Phytosterols and Phytostanols: Functional Food Cholesterol Busters
Growing recognition of their health benefits may lead to a boost in marketing of these cholesterol-lowering functional food ingredients in food products.
Few functional food ingredients have created more recent interest than phytosterols and phytostanols (also called plant sterols and stanols). When consumed, these natural phytonutrients significantly reduce levels of “bad” serum low-density-lipoprotein cholesterol (LDL-C) in humans. Yet in spite of their proven benefit, sales of phytosterol-containing products have not lived up to their promise. That may be changing, however, since the Food and Drug Administration has issued a rare “health claim” for sterol and stanol products. This growing recognition of health benefits and a growing number of unique products in the pipeline may lead to a boost in marketing trends. In fact, sales of a major brand of phytostanol products have been up by about 20% since the FDA announcement (Anonymous, 2000c).

Definition, Structures, and Occurrence
Just as cholesterol plays a critical role in stabilizing our cells’ membranes, so do phytosterols play the same essential role in plants. In animals, cholesterol is the most abundant sterol. In plants, more than 40 sterols have been identified, of which β-sitosterol, stigmasterol, and campesterol are the most abundant. In Western diets, we consume an average of 250 mg of phytosterols per day, largely derived from vegetable oils, cereals, fruits, and vegetables. This is roughly equivalent to the amount of cholesterol consumed (~300 mg/day). These phytosterols and cholesterol are all 4-desmethyl sterols that share identical ring structures. The various sterols only differ in their side chains. Surprisingly, these minor differences result in major changes in their biological function.
A less abundant class of related compounds found in plants are the phytostanols (or stanols). Phytostanols are completely saturated forms of phytosterols and lack the carbon–carbon double bonds found in cholesterol and phytosterols. We typically consume much lower amounts of stanols (~25 mg/day), which are derived primarily from corn, wheat, rye, and rice.
Fig. 1 compares the structures of cholesterol, phytosterols, and phytostanols.
Cholesterol-Lowering Ability
An elevated serum cholesterol level is a well-known risk factor for coronary heart disease. Most strategies for lowering serum cholesterol require unpopular dietary restrictions or the use of drugs. The prospect of lowering cholesterol levels by consuming foods fortified with natural phytonutrients is much more attractive.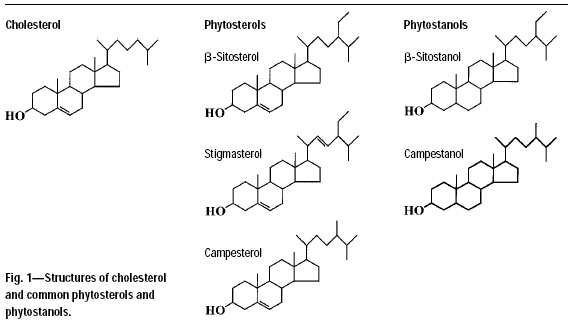
Contrary to popular belief, the use of phytosterols to lower serum cholesterol levels is not a new idea. b-Sitosterol, for instance, has been used since the 1950s (Pollak, 1953) as a supplement and as a drug (Cytellin, marketed by Eli Lilly) to lower serum cholesterol levels in hypercholesterolemic individuals. Because of poor solubility and bioavailability, doses as high as 25 g/day were sometimes required for efficacy. When “statin” drugs became available, the use of these products diminished rapidly. The crystalline nature and poor solubility of free phytosterols also limited their applications in foods. A major breakthrough occurred when scientists at Raisio Group in Finland discovered that phytostanol fatty acyl ester derivatives (stanol esters) could be readily incorporated into fatty foods, such as margarines (Miettinen et al., 1996). These fatty acid esters are thought to be hydrolyzed by intestinal enzymes, yielding the physiologically active free stanol form (Anonymous, 2000a). A recent in-vivo study (Miettinen, et al., 2000) confirmed that stanol esters were hydrolyzed by about 90% after passage through the small intestine.
