GRAS Flavoring Substances 20
The 20th publication by the Expert Panel of the Flavor and Extract Manufacturers Association on recent progress in the consideration of flavoring ingredients generally recognized as safe under the Food Additives Amendment
In 1958, Congress enacted the Food Additives Amendment (FAA) to the Federal Food, Drug, and Cosmetics Act amid growing concern over the safety of substances added to foods. The FAA set forth standards and guidelines by which the safety of food additives must be established before they can be added to foods (FAA, 1958).
The FAA contained an exclusion provided by Congress for substances “generally recognized, by experts qualified by scientific training and experience to evaluate its safety, as having been adequately shown through scientific procedures . . . to be safe under the conditions of intended use.” Based on this, substances “generally recognized as safe” (GRAS) are not considered to be food additives, and are excluded from mandatory premarket approval by the Food and Drug Administration (FDA). However, GRAS substances must meet strict criteria specified by Congress (Degnan, 1991).
As a result, in 1960, the Flavor and Extract Manufacturers Association of the United States (FEMA) created the FEMA GRAS program in which the safety of flavor ingredients would be evaluated for potential GRAS status by an independent panel of experts in the fields of chemistry, toxicology, pharmacology, medicine, pathology, and flavor safety assessment. The conclusions of the Expert Panel would be provided to FDA, the food and flavor industries, and the public. FDA has acknowledged the validity of the FEMA GRAS program and has recognized the FEMA GRAS publications as “reliable industry GRAS lists” within the context of the agency’s bulk labeling regulations for flavors codified at 21 CFR Sec. 101.22(b) (2001). FDA expressed significant support for the FEMA GRAS program in the preamble to its proposed voluntary GRAS notification program (FDA, 1997).
The FEMA GRAS lists have been published in Food Technology since 1960 (see sidebar on p. 44).
The GRAS assessment performed by the Expert Panel includes a rigorous evaluation of all the available data on flavor ingredients and structurally related substances. The analyses include a comprehensive evaluation of the potential exposure to the flavor ingredients through food compared with toxicologic and pharmacokinetic characteristics. As advancements are made in science, new information becomes available on existing FEMA GRAS flavoring substances. The dynamic FEMA GRAS assessment process incorporates this new information into the program by way of systematic reviews of all GRAS flavor ingredients.
