hypolipidemic drugs which are recognized peroxisome proliferators in rodents have failed to show any significant difference in cancer deaths between treated patients and a placebo-treated group (IARC, 1996).
Given these more recent data on corn oil gavage and the lack of any correspondence between bioassay results and human studies with peroxisome proliferators, it is concluded that the increased incidence of acinar-cell neoplasms in the F344/N male rat is associated with hepatic peroxisomal proliferation induced by CA administered in corn oil (5 mL/kg). The direct relationship of the use of corn oil as a gavage vehicle to the incidence of pancreatic acinar-cell neoplasms in the NTP study clearly compounds any interpretation of the relevance of these results to a human health assessment. In any event, the association of hepatic peroxisome proliferation and the incidence of pancreatic acinar-cell neoplasms is specific to the male F344/N rat and therefore is not relevant to the human health risk assessment of CA.
3. Hepatocellular Neoplasms in Mice. Neoplastic and non-neoplastic lesions associated with administration of CA to mice developed principally in the liver (Table 2). Treated groups of male and female mice showed evidence of lipoidosis, hemosiderosis, and hyperplasia of hepatocytes. There was a statistically significant increase in the incidence of combined hepatocellular adenomas and carcinomas [control, 14/48; 15,000 ppm or 2,250 mg/kg, 30/50 (P = 0.003); 30,000 ppm or 4,500 mg/kg, 37/47 (P < 0.001)] in male mice compared to that of the control group (Table 2). However, the increase in the incidence of hepatocellular carcinomas (control, 6/48; 15,000 ppm or 2,250 mg/kg, 7/50; 30,000 ppm or 4,500 mg/kg, 12/47) was not statistically significant. There was a statistically significant increase in the incidence of hepatocellular carcinomas [control, 1/50; 15,000 ppm or 2,250 mg/kg, 8/49 (P = 0.014); 30,000 ppm or 4,500 mg/kg, 14/49 (P < 0.001)] and combined adenomas and carcinomas [control, 3/50; 15,000 ppm or 2,250 mg/kg, 20/49 (P < 0.001); 30,000 ppm or 4,500 mg/kg, 33/49 (P < 0.001)] in dosed groups of female mice. Four high-dose and two low-dose females were diagnosed as having both adenomas and carcinomas.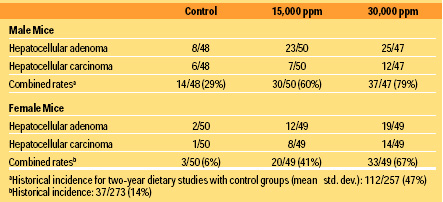
--- PAGE BREAK ---
The NTP report concluded that “Based on increased incidences of hepatocellular adenomas, and hepatocellular adenomas and carcinomas, cinnamyl anthranilate was considered carcinogenic for male and female B6C3F1 mice receiving 15,000 or 30,000 ppm in the diet” (NCI, 1980).
Since performance of the original bioassay (NCI, 1980), additional studies on more than 70 substances have established a direct correlation between the increased incidence of hepatocarcinogenicity and the induction of peroxisome proliferation in rodent livers (Ashby et al., 1994). Studies performed by the European Centre for Ecotoxicity and Toxicology of Chemicals (ECETOC, 1992) show that peroxisome proliferators form a discrete category of rodent liver carcinogens, the carcinogenicity of which does not involve direct genotoxic mechanisms.
Histological evidence of peroxisome proliferation in rodents is reflected by an increased peroxisome/mitochondrial ratio which is correlated with increases in target organ weights, total cytochrome P-450 content, and activities in microsomal lauric acid omega-hydroxylation, carnitine acetyl transferase, and cyanide (CN-) insensitive palmitoyl-CoA (Reddy et al., 1980, 1986; Reddy and Lalwai, 1983; Barber et al., 1987).Peroxisome proliferation is a transcription-mediated process involving the peroxisome proliferator–activated receptor (PPARα) in the hepatocyte nucleus. The role of PPARα in the induction of hepatocarcinogenicity in the mouse has been clearly established (Peters et al., 1997). Carcinogenicity studies with mice genetically modified to remove PPARα show no evidence of either peroxisome proliferation or carcinogenicity. Given that levels of expression of PPARα in humans is 1–10% of levels found in the rat or mouse (Palmer et al., 1994, 1998), humans are refractory to peroxisome proliferation following chronic exposure to potent rodent peroxisome proliferators. No significant evidence of peroxisome proliferation has been observed in human studies with several potent hypolipidemic drugs that are peroxisome proliferators (reviewed in Doull et al., 1999 and Ashby et al., 1994). Based on these observations, it is concluded that the hepatocarcinogenic response in rodents is not relevant to the human health assessment of CA.