--- PAGE BREAK ---
More than 20 clinical studies have been carried out on the effects of stanol esters on blood lipids. These studies, which have recently been reviewed (FDA, 2000), have shown that stanol esters lower “bad” LDL-C levels up to about 14% in adult males and females (with or without statin drugs) and children with elevated cholesterol levels. Levels of “healthy” high-density-lipoprotein cholesterol (HDL-C) and triglycerides were unaffected in these studies. A recent study (Blair et al., 2000) confirmed that patients already taking statin drugs could reduce their LDL-C levels by an additional ~10% by inclusion of stanol ester margarines in their diets.
Stanol esters are often derived from “tall oil,” a phytosterol-rich by-product from the pulping of pine and other trees (Miettinen et al., 1996). In that process, tall oil phytosterols are refined and purified, chemically hydrogenated to phytostanols, then esterified with food-grade fatty acids. A similar product can be produced starting with phytosterols derived from vegetable oil refining. Stanols derived from tall oil contain primarily sitostanol and campestanol in a ratio of about 92/8. Stanols derived from soy oil contain a sitostanol/campestanol ratio of about 68/32 (Plat and Mensink, 2000). Both blends conappear to have equal efficacy.
Phytosterol esters (sterol esters) have also been shown to lower serum LDL-C levels in more than 14 efficacy studies (FDA, 2000). Similar to the effect seen with stanol esters, consumption of sterol esters resulted in LDL-C reductions up to ~10% or more, with no change in HDL-C values. Sterol esters are made by esterifying vegetable oil–derived phytosterols with foodgrade fatty acids. Sterol esters are simpler and cheaper to synthesize than stanol esters because no hydrogenation is required. The phytosterol composition of sterol esters depends on their vegetable oil source and is frequently a mixture of b-sitosterol, campesterol, stigmasterol, brassicasterol, fucosterol, and others.
Phytosterols and phytostanols inhibit the uptake of dietary and endogenously produced cholesterol from the gut, causing a decrease in serum cholesterol levels (Ling and Jones, 1995). The exact mechanism (Anonymous, 2000a) by which this occurs is not completely understood, but several theories have been proposed (reviewed in Pollak and Kritchevsky, 1981). One theory suggests that cholesterol in the intestine, already marginally soluble, is precipitated into a nonabsorbable state by the presence of added phytosterols and stanols. A second theory is based on the fact that cholesterol must enter bile-salt and phospholipid-containing “mixed micelles” to be absorbed into the bloodstream. Cholesterol is only marginally soluble in these micelles and is displaced by phytosterols (and stanols), preventing its absorption. Phytosterols, and to a greater extent phytostanols, are very poorly absorbed (Heinemann et al., 1993), and the small amount that is absorbed is actively re-excreted in bile.
--- PAGE BREAK ---
Relative Efficacy of Stanols and Sterols
Until recently, it was commonly thought that stanols were superior to sterols in lowering serum cholesterol levels. These conclusions were drawn from the results of both animal studies (Sugano et al., 1976, 1977; Ikeda et al., 1979; Ntanios and Jones, 1999) and human studies (Becker et al., 1993; Heinemann et al., 1986, 1988; Jones et al., 1997). Because of this evidence, considerable effort and expense were invested into the development of stanol and stanol ester products. Many of the studies cited above, however, were not done with systematic cross-comparison of sterol and stanols in the same subjects.
More recently, three head-to-head comparisons of stanol esters vs sterol esters have been completed (Weststrate and Meijer, 1998; Hallikainen et al., 2000; Jones et. al., 2000). Weststrate and Meijer (1998) first compared the effects of margarines enriched with either vegetable sterol esters or sitostanol esters (at 3.3 g/day) in mildly hypercholesterolemic patients. Surprisingly, the two esters were equally effective in lowering blood total and LDL-C levels. A similar study confirmed these results in a low-fat diet regimen (Hallikainen et al., 2000). Jones et al. (2000) recently reported that sterol esters gave slightly improved efficacy over stanol esters in reducing plasma total and LDL-C levels with dosages of 1.84 g/day in 15 hypercholesterolemic patients on a controlled diet.