Between 1965 and 1985, the first comprehensive and systematic scientific literature reviews (SLRs) of flavoring substances were completed by FEMA. These SLRs served as the basis for a comprehensive review of substances already designated as FEMA GRAS. This GRAS status reassessment program was known as “GRAS affirmation” or “GRASa” and was completed in 1985.
In 1994, the Expert Panel initiated a second comprehensive reassessment program known as “GRAS reaffirmation” or “GRASr.” It is anticipated that this reaffirmation program will be completed in 2005. As part of the GRASr program, the Expert Panel regularly publishes key scientific data on structurally related groups of flavoring substances on which GRAS decisions are based. FEMA GRAS assessments of alicyclic substances, furfural, lactones, and trans-anethole have been published as part of the GRASr program. The fifth in the series, on pyrazine compounds, and the sixth, on methyl eugenol and estragole, have been accepted for publication (Adams et al., 1996, 1997, 1998; Newberne et al., 1999; Smith et al., 2001a, b).
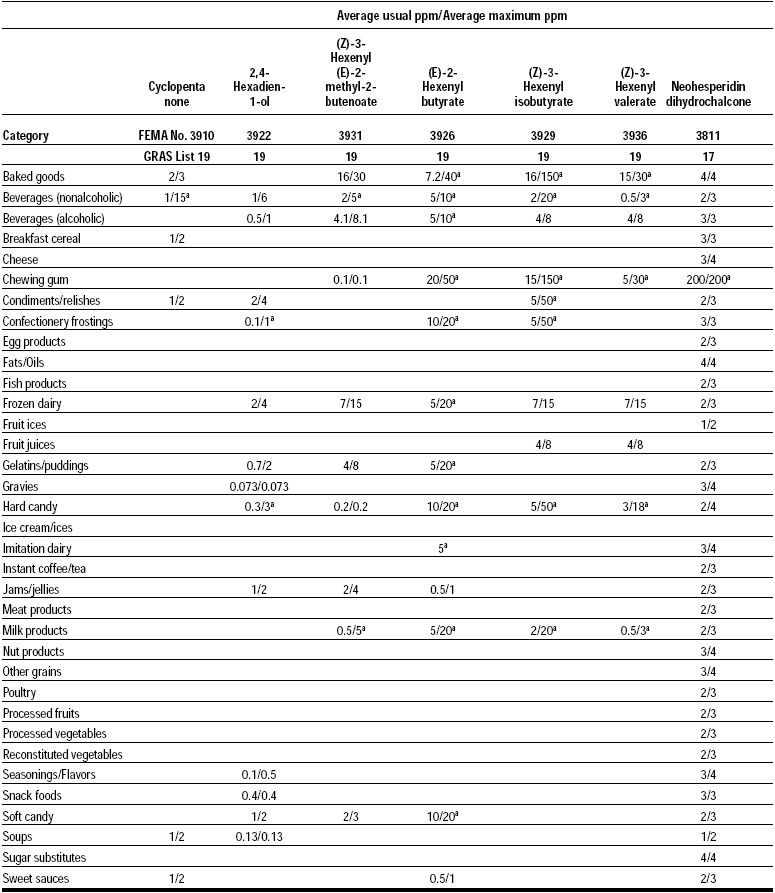
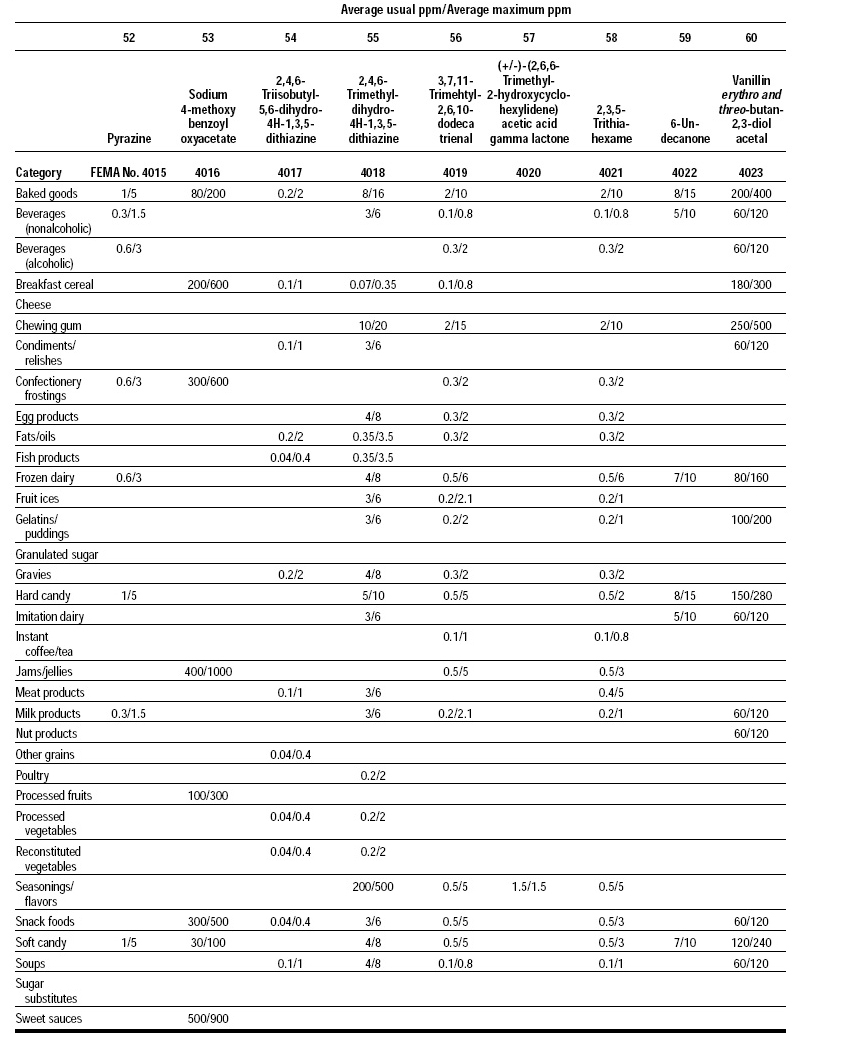
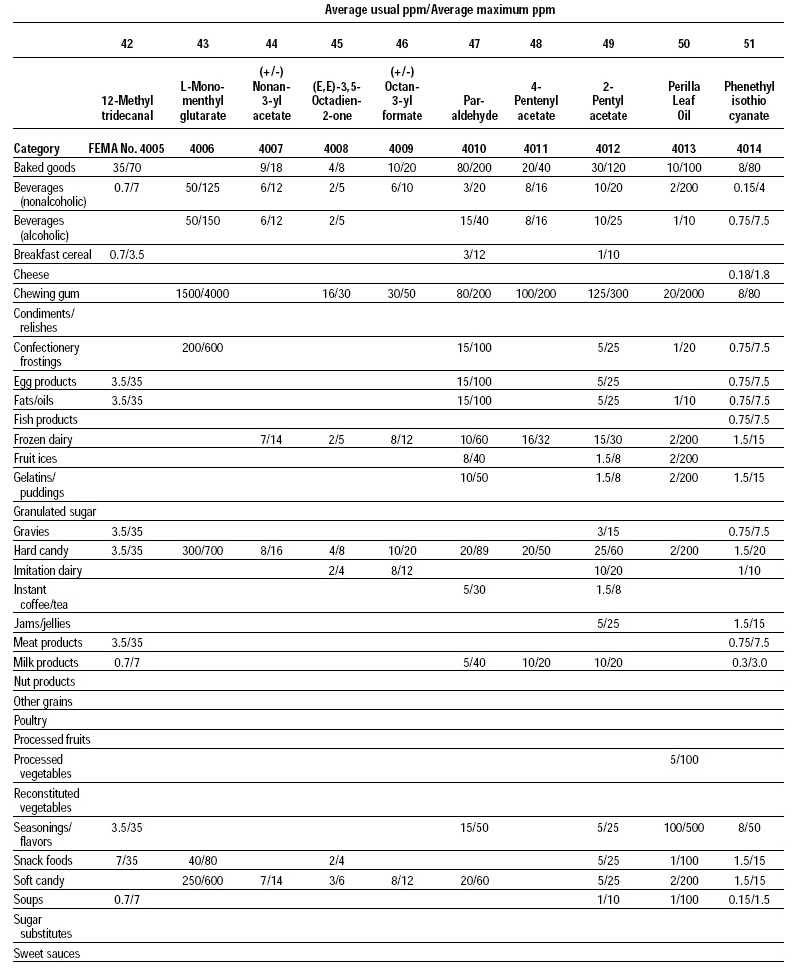
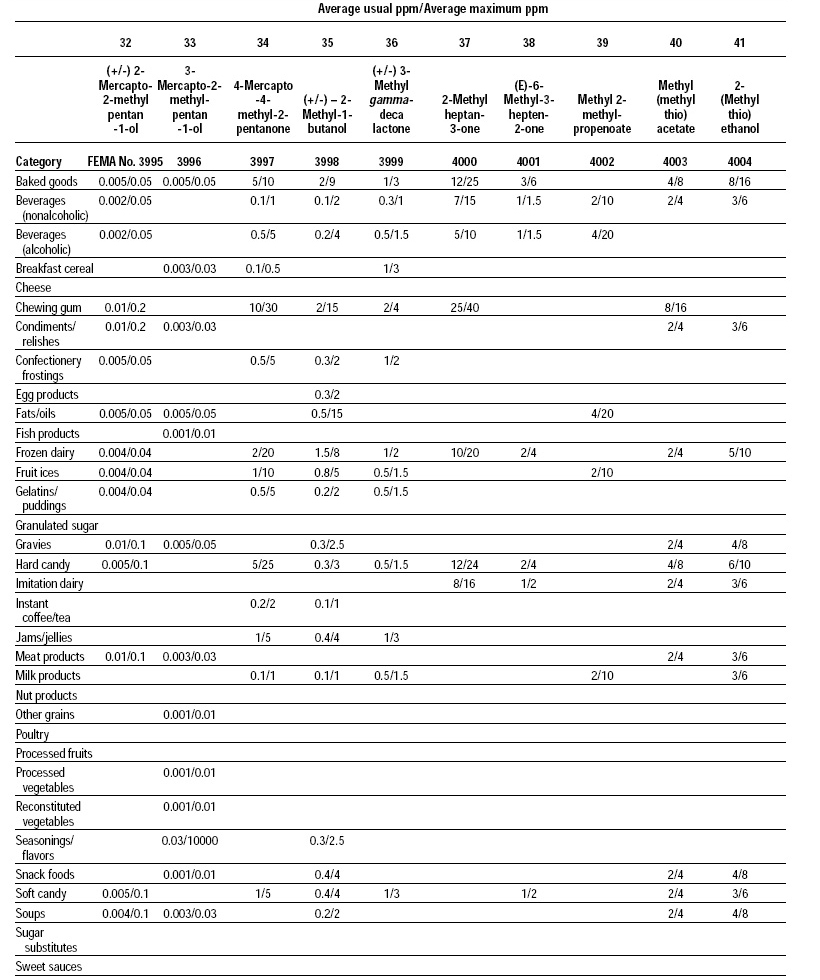
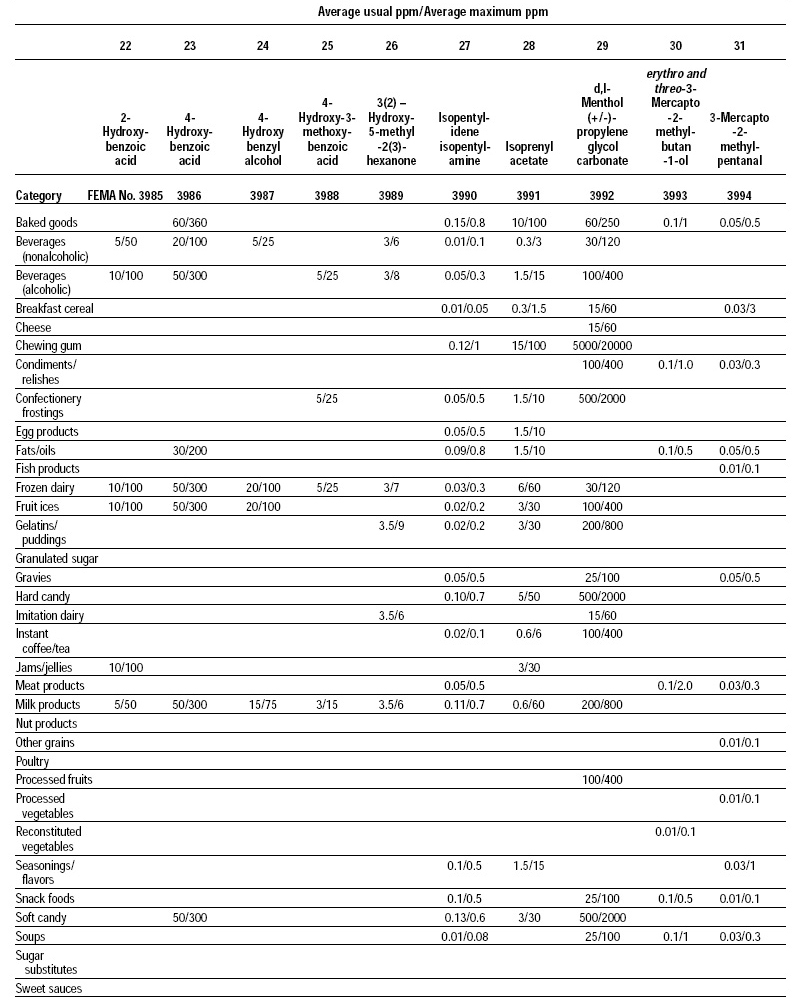
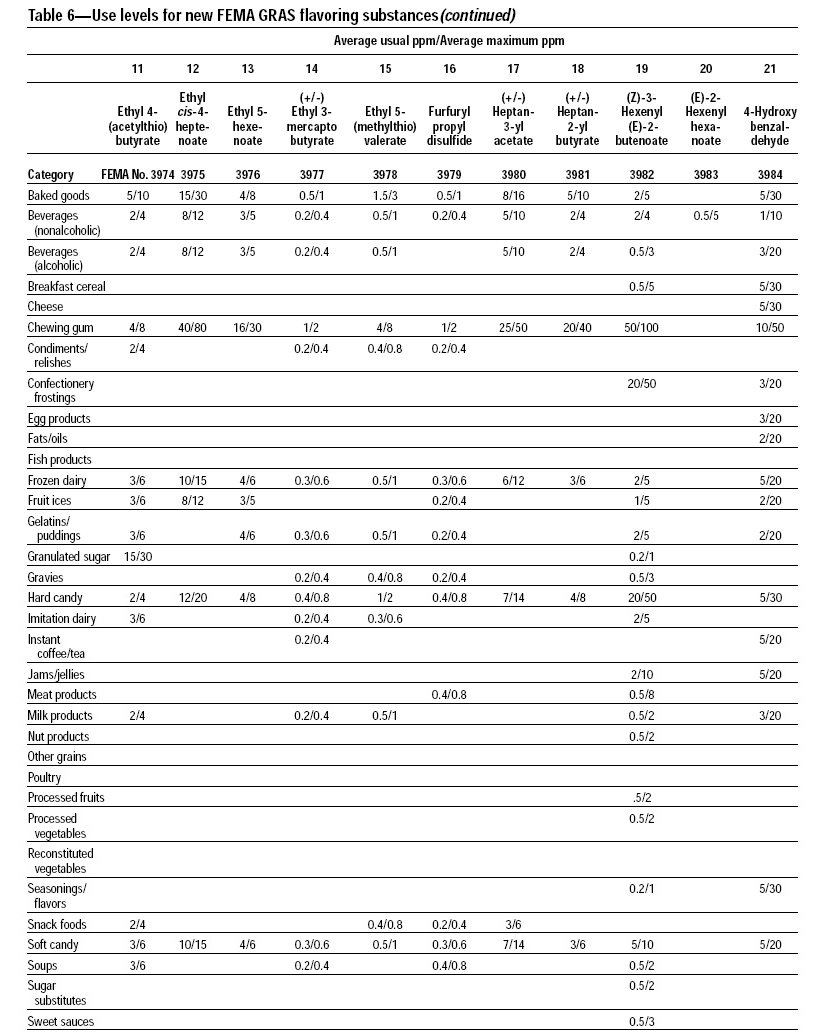
This, the 20th GRAS publication, includes the results of the Expert Panel’s review of 60 new GRAS flavoring substances (see pp. 38 and 40, and 45–50). The publication is a landmark in that it contains the 2,000th substance (FEMA No. 4000) to be recognized as GRAS by the FEMA Expert Panel. It also contains the Expert Panel’s determination that new use levels and food categories for seven flavoring substances previously considered GRAS are consistent with their current GRAS status (see p. 51). It also includes the panel’s views on methods of calculating human dietary exposure to flavoring substances and critically reviews the results of chronic two-year bioassay studies performed at the National Toxicology Program (NTP) for methyl eugenol (FEMA No. 2475) and citral (FEMA No. 2303).