Conclusion
When the above information is combined with recent data on metabolism and enzyme induction, it may be concluded that hepatic peroxisome proliferation is both a rodent-specific and a dose-dependent phenomenon induced by the intact ester CA (Viswalingam et al., 1988; Keyhanfar and Caldwell, 1996; Caldwell, 1992). Specifically, repeated-dose metabolism studies have shown that above a threshold dose of 500 mg/ kg bw/day, intact CA given i.p. or in the diet to mice shows a dose-dependent increase in liver weight, total cytochrome P-450, microsomal lauric acid omega-hydroxylation and cyanide-insensitive palmitoyl-CoA activity, and peroxisome/mitochondria ratio in hepatic cells (Caldwell, 1992; Viswalingam et al., 1988). These markers for peroxisome proliferation correspond to dose levels at which saturation of the hydrolysis pathway leads to the presence of the intact ester in vivo. Therefore, peroxisome proliferation caused by CA is a dose-dependent effect that is specific only to F344/N rats.
The FEMA Expert Panel considers that the report of pancreatic neoplasms in the male F344/N rat is related to use of corn oil as a gavage vehicle and is a secondary response to peroxisome proliferation. Likewise, hepatic neoplasms in the B6C3F1 mouse in the NTP bioassay are secondary responses to peroxisome proliferation. Since peroxisome proliferation is a dose-dependent effect specific to rodents, the results of the bioassay are not relevant to the safety of CA in humans at low levels of intake from its use as a flavoring substance. This conclusion is supported by the fact that CA is not genotoxic.
Finally, the continuum of chronic progressive nephropathy, focal hyperplasia, and renal neoplasms reported in the male F344/N rat are concluded to be a species-, strain-, and sex-specific phenomenon and is not relevant to the safety evaluation of cinnamyl anthranilate from its past use as a flavoring substance.
--- PAGE BREAK ---
Correction
The Expert Panel’s conclusion that 3-hydroxymethyl-2-heptanone (FEMA No. 2804) is considered GRAS for use as a flavoring substance was published in GRAS 3 (Hall and Oser, 1965) with the name 3-octenone-1-ol. This name is incorrect. The new name for FEMA No. 2804 should be 3-hydroxymethyl-2-heptanone.
Expert Panel Membership Changes
The FEMA Expert Panel would like to express their condolences at the passing of Carrol S. Weil. He served as an expert panel member from 1981 to 1998. He also served on numerous National Academy of Sciences committees, editorial boards of technical journals, and Society of Toxicology committees. He will be sadly missed.
In January 1999, Victor J. Feron, consultant to TNO Nutrition and Food Research Institute, Professor Emeritus, Utrecht University, The Netherlands, and William J. Waddell, Professor and Chair, Emeritus, Dept. of Pharmacology and Toxicology, University of Louisville School of Medicine, Louisville, Ky., joined the Expert Panel.
Author Newberne, Cochairman of the FEMA Expert Panel, is Professor, Dept. of Pathology, School of Medicine, Boston University, Boston, MA 01730
Author Smith, Cochairman of the FEMA Expert Panel, is Professor, Molecular Toxicology, Imperial College School of Medicine, University of London, South Kensington, London SW7 2AZ, United Kingdom.
Other members of the FEMA Expert Panel are John Doull, Professor Emeritus, University of Kansas Medical School, Kansas City, Kans.
Victor J. Feron, TNO Nutrition and Food Research Institute, Professor Emeritus, Utrecht University, The Netherlands.
Jay I. Goodman, Professor, Michigan State University, East Lansing, Mich
Ian C. Munro, Cantox Health Sciences, Inc., Mississauga, Ontario, Canada
Philip S. Portoghese, Professor, College of Pharmacy, University of Minnesota, Minneapolis, Minn.
William J. Waddell, Professor and Chair, Emeritus, Dept. of Pharmacology and Toxicology, University of Louisville School of Medicine, Louisville, Ky.