A unique recent study with ileostomy patients (Normen et al., 2000) showed that sterol and stanol esters were equally effective at inhibiting the absorption of cholesterol from the intestine. In view of these studies, stanol and sterol esters are currently thought to be roughly equivalent in their cholesterol-lowering efficacy (Law, 2000; Anonymous, 2000a). Several more studies are currently underway, however, and we may not have heard the final word on this subject.
Effects on Health
The effects of sterols and stanols on health have been the subject of various studies.
• Reduction in the Risk of Heart Disease. Consumers are interested in the cholesterol-lowering effects of phytosterols, but they are more interested in how this reduction might lessen their chances of getting heart disease. Weststrate and Meijer (1998) estimated that consumption of 3 g/day of stanols or sterols could reduce the risk of heart disease by 15–40%, depending on age and other dietary factors. A recent review (Law, 2000) indicated that consumption of 2 g/day of sterols or stanols could result in a reduction in the risk of heart disease by about 25%. This effect was considered to be more significant than reducing the intake of saturated fat.
• Toxicology and Estrogenic Potential. It is estimated that more than 2,400 subjects have taken part in clinical studies with phytosterols and stanols with dosages up to 25 g or more per day with no adverse effects noted. The drug Cytellin (primarily β-sitosterol) was prescribed for more than 20 years and had an excellent safety record. Concern had been expressed that plant sterols might have estrogenic effects. A series of studies (reviewed in Anonymous, 2000a) conducted over the past four years have discounted that view. If absorption of phytosterols is of concern, it’s important to note that blood levels of phytosterols and stanols in subjects consuming stanol ester products are much lower than blood levels of phytosterols and stanols in those consuming phytosterol ester products (Weststrate and Meijer, 1998; Hallikainen et al., 2000). Comprehensive studies conducted on phytosterols and stanols (reviewed in Anonymous, 2000a) have consistently demonstrated a lack of toxicity in animal models and humans, except for individuals with the extremely rare genetic condition called phytosterolaemia or sitosterolaemia (Allayee et al., 2000; Berge et al., 2000), and these patients are strongly urged to avoid intake of dietary phytosterols.
--- PAGE BREAK ---
• Absorption of Fat-Soluble Vitamins. Several studies (Gylling et al., 1996: Hallikainen et al., 2000; Hendriks et al., 1999; Weststrate and Meijer, 1998) have shown that consumption of stanol and sterol esters slightly reduces the absorption of β-carotene (pro-vitamin A), lycopene, and α-tocopherol (vitamin E). Law (2000) reviewed these data and stated that plant sterols and stanols lower blood concentrations of β-carotene by about 25%, concentrations of α-carotene by 10%, and concentrations of vitamin E by 8%. However, a key role for these vitamins is to protect LDL-C from oxidation. Since sterols and stanols reduce the amount of LDL-C, it may be appropriate to normalize the blood concentrations of these vitamins with respect to the lower LDL-C concentrations. With this adjustment, stanols and sterols did not significantly lower blood concentration of vitamin E, but concentrations of β-carotene were reduced by 8–19%. In view of these results, Hendriks et al. (1999) suggested limiting daily dosage of sterol esters to about 1.6 g/day, a dosage that gave good LDL-C reductions without seriously affecting plasma carotenoid concentrations.
Existing Products
Stanol and sterol ester products entered the United States market in 1999 after considerable regulatory discussions and delay. McNeil Consumer Healthcare (with North American marketing rights to Raisio’s Benecol™) intended to launch Benecol in fall 1998 as a dietary supplement which, under the Dietary Supplement Health and Education Act (DSHEA) is exempt from many FDA regulations. FDA, however, considered Benecol to be a food or food ingredient and threatened to seize any products McNeil planned to launch in a test market in Portland, Ore. Eventually, McNeil agreed to market Benecol as a food, but that required either a new food additive petition, or recognition of Generally Recognized As Safe (GRAS) status.