--- PAGE BREAK ---
Estimation of Intake/Exposure to Flavoring Substances
As food technology progresses, its impact on the human diet becomes more evident. The global food supply has grown to depend on the quantity, quality, and variety of wholesome and nutritious foods produced through scientific advancements in this field. The use of preservatives, color additives, and flavoring agents by manufacturers plays an important role in sustaining and extending the quality and quantity of food. As a direct result of these advancements, a variety of safety assessment procedures have developed and are currently in place to assure regulators and consumers that food additives and in particular flavoring agents are safe for human consumption (JECFA, 1968, 1996, 1998, 1999, 2000; NAS, 1970, 1980; Oser and Hall, 1977, FSC, 1980; FDA, 1982, 1993; WHO, 1987; SCF, 1991; Hallagan and Hall, 1995; Munro et al., 1999).
Exposure or intake of flavoring substances is defined as the amount of substance ingested and is essential to assessing the safety of food ingredients. Quantifying intake of flavoring substances is a daunting task and challenged by many technical and economic difficulties. More than 20,000 different food products are available for consumption in the Western diet (FMI, 1998). These products are occasionally consumed by a large heterogeneous population, which makes it difficult to determine any one individual’s intake of a food constituent. Added to this is the difficulty and expense of obtaining accurate intake data, requiring detailed dietary analysis of a large enough group of people to obtain statistically significant results for the diverse population of eaters.
For more than 40 years, government regulators, scientists, and food industry experts have proposed various methods of estimating exposure to flavoring substances in food. Initially, exposure was calculated using a method called “the possible average daily intake” (PADI), which is based on the levels of flavoring substances added to foods and the amounts of those foods consumed (see below). Unfortunately, this approach fails to incorporate the many complexities associated with human consumption patterns and the food supply, usually resulting in exaggerated overestimates of intake.
The current methods for determining exposure to flavoring substances are the estimated PADI in the U.S. and the theoretical added maximum daily intake (TAMDI) in Europe from use of the substance as a flavoring agent. The PADI is determined by (1) multiplying usual use levels of the substance in each of 33 food categories (e.g., baked goods and meat products) by the average amount of that food category consumed daily and (2) summing the intake over all 33 food categories (USDA/ARS, 1973).
For the vast majority of flavoring agents that have low reported annual volumes of use (Lucas et al., 1999; IOFI, 1995), the PADI is a gross exaggeration of the average daily intake. The PADI calculation assumes that all foods in a food category always contain that substance and that the food category is consumed daily (Oser and Hall, 1977). An example of how this assumption can be problematic is ethyl methylphenylglycidate. Since it is added to impart strawberry flavor to hard candy, the PADI method assumes that all hard candy, including peppermints, cherry-flavored lollipops, and butterscotch, contain ethyl methylphenylglycidate.
These methods for calculating intake do not take into account loss of flavoring substance by processing, cooking, or waste. For example, the majority of allyl disulfide, a volatile disulfide, that is added to garlic breads is lost during the baking process. More than 98% of the flavoring substances are low-molecular-weight compounds (<300 Da), so processing (heating) will lead to substantial loss and concomitant lower levels of intake.
Of the two comprehensive studies of flavor intake undertaken over the past three decades, one involves a detailed dietary analysis (DDA) of a panel of 12,000 consumers, and the other is based on a robust stochastic model (FSM).
The DDA method is based on the detailed reporting of dietary intake of all foods by a panel of consumers over a period of 14 days spread over a year to accommodate seasonal variations in diet (Hall, 1976; Hall and Ford, 1999). Market Research Corp. of America (MRCA) enlisted a diverse panel of 12,000 consumers that came from urban, suburban, and rural communities and ranged in age from infant to over 65 years. The results were statistically analyzed and sorted by age groups and consumption patterns. The participants had more than 4,000 descriptors to choose from for food eaten. Food categories were narrow and highly specific. For example, the baked goods category was divided into 500 subcategories, allowing for garlic bread to not be lumped in with cinnamon coffee cake.
--- PAGE BREAK ---
This study assessed the amount of each specific food eaten, the frequency of consumption of each food, the amount of flavoring agent in each food, and the classification of consumer by age, weight, or other pertinent characteristics. The amount of flavoring agent in foods was difficult to determine, since flavor formulas were proprietary. These levels were assured by a panel of food chemists and flavorists familiar with the flavor substance levels in particular food products. Once all the data were compiled, they were taken through eight steps of analysis (Table 1) to produce average intake levels for both eaters-only groups and non-eaters. Eaters are defined as participants who consumed foods containing specific flavoring substances, and non-eaters are consumers with zero reported intake for a particular flavoring substance.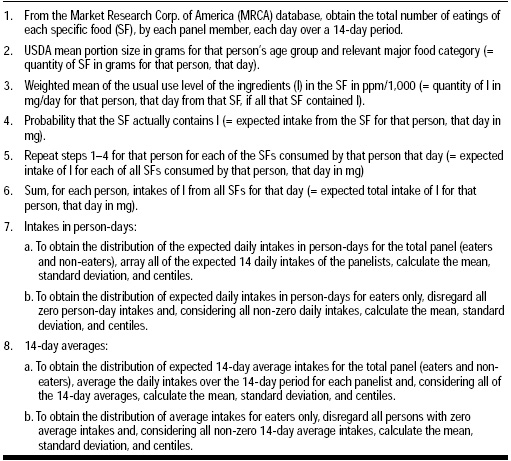
Although this data-intense method is accurate and reliable, it is expensive and time consuming. In the original 1970 survey data gathered on 12 key flavoring substances, two of these substances, cinnamaldehyde ethylene glycol acetal and allyl cyclohexylacetate, exhibited very low intake among the panelists.
Conservative estimates derived from these data are obtained by using the 99th percentile intake levels. In the vast majority of cases, estimates of intake are orders of magnitude lower than those obtained from PADI calculations.
Since DDA methods are economically burdensome, other methodologies to improve the estimation of intake were developed. Based on the results of the DDA study, it was determined that intake could be reliably estimated by applying the per capita intake × 10 method to the annual production volume of flavoring substances (Rulis et al., 1984; Woods and Doull, 1991). This method assumes that only 10% of the population consumes the total annual reported volume of use of a flavor ingredient. This approximation provides a practical and cost-effective approach to the estimation of intake for flavoring substances. The annual volumes of flavoring agents are relatively easy to obtain by industry-wide surveys, which can be performed on a regular basis to account for changes in food trends and flavor consumption. The 1995 poundage survey of U.S. flavor producers was published by FEMA in 1999 (Lucas et al., 1999).
