Preventing Degenerative Diseases by Anti-Angiogenic Functional Foods
Biologically active compounds naturally occurring in foods can inhibit angiogenesis,a key step in the development of degenerative diseases, and have the potential for inclusion in functional foods.
The concept that “food is medicine” is common to many cultures. Minora Shirota, the founder of Yakult Honsha, in Japan, had a straightforward philosophy: “Prevent disease rather than treat disease; a healthy intestine leads to a long life; and deliver health benefits to as many people as possible at an affordable price” (Heasman and Mellentin, 2001). This philosophy, elaborated almost half a century ago, is becoming more valid now than ever before. At the present level of scientific knowledge, the best strategy to cure chronic degenerative diseases is to prevent them.
Inflammation, basement membrane degradation, endothelial cell proliferation and migration, angiogenesis or neovascularization, metastasis, huge healthcare costs, and painful death are the hallmarks of degenerative diseases such as arthritis, coronary heart disease, stroke, diabetes, osteoporosis, age-related macular degeneration, and cancer. Most chronic diseases are the result of disrupted homeostatic balance between enzymes and their inhibitors, developing over a period of many years, with different enzymes being associated at different steps of disease onset and/or progression.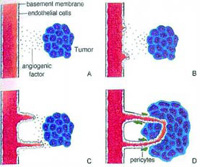
Our current understanding of the mechanism of degenerative disease progression has developed to a stage where the process of angiogenesis—the formation of new blood vessels from preexisting vascular ones—is fast becoming a target for prevention and possibly for therapy. This article presents an overview of the anti-angiogenic properties of naturally occurring physiologically active compounds in foods—phenolics, carotenoids, proteins, lipids, carbohydrates, saponins, acids, and amino acids—and the prospect of their use in anti-angiogenic functional foods.
 Angiogenesis and Its Inhibition
Angiogenesis and Its Inhibition
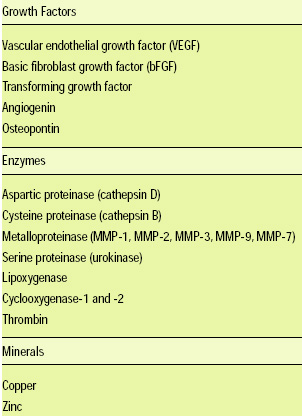
The process of angiogenesis is shown in Fig. 1, and the stimulators of angiogenesis are reported in Table 1. Angiogenesis is important in both normal and pathological processes.
In a normal healthy body, angiogenesis is involved in body development and wound healing and is tightly regulated by natural angiogenesis inhibitors. Examples of these inhibitors include tissue inhibitor of metalloproteinases (TIMPs), α2-macroglobulin, αi-anti-trypsin, lysozyme, and immunoglobulins.
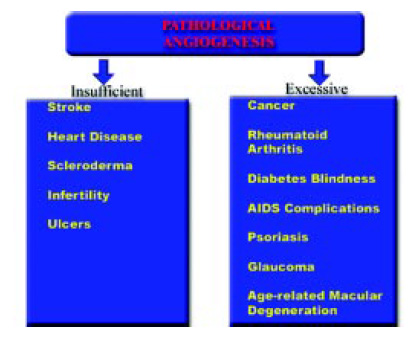 In many chronic pathological processes, such as arthritis and inflammatory bowel diseases, the body loses control over angiogenesis, and new blood vessels grow either excessively or insufficiently, with consequences shown in Fig. 2. Excessive serine, cysteine, and metalloproteinase activities have been linked to the onset and progression of pathological angiogenesis. Vascular endothelial growth factor (VEGF) has been recognized as a key mediator of angiogenesis.
In many chronic pathological processes, such as arthritis and inflammatory bowel diseases, the body loses control over angiogenesis, and new blood vessels grow either excessively or insufficiently, with consequences shown in Fig. 2. Excessive serine, cysteine, and metalloproteinase activities have been linked to the onset and progression of pathological angiogenesis. Vascular endothelial growth factor (VEGF) has been recognized as a key mediator of angiogenesis.
 Clinically, therapeutic alternatives to suppress excessive angiogenesis involve the use of anti-angiogenic compounds aimed at halting new blood vessel growth. Conversely, stimulation of new blood vessel growth with growth factors is being considered as a therapeutic approach for insufficient angiogenesis. Table 2 describes strategies to inhibit angiogenesis (Folkman and Ingber, 1992).