In 1999, a panel of independent experts in the U.S. concluded that plant sterol esters were GRAS for use as an ingredient in vegetable oil–based spreads in amounts not to exceed 20%. Based on this GRAS recognition, FDA approved spreads containing up to 20% of plant sterol and stanol esters. Benecol (Fig. 2), containing stanol esters, was finally launched in May 1999. 
In 1999, Lipton and its parent company, Unilever, also launched its product, Take ControlTM (Fig. 3) which contained vegetable oil–derived sterol esters. Because Take Control’s active ingredient, sterol esters, was simpler and less expensive to prepare (no hydrogenation required), Lipton’s product could be sold at a lower price than Benecol. This initially didn’t seem to be a major advantage, since sterol esters were considered by many to be inferior in efficacy to stanol esters. However, with the growing evidence that sterol and stanol esters are equivalent in reducing serum cholesterol levels, Take Control may begin to reap the benefits of its lower price. 
Despite the obvious benefits of including phytosterols in the diet, sales of Benecol and Take Control have been disappointing. U.S. retail sales for Benecol between May 1999 and August 2000 were reported to be only $42 million (Anonymous, 2000b). It was estimated that in 1999 McNeil spent $49 million on advertising and had only $17 million in sales (Schmitt and Walsh, 2000). This caused McNeil to modify its marketing strategy by mounting a large-scale information campaign about Benecol’s health merits with U.S. doctors. Unilever, on the other hand, reportedly spent $15 million for advertising and had $13 million in sales for Take Control in 1999 (Schmitt and Walsh, 2000). As of January 2000, Benecol had 1.2% of the U.S. margarine market, and Take Control held 1.6%. Sluggish sales in 2000 have resulted in a current oversupply of sterols and stanols and the resulting suspension of two sterol production ventures by Raisio. An analysis of the present market for sterol and stanols was recently published (Challener, 2000).
--- PAGE BREAK ---
New and Second-Generation Products
In addition to existing products on the market, a number of other products are being developed or are entering the market.
• Phytrol (Fig. 4). In October 2000, Forbes Medi-Tech Inc. announced the first consumer-product test market of its phytosterol-based cholesterol-lowering ingredient, Phytrol™ (unesterified tall oil phytosterols), in Australia and the U.S. Phytrol is exclusively licensed worldwide to Novartis Consumer Health, Inc., and in 2000 Novartis announced a joint venture with Quaker Oats to form Altus Foods, which will manufacture “healthy foods” containing Phytrol. The successful marketing of a free phytosterol product would be quite an accomplishment in view of previously published evidence that free sterols were difficult to formulate and that their poor bioavailability necessitated large dosages for significant reductions in serum LDL-C levels. 
Jones et al. (1999) fed 1.7 g/day of purified tall oil phytosterols (similar to Phytrol) suspended in pre-warmed margarine to 32 hypercholesterolemic men. After 30 days of treatment, LDL-C levels dropped about 15% compared to the test diet. From a marketing point of view, this information is significant, since, theoretically at least, free phytosterols should be less expensive to market than either sterol or stanol esters. Forbes also claims that Phytrol (consumer branded as Reducol™) can be used in a wide variety of food products besides fatty foods. If this is confirmed, it could also help marketing efforts for Phytrol.

 • Corn Fiber Oil. A unique oil was discovered in corn fiber (a fiber-rich by-product from corn wet-milling) by a team of scientists (Figs. 5 and 6) with USDA’s Agricultural Research Service (Moreau et al.,1996) and proposed as a natural cholesterol-lowering oil. Corn fiber oil, called Amaizing Oil, was shown to lower serum cholesterol levels in an animal model at the University of Massachusetts and was then patented (Moreau et al., 1998) as a joint invention of ARS and the university. Additional animal studies (Ramjiganesh et al.,2000; Wilson et al., 2000) have since confirmed the cholesterol-lowering effects.