This method can be evaluated by comparison to the data obtained by the DDA method discussed above. Since the dietary analyses were completed in 1970, it is necessary to use poundage information from that time (NAS, 1972). To correct for possible incompleteness in the poundage survey, these data are assumed to be 60% of the flavoring agents (0.6 correction factor in the equation below) actually used. The per capita daily intake (PCI) in micrograms/day is then calculated from the annual volume, in kilograms, for the U.S. population in 1970 (i.e., 210 million) by the following equation:
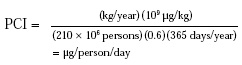
The calculated PCI is then multiplied by 10 to obtain a reasonably conservative estimate for intake by the eaters of the ingredient. The data obtained from PCI × 10 is more conservative than that obtained from the DDA method (see Table 2).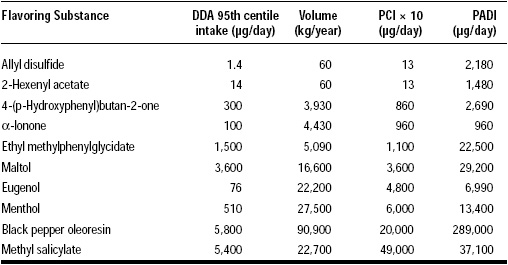
For the 10 substances studied in the panel survey, the PADI is a gross overestimation of the DDA intake. For two high-volume substances, α-ionone and methyl salicylate, PADI gave data comparable to PCI × 10. This demonstrates that PADI is a reasonable model to follow for intake estimation of high-volume substances that are used in many food categories. However, for low-volume substances (i.e., allyl disulfide), it gives an estimation three or four orders of magnitude higher than DDA and three orders of magnitude higher than PCI × 10.
--- PAGE BREAK ---
A second method that has been developed to improve on our understanding and estimation of exposure to flavoring agents is based on the theoretical full stochastic model, FSM (Cadby, 2001; Lambe, 2000). It was developed to assist the European Union in its goal to evaluate 2,800 flavoring agents by 2004. Some European intake estimates are based on the theoretical added maximum daily intake (TAMDI) paradigm, which is based on the same assumptions as the PADI estimate. It reduces levels of incorporation to 31 different categories of food or beverages. Dietary intake data were collected from British males age 16–24 years in the 1988 Dietary and Nutritional Survey of British Adults. These data provided the maximum concentrations, distributions of concentrations, and the maximum probability of encountering each substance in a flavored food or beverage in any one of the 31 different categories. TAMDI produces intake estimates which are on the same order of magnitude as PADI. If the model is refined and full stochastic treatment of the data is performed (see Table 3), the FSM data are lower than TAMDI estimates by three orders of magnitude for the 12 substances studied (Table 4).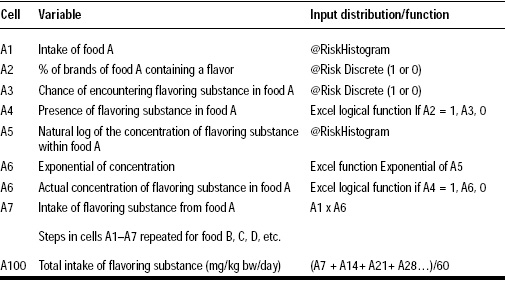
The FSM allows for the complete randomization of conventional intake data and assumes that only a small portion of the population consumes a given flavoring substance at its maximum level on a daily basis. This application of probability to dietary intake provides a more realistic estimation of intake in that it eliminates the exaggeration that the maximum level of added flavoring is consumed daily in each food category. To some extent, even the FSM method overestimates intake in that it does not account for loss due to processing (cooking) or manufacturing waste and the market share of flavored foods in that food category, which has the potential to skew the data depending on the concentration of the substance reported in that food. For example, if a soft drink containing a maximum concentration of isoamyl acetate has a market dominance at the time of data collection, intake for all similar soft drinks will estimate a higher concentration in all cases. If that brand dominance fades, then the familiar scenario of higher intake than manufacture of flavoring agent would occur.
Comparing the FSM and TAMDI methods to the PCI × 10 method (Table 4) reveals that TAMDI, like PADI, overestimates exposure to flavoring agents through food consumption. The PCI × 10 method is a reasonably conservative estimation for safety analysis when compared to the levels of exposure calculated by the FSM. The FSM estimates are comparable to those obtained for 10 different substances by the DDA method with respect to order of magnitude.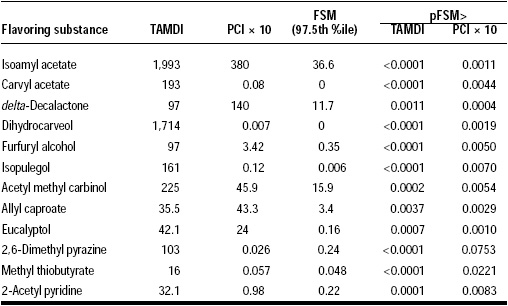
The authors of the FSM study pointed out that the PCI × 10 estimates were a close match to the FSM data, which are lower by one order of magnitude in most cases. The probability (pFSM>) of FSM overestimating either TAMDI or PCI × 10 is very small, as shown in Table 4. This analysis affirms that FSM estimates are in good agreement with PCI × 10 estimates.
An advantage to using PCI × 10 estimations is that the common problem shared by other methods of a decreasing supply of flavoring substance being eclipsed and surpassed by intake estimates based on food categories cannot occur. The exposure to flavoring agents is strictly limited to the volume distributed for the use in food. Industry poundage surveys are regularly updated, as are estimations of the population through census.
It can be concluded that the DDA and FSM approaches offer a more realistic assessment of intake of flavoring substances through consumption of food. The drawback to the DDA method is the cost and time needed to evaluate the data on a fairly regular basis. The FSM approach requires a computer and a fairly extensive food intake survey as well, which is economically challenging. The PCI × 10 method offers a simple calculation based on easily obtained data, and its results are consistent with those provided by the DDA and FSM methods. Therefore, PCI × 10 offers conservative intake estimates and would be easy to implement on a national and global basis. The Expert Panel uses the PCI × 10 method as a satisfactory means of assessing exposure to flavoring substances.
--- PAGE BREAK ---
Safety Assessment of Methyl Eugenol (FEMA 2475)
Methyl eugenol (CAS No. 93-15-2) is 3,4-dimethoxyallylbenzene. It belongs to a group of naturally occurring allylalkoxybenzene derivatives, including estragole and safrole. Methyl eugenol occurs in many foods but is present mainly in spices, including sweet basil, allspice, and nutmeg. It is used as a flavor ingredient in foods up to an average level of 50 ppm. Based on a reported annual volume of 620 kg (Lucas et al., 1999), the estimated per capita intake (“eaters only”) is approximately 0.001 mg/kg of body weight/day from use of methyl eugenol as a flavoring substance.
Groups (50 each) of male and female B6C3F1 mice and F344/N rats were administered 0, 37, 75, or 150 mg of methyl eugenol/kg bw in 0.5% methylcellulose by gavage daily, five days per week for two years (NTP, 2000). On completion of the study, NTP concluded:
“Under the conditions of these 2-year gavage studies, there was clear evidence of carcinogenic activity of methyl eugenol in male and female F344/N rats based on increased incidences of liver neoplasms and neuroendocrine tumors of the glandular stomach in male and female rats and increased incidences of kidney neoplasms, malignant mesotheliomas, mammary gland fibroadenoma, and subcutaneous fibroma and fibroma or fibrosarcoma (combined) in male rats. A marginal increase in squamous cell neoplasms of the forestomach may have been related to methyl eugenol administration in female rats. There was clear evidence of carcinogenic activity of methyl eugenol in male and female B6C3F1 mice based on the increased incidences of liver neoplasms in males and females. Neuroendocrine tumors of the glandular stomach in male mice were also considered related to exposure to methyl eugenol.
“In male and female mice and rats, methyl eugenol administration caused significant increases in nonneoplastic lesions of the liver and glandular stomach.”
High doses of methyl eugenol and structurally related allylalkoxybenzene derivatives (e.g., estragole and safrole) are carcinogenic in rodents. This has been observed in several different studies in mice and rats, newborns and adults. Repetitive intraperitoneal administration for 20 days of high concentrations of safrole, estragole, or methyl eugenol to preweanling or weanling mice induced liver tumors at approximately 10–12 months (Miller et al., 1982, 1983; Borchert et al., 1973; Wiseman et al., 1987). Preweanling animals were more sensitive to tumorigenesis. Similar effects are seen at higher dose levels with methyl eugenol and safrole administered either by gavage or in the diet (NTP, 2000; Hagan et al., 1967; Long and Jenner, 1963). In these studies, evidence of carcinogenicity was concurrent with evidence of chronic hepatotoxicity. The lowest dose of methyl eugenol administered by gavage at which carcinogenicity and hepatotoxicity were reported in rodents in the NTP two-year bioassay was 37 mg/kg/day. In a separate two-year dietary study, safrole was not carcinogenic when administered in the diet at 25 or 5 mg/kg bw/day, although mild hepatotoxicity was reported even at these dose levels (Long and Jenner, 1963). Therefore, no valid study has as yet been performed in the absence of hepatotoxicity using the oral route of exposure.