Clinically, therapeutic alternatives to suppress excessive angiogenesis involve the use of anti-angiogenic compounds aimed at halting new blood vessel growth. Conversely, stimulation of new blood vessel growth with growth factors is being considered as a therapeutic approach for insufficient angiogenesis. Table 2 describes strategies to inhibit angiogenesis (Folkman and Ingber, 1992).
--- PAGE BREAK ---
The rationale of ingesting anti-angiogenic compounds as food or food ingredients is that (1) potent naturally occurring anti-angiogenic compounds are present in most raw food; (2) current level of knowledge has established that angiogenesis can be overcome with a systemic nontoxic low dose of angiogenesis inhibitors over a long period of time; (3) foods would be an inexpensive way to deliver low doses of anti-angiogenic compounds over the lifespan of an individual; (4) medical intervention is always costly once a degenerative disease has erupted and current therapies are not totally efficient; (5) most people do not have the discipline and perseverance to take pills over a sustained period of time; and (6) epidemiological studies have suggested that people who regularly consume anti-angiogenic compounds in their diet have a reduced rate of some degenerative diseases that strike later in life.
Compounds that Inhibit Angiogenesis
Anti-angiogenic functional foods are foods or food ingredients that exert their disease-preventive activity, at least in part, by inhibiting angiogenesis (growth of new blood cells). The following are key examples of biologically active compounds that are naturally occurring in foods and have demonstrated angiogenesis inhibitory activity.
• Stilbenes and Polyphenols. Epidemiologic observations have associated the presence of stilbenes and polyphenols in the diet to low rate of chronic diseases. The beneficial properties of polyphenols and stilbenes are well documented and include inhibition of mutagenesis and tumor growth.
Resveratrol (3,5,4'-trihydroxystilbene), a secondary metabolite produced by plants as a defense mechanism against Botrytis cinerea, Plasmopara viticola, and predators or as a response to ultraviolet irradiation, inactivates cyclooxygenase-1 and -2 (COX-1 and -2), two enzymes heavily involved in inflammation and angiogenesis. Micromolar concentrations of resveratrol alone or in combination with quercetin inhibited angiogeneisis in vitro (Igura et al., 2001). Resveratol is bioavailable.
Epigallocatechin-3-gallate (EGCG) from green tea, at concentrations found in the plasma of moderate green tea drinkers, can inhibit endothelial cell growth in vitro, urokinase, metalloproteinases (MMP-2 and MMP-9), inflammation, and angiogenesis in vivo in animal models of human carcinogenesis (Garbisa et al., 2001).
Human trials involving the preventive effect of green tea on oral and prostate cancer are underway in clinics around the world. The potency of EGCG as an antioxidant is 25 and 100 times that of vitamins E and C, respectively (Webb, 2000). Epicatechin gallate was reported to be the best antimetastatic polyphenol devoid of cytotoxicity (Garbisa et al., 2001).
Human colonic microflora, under anaerobic conditions, are capable of degrading some polymeric tannins into phenolic acids. Phenylacetic acid and phenylbutyric acids are bioavailable and can inhibit cancer cells growth (Thibout et al., 1999). Cyanidin-3-glucosylrutinoside I and cyanidin-3-rutinoside 2 from raspberries and sweet cherries inactivate COX-1 and -2 (Seeram et al., 2001).
• Isoflavones. Genistein can down-regulate MMP-9 activity and up-regulate the activity of TIMP-MMP-1 (Shao et al., 1998). Increased activity of MMP-9 and decreased concentration of TIMP correlates with increased destruction of the basement membrane and angiogenesis. Genistein, alone at the nanogram level or in combination with luteolin, inhibited corneal angiogenesis without side effect (Joussen et al., 2000). It has been used in the treatment of acute childhood lymphoblastic leukemia, adult chronic lymphocytic leukemia, and pancreatic tumor. Other structurally related phytoestrogens with higher potency against angiogenesis include fisetin, apigenin, 3-hydroxyflavone, 3',4'-dihydroxyflavone, and 2',3'-dihydroxyflavone (Fotsis et al., 1998) Kaempferol, apigenin, and genistein are inhibitors of topoisomerases I and II, two crucial catalysts of cell proliferation.
Daidzein in a mixture of soy isoflavones (genistein, genistin, biochanin A, and daidzein) dose-dependently reduced angiogenesis in mice bearing murine or human bladder carcinoma cells (Zhou et al., 1998).