• Corn Fiber Oil. A unique oil was discovered in corn fiber (a fiber-rich by-product from corn wet-milling) by a team of scientists (Figs. 5 and 6) with USDA’s Agricultural Research Service (Moreau et al.,1996) and proposed as a natural cholesterol-lowering oil. Corn fiber oil, called Amaizing Oil, was shown to lower serum cholesterol levels in an animal model at the University of Massachusetts and was then patented (Moreau et al., 1998) as a joint invention of ARS and the university. Additional animal studies (Ramjiganesh et al.,2000; Wilson et al., 2000) have since confirmed the cholesterol-lowering effects.
Corn fiber oil has several unique components (Fig. 7) and functional properties not found in any current commercial phytosterol product. Unlike phytosterols in soy or tall oil, most of the phytosterols in corn fiber oil are naturally esterified with either fatty acids or phenolic acids, such as ferulic acid, a powerful antioxidant (Moreau et al., 1998). urthermore, corn fiber oil contains a high level of sitostanol in the ferulic acid ester fraction. In fact, corn fiber oil appears to be the richest source of natural stanols (and stanol esters) ever reported.Corn fiber oil also contains g-tocopherol and carotenoids, both with important antioxidant properties. 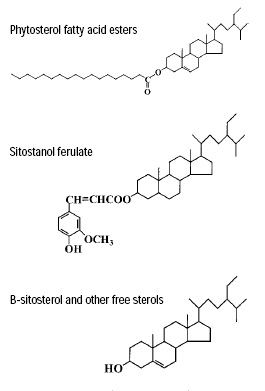
--- PAGE BREAK ---
The levels of total phytosterols in corn fiber oil range from about 15% to >50%, depending on extraction and fiber pretreatment conditions (Moreau et al., 1996). Levels of γ-tocopherol also vary with fiber pretreatment conditions (Moreau et al., 1999) and range from about 0.3% to 3%. Corn fiber oil’s combination of natural cholesterol-lowering components and antioxidants, which could potentially prevent oxidation of LDL-cholesterol, could give a “one-two punch” in the fight against heart disease. The ARS scientists have worked with several major U.S. corporations to move the oil toward commercial products, which could include spreads, chocolates, dairy products, cooking oils, and other products with health-promoting properties. An exclusive license for manufacturing and use of the oil has not yet been granted but could be in the next few months.
• Cooking Oil. Procter & Gamble has introduced a new line of phytosterol-containing cooking oils under the brand name, CookSmartTM. These oils contain soy phytosterol esters and can be purchased through the company’s Web site. P&G is the first company to market a phytosterol-containing cooking oil, which conceivably could add cholesterol-lowering phytosterols to fried foods such as French fries.
• Other “Designer” Oils. Forbes Medi-Tech Inc. announced in December 2000 its development of a “designer oil” that reduces LDL-C and increases energy expenditure, which may prevent people from gaining weight. The research was conducted at McGill University between October 1999 and May 2000. The oil contains Forbes’ phytosterol-based ingredient, Phytrol, which is incorporated into the oil by a proprietary process that preserves clarity of the oil.
• Getting the Fat Out. Some consumer resistance to current commercial products stems from the high fat content of the spreads and margarines in which they are formulated. Recent research shows that phytosterols delivered in a low-fat spread are as effective as those in higher-fat formulations (Hallikainen and Uusitupa,1999). Low-fat spreads containing phytosterols are now commercially available.
Another approach to delivering phytosterols in a nonfat form is taken by Traco Labs with its commercial product, Cholestatin, which contains a mixture of free phytosterols in a capsular form containing no added fat. Additional research studies need to be done to confirm that phytosterols taken as dietary supplements in capsular form are as effective as those consumed in spreads.
• Formulations that Expand Product Usage. There is a demand for phytosterol formulations that could be included in beverages, dairy drinks, and nonfat foods. ADM has developed a patent-pending phytosterol formulation that allows introduction of sterols in a dispersible form in an aqueous application (Spizzirri, 2000).
• Formulations that Enhance the Effects of Phytosterols. Monsanto recently received a patent on a “phytosterol protein complex” composed of phytosterols, proteins, and edible oil (Corliss et al., 2000). The complex is said to “increase the bioavailability of phytosterols.” Monsanto says that “It is most preferred to extract the phytosterols from corn fiber oil.”