 Based on the results of these studies, dose-dependent hepatotoxicity induced by methyl eugenol, safrole, and other allylalkoxybenzene derivatives is a necessary step in the formation of hepatic tumors. Daily intakes of methyl eugenol that are carcinogenic in rodents following chronic gavage administration are 1,000-fold higher than the typical dietary intake of methyl eugenol by humans (Lucas et al., 1999; NAS, 1970, 1975, 1981, 1987). Since the amount of methyl eugenol added as a flavoring constituent accounts for approximately 10% of dietary intake, its potential to induce hepatotoxicity is expected to be small, possibly zero. As with all substances administered at high dose in carcinogenic assays, there is uncertainty about the shape of the dose-response curve at low doses that are typical of normal human exposure. This uncertainty is compounded, in the case of methyl eugenol, by the fact that it was administered chronically in the NTP bioassay by gavage, which clearly induced gastric toxicity.
Based on the results of these studies, dose-dependent hepatotoxicity induced by methyl eugenol, safrole, and other allylalkoxybenzene derivatives is a necessary step in the formation of hepatic tumors. Daily intakes of methyl eugenol that are carcinogenic in rodents following chronic gavage administration are 1,000-fold higher than the typical dietary intake of methyl eugenol by humans (Lucas et al., 1999; NAS, 1970, 1975, 1981, 1987). Since the amount of methyl eugenol added as a flavoring constituent accounts for approximately 10% of dietary intake, its potential to induce hepatotoxicity is expected to be small, possibly zero. As with all substances administered at high dose in carcinogenic assays, there is uncertainty about the shape of the dose-response curve at low doses that are typical of normal human exposure. This uncertainty is compounded, in the case of methyl eugenol, by the fact that it was administered chronically in the NTP bioassay by gavage, which clearly induced gastric toxicity.
--- PAGE BREAK ---
Methyl eugenol has been detected in the blood plasma of humans (Barr et al., 2000), indicating that it and other structurally related substances are absorbed from the diet and distributed. It is rapidly metabolized by O-demethylation (Sangster et al., 1983, 1987), epoxidation (Delaforge et al., 1980), and 1'-hydroxylation, with the 1'-hydroxymetabolite being the proximate hepatotoxic and carcinogenic agent (Drinkwater et al., 1976; Solheim and Scheline, 1973; Zangouras et al., 1981). The daily production of 1'-hydroxymetabolite by rodents at high dose levels in chronic studies is orders of magnitude greater than those formed in humans at typical dietary intake. Less than 0.3% of a typical dietary dose of estragole is metabolized and excreted in the urine of humans as the 1'-hydroxymetabolite, while as much as 40% of carcinogenic doses of safrole can be accounted for in the urine of mice as the 1'-hydroxymetabolite. The increase in 1'-hydroxylation has been related to dose-dependent induction of selected CYP-450 isoenzymes (2E1) (Sharma et al., 2001).
Following metabolism, methyl eugenol forms adducts to DNA (Miller et al., 1983; Phillips et al., 1984; Randerath et al., 1984; Wiseman et al., 1987) and protein (Gardner et al., 1997; Sangster et al., 1983, 1987; Zangouras et al., 1981). Dose-dependent protein adducts have been isolated from rats receiving repeated doses of methyl eugenol (Gardner et al., 1997). These have not been chemically characterized. The principal DNA adduct originates from the 1'-hydroxymetabolite through coupling of an allylic carbocation to the exocyclic amino group (N2) of deoxyguanosine residues. DNA adducts have been detected in the livers of mice at doses that induce tumor formation. Comparison of adduct levels induced in preweanling and weanling mice and the kinetics of their disappearance indicate that these adducts are more slowly removed from preweanling animals. This may explain the increased sensitivity of newborns to the carcinogenic effects of high dose levels of methyl eugenol.
Curiously, methyl eugenol is not strongly mutagenic in bacterial or yeast test systems with metabolic activation (Dorange et al., 1977; Mortelmans et al., 1986; Schiestl et al., 1989; Sekizawa and Shibamoto, 1982; To et al., 1982). 1'-Acetoxymethyleugenol, a chemical model for the ultimate activation metabolite, 1'-sulfooxymethyleugenol, is mutagenic in Salmonella, although a nonlinear dose-response was reported (Boberg et al., 1983; Drinkwater et al., 1976; Gardner et al., 1995, 1996; Miller et al., 1982, 1983; Swanson et al., 1981). The mutagenic potencies of the N2-deoxyguanosine adducts of methyl eugenol have not been directly tested in site-specific mutagenesis assays. Structurally analogous DNA adducts formed by reaction of the epoxide of sytrene oxide with the exocyclic amino group of deoxyadenosine are weakly mutagenic in site-specific assays (Latham et al., 1993). Their activities in comparable as-says are approximately 10- to 100-fold lower than that of strongly mutagenic DNA adducts such as O6-methyldeoxyguanosine. It would be highly desirable to quantify, in parallel, the levels of methyleugenol-DNA adducts in the liver as a surrogate for genotoxicity.
In conclusion, the qualitative and quantitative aspects of the molecular disposition of methyl eugenol and estragole and their associated toxicological sequelae have been relatively well defined from mammalian studies. Several studies have clearly established that the profiles of metabolism, metabolic activation, and covalent binding are dose dependent and that their relative importance diminishes markedly at low levels of exposure (i.e., these events are not linear with respect to dose). In particular, rodent studies show that these events are minimal, probably in the dose range of 1–10 mg/kg bw, which is approximately 100–1,000 times the anticipated human exposure to these substances. For these reasons, it is concluded that present exposure to methyl eugenol and estragole resulting from consumption of food, mainly spices and added as such, does not pose a significant cancer risk. Nevertheless, in the interim, further studies are needed to confirm both the nature and implications of the dose-response curve in rats at low levels of exposure to methyl eugenol and estragole.
--- PAGE BREAK ---
Safety Assessment of Citral (FEMA No. 2045)
Citral is an aliphatic terpene aldehyde that occurs naturally in lemons, oranges, tomatoes, and many other fruits. Chemically, it is a mixture of the cis and trans isomers of 3,7-dimethyl-2,6-octadienal (CAS No. 5392-40-5). It is used as a flavor ingredient in foods up to an average level of 200 ppm. Based on a reported annual volume of 53,200 kg (Lucas et al., 1999), the estimated per capita intake (“eaters only”) of citral is 0.092 mg/kg bw/day.
A bioassay on citral was conducted by the Battelle Columbus Laboratory under contract to the National Toxicology Program (NTP, 2001). Groups of 50 F344 rats of both sexes were administered diets containing 0, 1,000, 2,000, or 4,000 ppm of microencapsulated citral for two years. These dietary levels were estimated to provide an average daily intake of 0, 50, 100, or 210 mg/kg. Groups of B6C3F1 mice were administered diets containing 0, 500, 1,000, or 2,000 ppm of citral, estimated to provide average daily intake levels of 0, 60, 120, or 260 mg/kg, for two years. On May 3, 2001, the NTP Board of Scientific Counselors Technical Report Review Subcommittee met for a peer review of the recently issued draft “NTP Technical Report on Citral” (NTP, 2001). The subcommittee concluded:
“Under the conditions of these 2-year feed studies there was no evidence of carcinogenic activity of citral in male or female rats exposed to 1,000, 2,000, or 4,000 ppm. There was no evidence of carcinogenic activity of citral in male B6C3F1 mice exposed to 500, 1,000, or 2,000 ppm. There was equivocal evidence of carcinogenic activity in female B6C3F1 mice based on increased incidences of malignant lymphoma.”
The neoplastic response reported in the NTP study was a dose-related increase in the incidence of lymphoma that was statistically significant in the high dose in B6C3F1 female mice—P = 0.011 by Fisher exact test; 12/50 (24%) at 2,000ppm vs 3/50 (6%) in controls. There was no evidence of increased incidence of malignant lymphoma in either sex of F344/N rats, in male B6C3F1 mice, or in the low-and mid-dose levels in female B6C3F1 mice.
The background incidence of malignant lymphoma in control female B6C3F1 mice maintained on an NTP-2000 diet is high (98/659), with a historical incidence of 14.0% (standard deviation, ±7.1%) and a range of 6–30% (NTP, 2001). The incidence of spontaneous malignant lymphoma in female B6C3F1 mice in all two-year rodent carcinogenicity studies carried out by NTP is also high (20.9%) (Haseman et al., 1998). The historical incidence in controls maintained on the NIH-07 diet at the same contract laboratory performing the citral study was high (167/953), with a historical incidence of 17.5% (standard deviation, 7.7%) and a range of 6–30%.