Bernard M. Wagner, Emeritus Research Professor of Pathology, New York University Medical Center, New York, N.Y.
Carrol S. Weil (deceased), Carrol S. Weil Inc., Pittsburgh, Pa.
Authors Adams and Hallagan are affiliated with the Flavor and Extract Manufacturers’ Association, 1620 I St., N.W., Suite 925, Washington, DC 20006.
Send reprint requests to author Smith.


References
Adams, T.B., Hallagan, J.M., Putnam, Gierke, T.L., Doull, J., Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1996. The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. Food Chem. Toxicol. 34: 763-828.
Adams, T.B., Doull, J., Goodman, J.I., Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1997. The FEMA GRAS assessment of furfural used as a flavor ingredient. Food Chem. Toxicol. 35: 739-751.
Adams, T.B., Greer, D.B., Doull, J., Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1998. The FEMA GRAS assessment of lactones used as a flavor ingredient. Food Chem. Toxicol. 36: 249-278.
Ashby, J., Brady, A., Elcombe, C.R., Elliot, B.M., Ismael, J., Odum, J., Tugwood, J.D., Kettle, S., and Purchase, I.F.H. 1994. Mechanistically-based human hazard assessment of peroxisome proliferator-induced hepatocarcinogenesis. Human Exp. Toxicol. 13(Suppl. 2): S1-S118.
Barber, E.D., Astill, B.D., Moran, E.J., Schneider, B.F., Gray, T.J.B., Lake, B.G., and Evans, J.G. 1987. Peroxisome induction studies on seven phthalate esters. Toxicol. Ind. Health 3(2): 7-24.
Boorman, G.A., Eustis, S.L., Montgomery, C., and Elwell, M. 1990. “Pathology of the F344 Rat.” Academic Press, San Diego.
Burdock, G.A., Wagner, B.M., Smith, R.L., Munro, I.C., and Newberne, P.M. 1990. Recent progress in the consideration of flavor ingredients under the food additives amendment. 15. GRAS substances. Food Technol. 44(2): 78-86.
Caldwell, J. 1992. Problems and opportunities in toxicity testing arising from species differences in xenobiotic metabolism. In “Toxicology from Discovery to Experimentation to the Human Perspective,” ed. P.L. Chambers, C.M. Chambers, H.M. Bolt, and P. Preziosi, pp. 651-659. Elsevier, London.
Caldwell, J. and Viswalingam, A. 1989. A comparative study of the hepatic effects of cinnamyl esters in mice. Presented at 5th International Congress of Toxicology.
Doull, J., Cattley, R., Elcombe, C., Lake, B.G., Swenburg, J., Wilkinson, C., Williams, G., and van Gemert, M. 1999. A cancer risk assessment of di(2-ethyhexyl)phthalate: Application of the new U.S. EPA risk assessment guidelines. Reg. Toxicol. Pharmacol. 29: 327-357.
ECETOC. 1992. Hepatic peroxisome proliferation. Monograph 17. European Centre for Ecotoxicity and Toxicology of Chemicals, Brussels.
FDA. 1993. Priority-based assessment of food additives (PAFA) database (p. 58). Center for Food Safety and Applied Nutrition, Food and Drug. Admin., Washington, D.C.
FEMA. 1999. 1995 Poundage and technical effects update survey. Compiled by C. Lucas, J. Putnam, J. Hallagan, and the Flavor Ingredients Committee. Flavor and Extract Manufacturers’ Assn., Washington, D.C.
Gross, G.A. 1990. Simple methods for quantifying mutagenic heterocyclic aromatic amines in food products. Carcinogenesis 11: 1597-1603.
Hall, R.L. and Oser, B.L. 1965. Recent progress in the consideration of flavoring ingredients under the food additives amendment. III. GRAS substances. Food Technol. 19(2): 151-197.
IARC. 1996. Hypolipidaemic drugs. In IARC Monograph on the evaluation of carcinogenic risk to humans, 66: 390-485. Intl. Agency for Research in Cancer, Lyon, France.
Jackson L.S, Hargraves W.A., Stroup W.H., and Diachenko G.W. 1994. Heterocyclic aromatic amine content of selected beef flavors. Mutat. Res. 320, 113-124.