Lecithin also plays a valuable role in increasing the bioavailability of free phytosterols. Ostlund et al. (1999) showed that 1 g of sitostanol powder reduced cholesterol absorption in human subjects by about 11%. In contrast, only 300 mg of sitostanol administered in lecithin micelles was required to reduce cholesterol absorption by 34%.
--- PAGE BREAK ---
Impact of the New Health Claim Phytosterol-containing products had a stormy introduction into the U.S. market because of the dispute about whether they were dietary supplements or foods. Resolving the dispute cost manufacturers considerable time and market revenue. Recently, FDA issued a rare interim final rule for allowing a health claim for reducing the risk of coronary heart disease for foods containing plant stanol and sterol esters (FDA, 2000), as long as the foods are low in saturated fat and low in cholesterol. This is only the 12th time that FDA has allowed a health claim. Having such a powerful endorsement by FDA may now boost the awareness and consumption of phytosterol-containing products on the market. The claim includes stanol esters in spreads, salad dressings, snack bars, and dietary supplements (soft gels). It includes sterol esters in spreads and salad dressings only.
To qualify for the health claim, a product must also contain no more than 13 g of total fat per serving or per 50 g. Spreads and salad dressings, however, are exempt from this requirement. Foods must contain at least 0.65 g of plant sterol ester or 1.7 g of plant stanol ester per serving, and at least two servings must be eaten at different times of the day for a total consumption of 1.3 and 3.4 g/day of sterol and stanol esters, respectively. The requirement for more than twice the amount of stanol esters than sterol esters seems contradictory to the preponderance of the scientific literature that indicates similar efficacy for both chemical species. The ruling was based in part because some studies on stanol esters showed lower than expected cholesterol reductions. Those studies, however, may have been confounded by problems with the formulations that were used or by factors related to the type of study performed. FDA allowed a 75-day period for comments on the interim rule. It is likely that the dosage issue as well as the list of covered food products will be reevaluated in the final rule, which should be issued by FDA sometime in 2001.
Once, twice, or three times a day? Another issue that likely may be addressed in the FDA comment period is the number of servings per day required for effect. While most studies to date have indicated better effect from consuming stanols and sterols more than two or three times per day, a new study (Plat et al., 2000) indicates that the cholesterol-lowering effect of plant stanols appears to be independent of whether they are consumed once per day or divided over three meals.
This finding is very significant from at least two points of view. First, most Western consumers’ lifestyles do not permit the incorporation of phytosterols into foods in two or three meals per day. Being able to benefit from consumption of a phytosterol-enriched food at only one meal greatly increases the chances of consumers’ “buying in” to the use of these functional foods.
The other significant aspect of these findings is that it means we need to reexamine the accepted mechanisms by which phytosterols reduce serum cholesterol levels. The study suggests that plant stanols remain either in the intestinal lumen or possibly in or associated with enterocytes. Furthermore, the researchers hypothesized that plant stanols have effects on lipoprotein metabolism beyond their effect on the micellar solubility of cholesterol. This is an unexpected finding, but if history tells us anything about phytosterols, it is to expect the unexpected.
The authors dedicate this article to the memory of Robert A. Norton.
Kevin B. Hicks and Robert A. Moreau
The authors are, respectively, Research Leader and Research Chemist, Eastern Regional Research Center, Agricultural Research Service, U.S. Dept. of Agriculture, 600 E. Mermaid Ln., Wyndmoor, PA 19038. Send reprint requests to author Hicks.
Edited by Neil H. Mermelstein
Senior Editor
References
Allayee, H., Laffitte, B., and Lusis, A. 2000. An absorbing study of cholesterol. Science 290: 1709-1711.
Anonymous. 2000a. Phytosterol esters (plant sterol and stanol esters). IFST: Current Hot Topics. www.ifst.org/hottop29.htm.
Anonymous. 2000b. Raisio’s Benecol U.S. sales below expectations. Johnson & Johnson Unit, McNeil. AFX Europe, Aug.21.
Anonymous. 2000c. Benecol sales in U.S. up pct. on FDA clearance of health claims. AFX Europe, Dec 14.