Therefore, these tumors occur at a high and variable rate in control animals. It is recommended (Haseman et al., 1986) that a compound is anticipated to exhibit a carcinogenic potential if the highest dose is associated with an increased incidence of a common tumor that is significant at the 1% (P <0.01) level, or an increased incidence in a rare tumor at the 5% (P <0.05) level. Therefore, statistical analysis should apply a significance level of 1% (P <0.01) to account for the high background incidence of lymphomas in female B6C3F1 mice. Based on pair-wise comparisons of the incidence of malignant lymphoma in the NTP study by a Fisher exact test, the incidence of this commonly observed neoplasm is not considered to be statistically significant (P = 0.011) for female mice at the 1% level.
Decreased body weights in female mice exposed to 500 (after week 30), 1,000, or 2,000 ppm of citral in the diet also confounded the interpretation of the neoplastic response in female mice. The lack of any significant decrease in feed consumption in these groups suggests that the dose-dependent decrease in body weights is evidence of toxicity.
Based on the high frequency of this neoplastic response in historical controls in NTP studies (Haseman et al., 1998), the fact that toxicity was observed at all dose levels in female B6C3F1 mice, and the observation that the incidences of lymphoma reported in the NTP study were not significant at the 1% level (P <0.01) (Haseman et al., 1986), the FEMA Expert Panel concludes that the results of the NTP bioassay do not provide evidence that citral is a carcinogenic risk to humans. The lack of any evidence of carcinogenicity in both sexes of F344 rats and male B6C3F1 mice support this conclusion.
The FEMA Expert Panel concludes that citral is generally recognized as safe (GRAS) under conditions of intended use as a flavoring substance and that use does not present a carcinogenic hazard to humans.
--- PAGE BREAK ---
Expert Panel Member Changes
In January 2000, Lawrence J. Marnett, Professor of Biochemistry at Vanderbilt University School of Medicine, joined the panel. In December 1999, Paul M. Newberne, Professor Emeritus in the Dept. of Pathology at Boston University School of Medicine and former Co-Chair of the Expert Panel, retired from the panel after a distinguished tenure. John Doull, Professor Emeritus, University of Kansas Medical School, retired from the panel in December 1999 but continues on as a consultant to the panel in key areas of expertise. Ian C. Munro, Consultant, Toxicologist and Principal, Cantox Health Sciences, Inc., retired from the panel in May 2000 but also continues as a consultant to the panel in areas of key expertise.
PREVIOUS FEMA GRAS LISTS PUBLISHED IN FOOD TECHNOLOGY, IN CHRONOLOGICAL ORDER
Hall, R.L. 1960. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. Food Technol. 14: 488.
Hall, L. and Oser, B.L. 1961. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. II. Food Technol. 15(12): 20.
Hall, R.L. and Oser, B.L. 1965. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 3. GRAS substances. Food Technol. 19(2, Part 2): 151-197.
Hall, R.L. and Oser, B.L. 1970. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 4. GRAS substances. Food Technol. 24(5): 25-34.
Oser, B.L. and Hall, R.L. 1972. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 5. GRAS substances. Food Technol. 26(5): 35-42.
Oser, B.L. and Ford, R.A. 1973a. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 6. GRAS substances. Food Technol. 27(1): 64-67.
Oser, B.L. and Ford, R.A. 1973b. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 7. GRAS substances. Food Technol. 27(11): 56-57.
Oser, B.L. and Ford, R.A. 1974. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 8. GRAS substances. Food Technol. 28(9): 76-80.
Oser, B.L. and Ford, R.A. 1975. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 9. GRAS substances. Food Technol. 29(8): 70-72.
Oser, B.L. and Ford, R.A. 1977. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 10. GRAS substances. Food Technol. 31(1): 65-74.
Oser, B.L. and Ford, R.A. 1978. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 11. GRAS substances. Food Technol. 32(2): 60-70.
Oser, B.L. and Ford, R.A. 1979. Recent progress in the consideration consideration of flavoring ingredients under the Food Additives Amendment. 12. GRAS substances. Food Technol. 33(7): 65-73.
Oser, B.L., Ford, R.A., and Bernard, B.K. 1984. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 13. GRAS substances. Food Technol. 38(10): 66-89.
Oser, B.L., Weil, C.L., Woods, L.A., and Bernard, B.K. 1985. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 14. GRAS substances. Food Technol. 39(11): 108-117.
Burdock, G.A., Wagner, B.M., Smith, R.L., Munro, I.C., and Newberne, P.M. 1990. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 15. GRAS substances. Food Technol. 44(2): 78,80, 82, 84, 86.
Smith, R.L. and Ford, R.A. 1993. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 16. GRAS substances. Food Technol. 47(6): 104-117.
Smith, R.L., Newberne, P., Adams, T.B., Ford, R.A., Hallagan, J.B., and the FEMA Expert Panel. 1996a. GRAS flavoring substances 17. Food Technol. 50(10): 72-78, 80-81.
1996b. Correction to GRAS flavoring substances 17. Food Technol. 51(2): 32.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Hallagan, J.B., and Ford, R.A. 1998. GRAS flavoring substances 18. Food Technol. 52(9): 65-66, 68, 70, 72, 74, 76, 79-92.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Hallagan, J.B., and Ford, R.A. 1999. Correction to GRAS flavoring substances 18. Food Technol. 53(3): 104.
Newberne, P., Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Waddell, W.J., Wagner, B.M., Weil, C.S., Adams, T.B., and Hallagan, J.B. 2000. GRAS flavoring substances 19. Food Technol. 54(6): 66, 68-69, 70, 72-74, 76-84.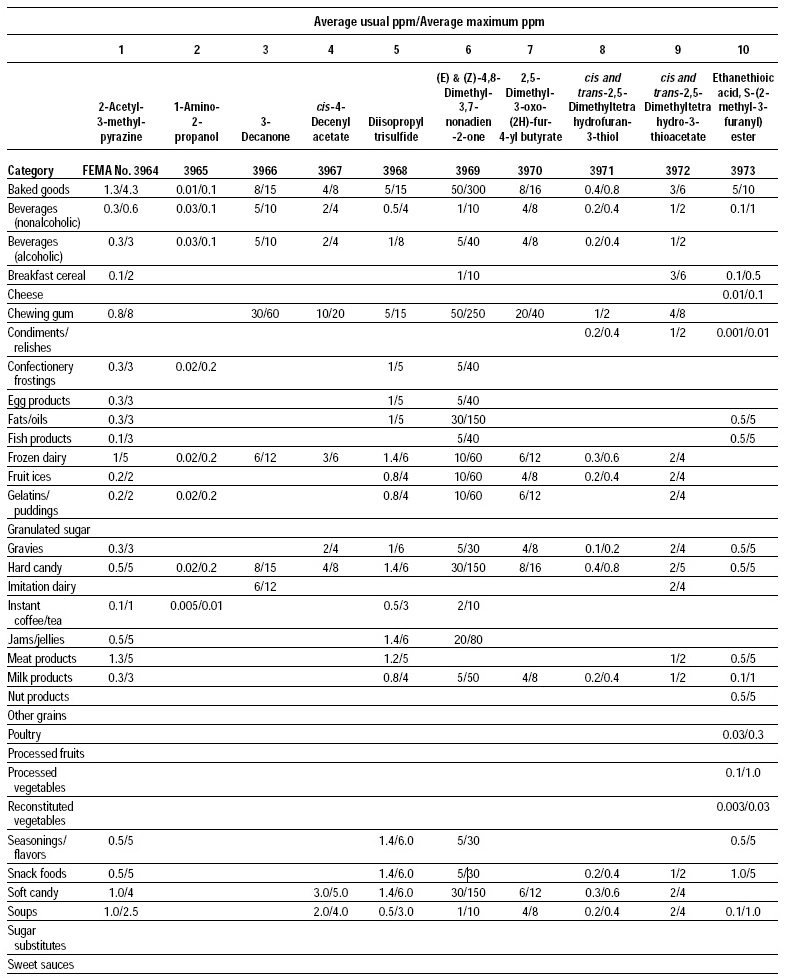
--- PAGE BREAK ---
 by R.L. Smith, J. Doull, V.J. Feron, J.I. Goodman, I.C. Munro, P.M. Newberne, P.S. Portoghese, W.J. Waddell, B.M. Wagner, T.B. Adams, and M.M. McGowen
by R.L. Smith, J. Doull, V.J. Feron, J.I. Goodman, I.C. Munro, P.M. Newberne, P.S. Portoghese, W.J. Waddell, B.M. Wagner, T.B. Adams, and M.M. McGowen
Robert L. Smith, Chairman of the FEMA Expert Panel, is Professor, Molecular Toxicology, Imperial College School of Medicine, University of London, South Kensington, London SW7 2AZ, United Kingdom. Other members of the FEMA Expert Panel are: John Doull, Professor Emeritus, University of Kansas Medical School, Kansas City; Victor J. Feron, TNO Nutrition and Food Research Institute, Professor Emeritus, Utrecht University, The Netherlands; Jay I. Goodman, Professor, Michigan State University, East Lansing; Ian C. Munro, Consultant, Toxicologist and Principal, Cantox Health Sciences, Inc., Mississauga, Ontario, Canada; Paul M. Newberne, former Co-Chairman of the FEMA Expert Panel and now retired, Professor Emeritus, Dept. of Pathology, School of Medicine, Boston University, Boston, Mass.; Philip S. Portoghese, Professor, College of Pharmacy, University of Minnesota, Minneapolis; William J. Waddell, Professor and Chair, Emeritus, Dept. of Pharmacology and Toxicology, University of Louisville School of Medicine, Louisville, Ky.; and Bernard M. Wagner, Emeritus Research Professor of Pathology, New York University Medical Center, New York, N.Y.; Timothy B. Adams is Scientific Director of, and Margaret M. McGowen is associated with, the Flavor and Extract Manufacturers Association, 1620 I St., N.W., Suite 925, Washington, DC 20006. Send reprint requests to author Smith.