JECFA 1996. Safety evaluation of certain food additives and contaminants. Prepared by the 46th Meeting of the Joint FAO/WHO Expert Committee on Food Additives. World Health Org., Geneva.
JECFA. 1998. Safety evaluation of certain food additives and contaminants. Prepared by the 49th Meeting of the Joint FAO/WHO Expert Committee on Food Additives. World Health Org., Geneva.
JECFA. 1999. Safety evaluation of certain food additives and contaminants. Prepared by the 51st Meeting of the Joint FAO/WHO Expert Committee on Food Additives. World Health Org., Geneva.
Keyhanfar, F. and Caldwell, J. 1996. Factors affecting the metabolism of cinnamyl anthranilate in the rat and mouse. Food Chem. Toxicol. 34: 241-249.
Knize, M. and Salmon, C. 1998. Heterocyclic amine analysis of process flavors. Unpublished data, Biology and Biotechnology Research Program, Lawrence Livermore Natl. Lab., Livermore, Calif.
Lhoste, E.F., Roebuck, B.D., Brinck-Johnsen, T., and Longnecker, D.S. 1987a. Effect of castration and hormone replacement on azaserine-induced pancreatic carcinogenesis in male and female Fischer rats. Carcinogenesis 8: 699-703.
Lhoste, E.F., Roebuck, B.D., Stern, J.E., and Longnecker, D.S. 1987b. Effect of orchiectomy and testosterone on the early stages of azaserine-induced pancreatic carcinogenesis in the rat. Pancreas 2: 38-43.
Longnecker, D.S. and Sumi, C. 1990. Effects of sex steroid hormones on pancreatic cancer in the rat. Intl. J. Pancreatol. 7: 159-165.
Malley, L.A., Carakostas, M., Hansen, J.F., Rusch, G.M., Kelly, D.P., and Trochimowicz, H.J. 1995. Two-year inhalation toxicity study in rats with hydrochlorofluorocarbon 123. Fundamental Appl. Toxicol. 25: 101-114.
Munro, I.C., Kennepohl, E., Erickson, R.E., Portoghese, P.S., Wagner, B.M., Easterday, O.D., and Manley, C.H. 1993. Safety assessment of ingested heterocyclic amines: Initial report. Reg. Toxicol. Pharmacol. 17(2): S1-S109.
Munro, I.C., Shubik, P., and Hall, R. 1998. Principles for the safety evaluation of flavouring substances. Food Chem. Toxicol. 36: 529-540.
NAS. 1972. Evaluating the safety of food chemicals. Natl. Acad. of Sciences, Washington, D.C.
NAS. 1975. Evaluating the safety of food chemicals. Natl. Acad. of Sciences, Washington, D.C.
NAS. 1982. Evaluating the safety of food chemicals. Natl. Acad. of Sciences, Washington, D.C.
NAS. 1987. Evaluating the safety of food chemicals. Natl. Acad. of Sciences, Washington, D.C.
NCI. 1980. Bioassay of cinnamyl anthranilate for possible carcinogenicity. NCI-CG-TR-196; NTP-80-10. Natl. Cancer Inst., Washington, D.C.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Hallagan, J.B., and Ford, R.A. 1998. GRAS flavoring substances 18. Food Technol. 52(9): 65-92.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Lucas, C.D., and Ford, R.A. 1999. The FEMA GRAS assessment of trans-anethole used as a flavoring substance. Food Chem. Toxicol. 37: 545-554.
NTP. 1992. Toxicology and carcinogenesis studies of quercetin in F344/N rats. NTP-TR-409, NIH Pub. 92-3140, Natl. Toxicology Program, Research Triangle Park, N.C.
NTP. 1993a. Toxicology and carcinogenesis studies of coumarin in F344/N rats and B6C3F mice (gavage studies). NTP-TR-422; NIH Pub. 93-3153, Natl. Toxicology Program, Research Triangle Park, N.C.
NTP. 1993b. Toxicology and carcinogenesis studies of 3,4-dihydrocoumarin in F344/N rats and B6C3F1 mice (gavage studies). NTP-TR-423; NIH Publication No. 93-3154, Natl. Toxicology Program, Research Triangle Park, N.C.
NTP. 1994. Comparative toxicology studies of corn oil, safflower oil, and tricaprylin (CAS No.s 8001-30-7, 8001-23-8, and 538-23-8) in male F344/ N rats as vehicles for gavage. NTP-TR-426; NIH Pub. 94-3157, Natl. Toxicology Program, Research Triangle Park, N.C.