Becker, M., Staab, D., and von Bergmann K. 1993. Treatment of severe familial hypercholesterolemia in childhood with sitosterol and sitostanol. J. Pediatr. 122: 292-296.
Berge, K., Tian, H., Graf, G., Yu, L., Grishin, N., Schultz, J., Kwiterovich, P., Shan, B., Barnes, R., and Hobbs, H. 2000. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290: 1771-1775.
Blair, S., Capuzzi, D., Gottlieb, S., Nguyen, T., Morgan, J., and Cater, N. 2000. Incremental reduction of serum total cholesterol and low-density lipoprotein cholesterol with the addition of plant stanol ester-containing spread to statin therapy. Am.J. Cardiol. 86: 46-52.
Challener, C. 2000. Health claim may bolster plant stanol and sterol esters. Chem. Mktg. Reporter, Oct. 9.
Corliss, G., Finley, Basu, H., Kincs, F., and Howard, L. 2000. Phytosterol protein complex. U.S. patent 6,113,972.
FDA. 2000. Food labeling: Health claims; Plant sterol/stanol esters and coronary heart disease. Food and Drug Admin., Fed. Reg. 65: 54686-54739. www.access.gpo.gov/su_docs/fedreg/a000908c.html.
Gylling, H.K., Puska, P., Vartianen, E., and Miettinen, T.A. 1996. Serum retinol, -tocopherol, carotenes, and lipid peroxide production during serum cholesterol lowering by sitostanol ester margarine in a mildly hypercholesterolemic population. Circulation 94: Suppl. I-578, abstract 3379.
Hallikainen, M.A. and Uusitupa, M.I.J. 1999. Effects of 2 lowfat stanol ester-containing margarines on serum cholesterol concentrations as part of a low-fat diet in hypercholesterolemic subjects. Am. J. Clin. Nutr. 69: 403-410.
Hallilainen, M.A., Sarkkinen, E.S., Gylling, H., Erkkila, A.T., and Uusitupa, M.I.J. 2000. Eur. J. Clin. Nutr. 54: 715-725.
Heinemann, T., Leiss, O., and von Bergmann, K. 1986. Effect of low dose sitostanol on serum cholesterol in patients with hypercholesterolemia. Atherosclerosis 61: 219-223.
Heinemann, T., Pietruck, B., Kullak-Ublick, G., and von Bergmann, K. 1988. Comparison of sitosterol and sitostanol on inhibition of intestinal cholesterol absorption. Agents Actions 26: S117-S122.
Heinemann, T, Axtmann, G., and Von Bergmann, K. 1993. Comparison of intestinal absorption of cholesterol with different plant sterols in man. Eur. J. Clin. Invest. 23: 827-831.
Hendriks, H.F.J., Weststrate, J.A., van Vliet, T., and Meijer, G.W. 1999. Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur. J. Clin. Nutr. 53: 319-327.
Ikeda, I., Morioka, H., and Sugano, M. 1979. The effect of dietary alpha-sitosterol and alpha-sitostanol on the metabolism of cholesterol in rats. Agric. Biol. Chem. 43: 1927-1933.
Jones, P.J.H., MacDougall, D.E., Ntanios, F.Y., and Vanstone, C.A.. 1997. dietary phytosterols as cholesterol-lowering agents in humans. Can. J. Physiol. Pharm. 75: 217-227.
Jones, P., Ntanios, F., Raeini-Sarjaz, M., and Vanstone, C. 1999. Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic men. Am. J. Clin. Nutr. 69: 1144-50.
Jones, P.J., Raeini-Sarjaz, M., Ntanios, F.Y., Vanstone, C.A., Feng, J.Y., and Parsons, W.E. 2000. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J. Lipid Res. 41: 697-705.
Law, M. 2000. Plant sterol and stanol margarines and health. Brit. Med. J. 320: 861-864.
Ling, W.H. and Jones, P.J.H. 1995. Minireview of dietary phytosterols: A review of metabolism, benefits, and side effects. Life Sci. 57: 195-206.