Edited by Neil H. Mermelstein
Editor
References
Adams, T.B., Hallagan, J.M., Putnam, Gierke, T.L., Doull, J., Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1996. The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. Food Chem. Toxicol. 34: 763-828.
Adams, T.B., Doull, J., Goodman, J.I., Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1997. The FEMA GRAS assessment of furfural used as a flavor ingredient. Food Chem. Toxicol. 35: 739-751.
Adams, T.B., Greer, D.B., Doull, J., Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1998. The FEMA GRAS assessment of lactones used as a flavor ingredient. Food Chem. Toxicol. 36: 249-278.
Barr, D.B., Barr, J.R., Bailey, S.L., Lapeza, C.R. Jr., Beeson, M.D., Caudill, S.P., Maggio, V.L., Schecter, A., Masten, S.A., Lucier, G.W., Needham, L.L., and Sampson, E.J. 2000. Levels of methyleugenol in a subset of adults in the general U.S. population as determined by high resolution mass spectrometry. Environ. Health Perspectives 108: 323-328.
Boberg, E.W., Miller, E.C., Miller, J.A., Poland, A., and Liem, A. 1983. Strong evidence from studies with brachymorphic mice and pentachlorophenol that 1'-sulfooxysafrole is the major ultimate electrophilic and carcinogenic metabolite of 1'-hydroxysafrole in mouse liver. Cancer Res. 43: 5163-5173.
Borchert, P., Wislocki, P.G., Miller, J.A., and Miller, E.C. 1973. Metabolism of the naturally occurring hepatocarcinogen safrole to l-hydroxysafrole and the electrophilic reactivity of l-acetoxysafrole. Cancer Res. 33: 575-589.
Cadby, P. 2001. Stochastic modeling techniques as a means of verifying the reliability of different methods of estimating extreme consumer exposure to flavoring substances that are intentionally added to foods and beverages in Europe. Personal communication. Firmerich, Geneva, Switzerland.
Degnan, F.H. 1991. Rethinking the applicability and usefulness of the GRAS concept. Food Drug Cosmet. Law J. 46: 553-582.
Delaforge, M., Janiaud, P., Levi, P., and Morizot, J.P. 1980. Biotransformation of allylbenzene analogues in vivo and in vitro through the epoxide-diol pathway. Xenobiotica 10: 737-744.
Dorange J.L., Delaforge, M., Janiaud, P., and Padieu, P. 1977. Mutagenicity of the metabolites of the epoxide diol pathway of safrole and analogs. Study on Salmonella typhimurium. Societe de Biologie de Dijon 171: 1041-1048.
Drinkwater, N.R., Miller, E.C., Miller, J.A., and Pitot, H.C. 1976. Hepatocarcinogenicity of estragole (1-allyl-4-methoxybenzene) and 1'-hydroxyestragole in the mouse and mutagenicity of 1'-acetoestragole in bacteria. J. Natl. Cancer Inst. 57: 1323-1331.
FAA. 1958. Food Additives Amendment to the Federal Food, Drug, and Cosmetic Act. Pub. L. No. 85-929, 72 Stat. 1784 (1958). Codified at 21 USC Sec. 348 (1988).
FDA. 1982. “Toxicological Principles for the Safety Assessment of Direct Food Additives and Color Additives Used in food.” Redbook. Food and Drug Admin., Washington, D.C.
FDA. 1993. “Toxicological Principles for the Safety Assessment of Direct Food Additives and Color Additives Used in food.” Redbook II Draft. Food and Drug Admin., Washington, D.C.
FDA. 1997. Substances generally recognized as safe—Proposal notification. Food and Drug Admin., Fed. Reg. 62: 18938.
FMI. 1998. “The Food Marketing Industry Speaks,” pp. 1-18. Food Mktg. Inst., Washington, D.C.
Gardner, I., Bergin, P., Stening, P., Kenna, J.G., and Caldwell, J. 1995. Protein adducts derived from methyleugenol. ISSX Intl. Mtg., 8: 208.
Gardner, I., Bergin, P., Stening, P., Kenna, J.G., and Caldwell, J. 1996. Immunochemical detection of covalently modified protein adducts in livers of rats treated with methyleugenol. Crit. Rev. Toxicol. 9: 713-721.
Gardner, I.B., Blench, I., Morris, H.R., Caldwell, J., and Kenna, J.G. 1997. Covalent modification of the laminin receptor precursor protein by reactive metabolites of methyleugenol. ISSX Intl. Mtg. 11: 244.
FSC. 1980. Proposed system for food safety assessment. Food Safety Council, Washington, D.C.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I., Taylor, J.M., Long, E.L., Nelson, A.M., and Brouwer, J.B. 1967. Food flavorings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet. Toxicol. 5: 141-157.
Hall, R.L. 1976. Estimating the distribution of daily intakes of certain GRAS substances. Comm. on GRAS list survey—Phase III. Natl. Acad. Of Sciences/Natl. Res. Council, Washington, D.C.
Hall, R.L. and Ford, R.A. 1999. Comparison of two methods to assess the intake of flavoring substances. Food Additives Contaminants 16: 481-495.
Hallagan, J.B. and Hall, R.L. 1995. FEMA-GRAS—A GRAS assessment program for flavor ingredients. Reg. Toxicol. Pharmacol. 21: 422-430.
Haseman, J.K., Winbush, J.S., and O’Donnell, M.W. Jr. 1986. Use of dual control groups to estimate false positive rates in laboratory animal carcinogenicity studies. Fundamental Applied Toxicol. 7: 573-584.
Haseman, J.K., Hailey, J.R., and Morris, R.W. 1998. Spontaneous neoplasm incidences in Fisher 344 rats and B6C3F1 mice in two-year carcinogenicity studies: A National Toxicology Program Update. Toxicol. Pathol. 26: 428-441.
IOFI. 1995. European inquiry on volume use. Personal communication. Intl. Org. of the Flavor Industry, Brussels, Belgium.
JECFA. 1968. Specifications for the identity and purity of food additives and their toxicological evaluation: Some flavoring substances and non-nutritive sweetening agents. 11th Rept. of the Joint FAO/WHO Expert Committee on Food Additives, FAO Nutrition Meetings Rept. Series 44, WHO Technical Rept. Series 383. Food and Agric. Org., Geneva.
JECFA. 1996. Safety evaluation of certain food additives and contaminants. Prepared by 46th Mtg. of the Joint FAO/WHO Expert Committee on Food Additives. World Health Org., Geneva.
JECFA. 1998. Safety evaluation of certain food additives and contaminants. 49th Rept. of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series 35. World Health Org., Geneva.
JECFA. 1999. Safety evaluation of certain food additives and contaminants. Prepared by 51st Mtg. of the Joint FAO/WHO Expert Committee on Food Additives.World Health Org., Geneva.
JECFA. 2000. Safety evaluation of certain food additives and contaminants. Prepared by 53rd Mtg. of the Joint FAO/WHO Expert Committee on Food Additives. World Health Org., Geneva.
Lambe, J. 2000. Application of stochastic modeling to exposure assessments of intentionally added flavoring substances. Personal communication. Inst. of European Food Studies at Trinity College, Dublin, Ireland.