NTP. 1997a. Toxicology and carcinogenesis studies of Buty benzyl phthalate in F344/N rats (feed studies). NTP-TR- 458; NIH Pub. 97-3374. Natl. Toxicology Program, Research Triangle Park, N.C.
NTP. 1997b. Effect of dietary restriction on toxicology and carcinogenesis studies in F344/N rats and B6C3F1 mice. NTP-TR –460: NIH Pub. 97-3376. Natl. Toxicology Program, Research Triangle Park, N.C.
Opdyke, D.L.J. 1975. Monographs on fragrance raw materials. Cinnamyl anthranilate. Food Cosmet. Toxicol. 13(Suppl. 2): 751.
Oser, B.L., Weil, C.S., Woods, L.A., and Bernard, B.K. 1985. Recent progress in the consideration of flavoring ingredients under the food additives amendment. 14. GRAS substances. Food Technol. 39(11): 108-117.
Palmer, C.A.N., Hsu, M.-H., Muerhoff, A.S., Griffith, K.J., and Johnson, E.F. 1994. Interaction of the peroxisome proliferator-activated receptor A with the retinoid X receptor A unmasks a cryptic peroxisome proliferator response element that overlaps an ARP-1-binding site in the CYP4A6 promoter. J. Biol. Chem. 269: 18083-18089.
Palmer, C.A.N., Hsu, M.-H., Griffith, K.J., Raucy, J.L., and Johnson, E.F. 1998. Peroxisome proliferator activated receptor-a expression in human liver. Molec. Pharmacol. 53: 14-22.
Peters, J.M., Cattley, R.C., and Gonzalez, F.J. 1997. Role of PPARa in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator WY-14, 643. Carcinogenesis. 18: 2029-2033.
Reddy, J.K. and Lalwai, N.D. 1983. Carcinogenesis by hepatic peroxisome proliferators: Evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. CRC Crit. Rev. Toxicol. 12(1): 1-58.
Reddy, J.K. and Qureshi, S.A. 1979. Tumorigenicity of the hypolipidaemic peroxisome proliferator ethyl-alpha-chlorophenoxyisobutyrate (clifibrate) in rats. Brit. J. Cancer 40: 476.
Reddy, J.K., Azarnoff, D.L., and Hignite, C.E. 1980. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature 283: 397-398.
Reddy, J.K., Reddy, M.K., Usman, M.I., Lalwani, N.D., and Rao, M.S. 1986. Comparison of hepatic peroxisome proliferative effect and its implication for hepatocarcinogenicity of phthalate esters, di(2-ethylhexyl) phthalate, and di(2-ethylhexyl) adipate with a hypolipidemic drug. Environ. Health Perspectives 65: 317-327.
Skog, K. 1993. Cooking procedures and food mutagens: A literature review. Food Chem. Toxicol. 31(9): 655-675.
Smith, R.L. and Ford, R.A. 1993. Recent progress in consideration of flavoring ingredients under the Food Additives Amendment. 16. GRAS substances. Food Technol. 47(6): 104-117.
Smith, R.L. and Ford, R.A. 1996. Recent progress in consideration of flavoring ingredients under the Food Additives Amendment. 17. GRAS substances. Food Technol. 50(10): 72-78, 80-81.
Smith, R.L. and Ford, R.A. 1997. Correction. Food Technol. 51(2): 32.
Sumi, C., Longnecker, D.S., Roebuck, B.D., and Brinck-Johnsen, T. 1989. Inhibitory effects of estrogen and castration on the early stage of pancreatic carcinogenesis in Fisher rats treated with azaserine. Cancer Res. 49: 2332-2336.
Svoboda, D.J. and Azarnoff, D.L. 1979. Tumors in male rats fed ethyl chlorophenoxyisobutyrate, a hypolipidemic drug. Cancer Res. 39: 3419-3428.
Viswalingam, A., Caldwell, J., Fournel, S., Schladt, L., and Oesch, F. 1988. Hepatic effects of cinnamyl anthranilate resemble those of a peroxisome proliferator in mouse, but not in rat. Brit. J. Pharmacol. 93: 32.
Edited by Neil H. Mermelstein,
Senior Editor