Miettinen, T, Vanhanen, H., and Wester, I. 1996. Use of a stanol fatty acid ester for reducing serum cholesterol level. U.S. patent 5,502,045.
Miettinen, T., Vuoristo, M., Nissinen, M., Jarvinen, H., and Gylling, H. 2000. Serum, biliary, and fecal cholesterol and plant sterols in colectomized patients before and during consumption of stanol ester margarine. Am. J.Clin Nutr. 71: 1095-1102.
Moreau, R.A., Powell, M.J., and Hicks, K.B. 1996. Extraction and quantitative analysis of oil from commercial corn fiber. J. Agric. Food Chem. 44: 2149-2154.
Moreau, R.A. , Hicks, K. B., Nicolosi, R.A., and Norton, R.A. 1998. Corn fiber oil, Its preparation and use. U.S. patent 5,843,499.
Moreau, R.A., Hicks, K.B., and Powell, M.J. 1999. Effect of heat pretreatment on the yield and composition of oil extracted from corn fiber. J. Agric. Food Chem. 47: 2869-2871.
Normen, L., Dutta, P., Lia, A., and Andersson, H., 2000, Soy sterol esters and beta-sitosterol ester as inhibitors of cholesterol absorption in human small bowel. Am. J. Clin. Nutr. 71: 908-913.
Ntanios, F.Y. and Jones, P.J.H. 1999. Dietary sitostanol reciprocally influences cholesterol absorption and biosynthesis in hamsters and rabbits. Atherosclerosis 143: 341-351.
Ostlund, R., Spilburg, C., and Stenson, W. 1999. Sitostanol administered in lecithin micelles potently reduces cholesterol absorption in humans. Am. J. Clin Nutr. 70: 826-831.
Plat, J. and Mensink, R.P. 2000. Vegetable oil based versus wood based stanol ester mixtures: Effects on serum lipids and hemostatic factors in non-hypercholesterolemic subjects. Artherosclerosis 148: 101-112.
Plat, J., van Onselen, E., van Heugten, M., and Mensink, R. 2000. Effects on serum lipids, lipoproteins and fat soluble antioxidant concentrations on consumption frequency of margarines and shortenings enriched with plant stanol esters. Eur. J. Clin. Nutr. 54: 671-677.
Pollak, O.J. 1953. Reduction of blood cholesterol in man. Circulation 7: 702-706
Pollak, O.J. and Kritchevsky, D. 1981. Sitosterol. In “Monographs on Atherosclerosis,” ed. T.B. Clarkson, D. Kritchevsky, and O.J. Pollak, Vol. 10, pp. 139-157. S. Karger, New York.
Ramjiganesh, T., Roy, S., Nicolosi, R., Young, T., McIntyre, J., and Fernandez, M. 2000. Corn husk oil lowers plasma LDL cholesterol concentrations by decreasing cholesterol adsorption and altering hepatic cholesterol metabolism in guinea pigs. J. Nutr. Biochem. 11: 358-366.
Schmitt, W. and Walsh, K. 2000. Slim pickings for cholesterol reducers. Chem. Week, Jan. 19, p. 38.
Spizzirri, J. 2000. The phytosterol story. Food Prod. Design, Sept., pp. 65-71.
Sugano, M., Kamo, F., Ikeda, I., and Morioka, H. 1976. Lipid-lowering activity of phytostanols in rats. Atherosclerosis 24: 301-309.
Sugano, M., Morioka, H., and Ikeda, I. 1977. A comparison of hypocholesterolemic activity of beta-sitosterol and betasitostanol in rats. J. Nutr. 107: 2011-2019.
Weststrate, J. and Meijer, G. 1998. Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur. J. Clin. Nutr. 52: 334-343.
Wilson, T., DeSimone, A., Romano, C., and Nicolosi, R. 2000. Corn fiber oil lowers plasma cholesterol levels and increases cholesterol excretion greater than corn oil and similar to diets containing soy sterols and soy stanols in hamsters. J. Nutr. Biochem. 11: 443-449.