Latham, G.J., Zhou, L., Harris, C.M., Harris, T.M., and Lloyd, R.S. 1993. The replication fate of R- and S-styrene oxide adducts on adenine N6 is dependent on both the chirality of the lesion and local sequence context. J. Biol. Chem. 268: 23427-23434.
Long, E.L. and Jenner, P.M. 1963. Esophageal tumors produced in rats by the feeding of dihydrosafrole. Fed. Proceedings Abstr. 22(2): 275.
Lucas, C.D., Putnam, J.M., and Hallagan, J.B. 1999. 1995 poundage and technical effects update survey. Flavor and Extract Mfrs. Assn. of the United States, Washington, D.C.
Miller, J.A., Miller, E.C., and Phillips, D.H. 1982. The metabolic activation and carcinogenicity of alkenylbenzenes that occur naturally in many spices. Carcinogens Mutagens in Environ. 1: 83-96.
Miller, E.C., Swanson, A.B., Phillips, D.H., Fletcher, T.L., Liem, A., and Miller, J.A. 1983. Structure-activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 43: 1124-1134.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B., and Zeiger, E. 1986. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ. Mutagenesis 8(Suppl.7): 1-119.
Munro, I.C., Shubik, P., and Hall, R. 1998. Principles for the safety evaluation of flavoring substances. Food Chem. Toxicol. 36: 529-540.
Munro, I.C., Kennepohl, E., and Kroes, R. 1999. A procedure for the safety evaluation of flavoring substances. Food Chem. Toxicol. 37: 207-232.
NAS. 1970. “Evaluation of the Safety of Food Chemicals.” Natl. Acad. of Sciences. Natl. Acad. Press, Washington, D.C.
NAS. 1975. “Evaluation of the Safety of Food Chemicals.” Natl. Acad. of Sciences. Natl. Acad. Press, Washington, D.C.
NAS. 1980. “Risk Assessment/Safety Evaluation of Food Chemicals.” Natl. Acad. of Sciences. Natl. Acad. Press, Washington, D.C.
NAS. 1981. “Evaluation of the Safety of Food Chemicals.” Natl. Acad. of Sciences. Natl. Acad. Press, Washington, D.C.
NAS. 1987. “Evaluation of the Safety of Food Chemicals.” Natl. Acad. of Sciences. Natl. Acad. Press, Washington, D.C.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams T.B., Lucas, C.D., and Ford, R.A. 1999. The FEMA GRAS assessment of trans-anethole used as a flavoring substance. Food Chem. Toxicol. 37: 789-811.
NTP. 1998. Technical report on the toxicology and carcinogenesis studies of methyl eugenol in F344/N rats and B6C3F1 mice (gavage studies). Tech. Rept. Series 491. Natl. Toxicology Program, Research Triangle Park, N.C.
NTP. 2000. Toxicology and carcinogenesis studies of methyleugenol (CAS No. 93-15-12) in F344/n rats and B6C3F1 mice (gavage studies). NTP-TR-491; NIH Pub. 98-3950. Natl. Toxicology Program, Research Triangle Park, N.C.
NTP. 2001. Draft report on the toxicology and carcinogenesis studies of citral (microencapsulated) (CAS No. 5392-40-5) in F344/N rats and B6C3F1 mice (feed studies). Tech. Rept. Series 505. Natl. Toxicology Program, Research Triangle Park, N.C.
Oser, B.L. and Hall, R.L. 1977. Criteria employed by the expert panel of FEMA for the GRAS evaluation of flavoring substances. Food Cosmet. Toxicol. 15: 457-466.
Phillips, D.H., Miller, J.A., Miller, E.C., and Adams, B. 1981. Structures of the DNA adducts formed in mouse liver after administration of the proximate hepatocarcinogen 1'-hydroxyestragole. Cancer Res. 41: 176-186.
Phillips, D.H., Reddy, M.V., and Randerath, K. 1984. 32P-Post-labeling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally occurring alkenylbenzenes. II. Newborn male B6C3F1 mice. Carcinogenesis 5: 1623-1628.
Randerath, K., Haglund, R.E., Phillips, D.H., and Reddy, M.V. 1984. 32P-Post-labeling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occuring alkenylbenzenes. I. Adult female CD-1 mice. Carcinogenesis 5: 1613-1622.
Rulis, A.M., Hattan, D.G., and Morgenroth, V.H. 1984. FDA’s priority-based assessment of food additives. I. Preliminary results. Reg. Toxicol. Pharmacol. 26: 44-51.
Sangster, S.A., Caldwell, J., Anthony, A., Hutt, A.J., and Smith, R.L. 1983. The dose dependent metabolism of anethole, estragole and p-propylanisole in relation to their safety evaluation. In “Extrahepatic Drug Metabolism and Chemical Carcinogenesis,” Proc. of Intl. Mtg. on Extrahepatic Drug Metabolism, Stockholm, Sweden, May 17-20, 1983, ed. J. Rydstrom, J. Montelius, and M. Benlytsson, pp. 213-214. Elsevir, Amsterdam and New York.
Sangster, S.A., Caldwell, J., Hutt, A.J., Anthony, A., and Smith, R.L. 1987. The metabolic disposition of [methoxy-14C]-labeled trans-anethole, estragole and p-propylanisole in human volunteers. Xenobiotica 17: 1223-1232.
SCF. 1991. Guidelines for the evaluation of flavorings use in foodstuffs: I. Chemically defined flavoring substances. Scientific Committee for Food, Commission of the European Communities, Brussels, Belgium.
Schiestl, R.H., Chan, W.S., Gietz, R.D., Mehta, R.D., and Hastings, P.J. 1989. Safrole, eugenol and methyleugenol induce intrachromosomal recombination in yeast. Mutation Res. 224: 427-436.
Sekizawa, J. and Shibamoto, T. 1982. Genotoxicity of safrolerelated chemicals in microbial test systems. Mutation Res. 101: 127-140.
Sharma, R.A., Ireson, C.R., Verschoyle, R.D., Hill, K.A., Williams, M.L., Leuratti, C., Manson, M.M., Marnett, L.J., Steward, W.P., and Gescher, A. 2001. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: Relationship with drug levels. Clinical Cancer Res. 7: 1452-1458.
Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Munro, I.C., Newberne, P.M., Portoghese, P.S., Waddell, W.J., Wagner, B.M., and Adams, T.B. 2001a. The FEMA GRAS assessment of pyrazine derivatives used as flavor ingredients. Food Chem. Toxicol. In press.
Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Munro, I.C., Newberne, P.M., Portoghese, P.S., Waddell, W.J., Wagner, B.M., and Adams, T.B. 2001b. Safety assessment of methyl eugenol and estragole as flavoring ingredients. Food Chem. Toxicol. In press.
Solheim, E. and Scheline, RR. 1973. Metabolism of alkylenebenzene derivatives in the rat. II. Eugenol and isoeugenol methyl ethers. Xenobiotica 6: 137-150.
Swanson, A.B., Miller, E.C., and Miller, J.A. 1981. The sidechain epoxidation and hydroxylation of the hepatocarcinogens safrole and estragole and some related compounds by rat and mouse liver microsomes. Bioch. Biophys. Acta 673: 504-516.
To, L.P., Hunt, T.P., and Andersen, M.E. 1982. Mutagenicity of trans-anethole, estragole, eugenol and safrole in the Ames Salmonella typhimurium assay. Bull. Environ. Contam. Toxicol. 28: 647-654.
USDA/ARS. 1972. Food and nutrient intake of individuals in the United States, Spring 1965. Household food consumption survey 1965-66. Rept. 11. U.S. Dept. of Agriculture, Agricultural Research Service. U.S. Govt. Print. Office, Washington, DC.
WHO. 1987. Principles for the safety assessment of food additives and contaminants in food. WHO environmental health criteria. Environmental Health Criteria 70. WHO Intl. Program on Chemical Safety in cooperation with the Joint FAO/WHO Expert Committee on Food Additives (JECFA). World Health Org., Geneva, Switzerland.
Wiseman, R.W., Miller, E.C., Miller, J.A., and Liem, A. 1987. Structure-activity studies of the hepatocarcinogenicities of alkenylbenzene derivatives related to estragole and safrole on administration to preweanling male C57BL/6J × C3H/HeJ F1 mice. Cancer Res. 47: 2275-2283.
Woods, L.A. and Doull, J. 1991. GRAS evaluation of flavoring substances by the Expert Panel of FEMA. Reg. Toxicol. Pharmacol. 14: 48-58.
Zangouras, A., Caldwell, J., Hutt, A.J., and Smith, R.L. 1981. Dose dependent conversion of estragole in the rat and mouse to the carcinogenic metabolite, 1'-hydroxyestragole. Biochem. Pharmacol. 30: 1383-1386.