--- PAGE BREAK ---
• Lignans. Enterolactone or enterodiol added to cell lines (LS174T, Caco-2, HCT-15, T-84) reduced cell proliferation, and secoisolariciresinol diglycoside in rat’s pellet reduced tumor size and number (Sung et al., 1998). Flaxseed (Linum usitatissimum) is the richest source of the lignan precursors secoisolariciresinol diglycoside (SDG) and matairesinol in the plant kingdom (Thompson et al., 1991). However, large-scale human studies using purified flax lignans do not exist because of the difficulty in obtaining large quantities of lignans for clinical intervention.
• Other Phenolic Compounds. Curcumin (diferuloylmethane), a component of turmeric, is a natural anti-inflammatory used as a dietary ingredient in India and Southeast Asia. Curcuminoids are reported to inhibit endothelial cell proliferation in the mouse, and angiogenesis of LNCaP prostate cancer cells (Dorai et al., 2001). Tricin, a component of rice bran, fenugreek, and sugar cane, inhibits cancer cell viability, curtailing tumor growth. Other phenolics with variable antitumor cancer properties are protocatechuic acid, p-coumaric acid, caffeic acid, ferulic acid, sinapic acid, vanillic acid, methoxycinnamic acid, and tangerin. The flavonoids fisetin, quercetin, and luteolin were among the most potent inhibitors of protein kinase C. Tannic acid is more potent against the gelatinolytic activity of MMP-2 and MMP-9 than EGCG (Garbisa et al., 2001).
• Saponins and Terpenes. Saponins induce apoptosis (cell death) of premalignant and malignant human cells. Most well-studied saponins that possess anti-angiogenic, anti-inflammatory, and anti-apoptosis activities include ginseng and soybean saponins, oleanolic acid, and ursolic acid (Shibata, 2001). Phase II clinical trials of ginseng saponins showed promising results against lung cancer (Shibata, 2001); orally administered deglycosylated ginseng saponins reached the tumor site and were more effective than intraperitoneally injected saponin. The sweet-tasting saponin glycyrrhizin, from licorice, and its deglycosylated sapogenin form, glycyrrhetinic acid, possess anti-inflammatory, anti-allergic, anti-ulcer, and anti-hepatitic activities.
• Phytic Acid. Phytic acid (myo-inositol-hexaphosphate) is present in cereals and many legumes and may prevent the onset/progression of several types of chronic diseases. It inhibited the activation of pro-MMP-1, pro-MMP-2, and pro-MMP-9 by serine proteinases (Bansode and Losso, 2002) by chelating the divalent cations that catalyze pro-MMP activation. The lining of the gastrointestinal membrane is rich in pro-MMPs activity. Colon cancer was reduced in rats and mice when phytate was added in the drinking water (Pretlow et al., 1992).
• Vitamins. Vitamins B-3, D, and E have been reported as anti-angiogenic. Vitamin B-3 (nicotinamide and nicotinic acid) is the principal vitamin necessary for the prevention of esophageal cancer (Troll, 1993). Nicotinamide inactivates chymotrypsin and trypsin and suppresses ornithine decarboxylase, the early and late stage of tumor promotion, and the reduction of oxygen radicals, all of which have been identified as contributors to tumor promotion (Troll, 1993). At 500 mg/kg, nicotinamide was angiostatic in a C3H mouse model of mammary carcinoma (Horsman et al., 1995).
Vitamin D-3 can suppress VEGF-induced angio-genesis and inhibit the formation of a network of elongated endothelial cells (Mantell et al., 2000). In the body, vitamin D is transformed into a dihydroxy compound which is very active against cancer cells. However, cancer cells have the ability to express a hydroxylase enzyme CYP24 which catalyzes the conversion of dihydroxy vitamin D into an inactive form. Genistein inhibits CYP24 and may protect vitamin D-3 from degradation. Vitamin D in combination with retinoic acids can inhibit breast cancer cell growth and human umbilical vascular endothelial cell (HUVEC) proliferation (Dawson et al., 1998). Shklar and Schwartz (1996) observed less angiogenesis from the tumor of hamsters on dimethylbenz(a)anthracene (DMBA) + vitamin E compared to animals treated with DMBA only. The anticarcinogenicity property ascribed to vitamin E may derive from its anti-angiogenic property as well.
--- PAGE BREAK ---
• Lipids and Lipophilic Compounds. A number of bioactive lipids or lipophilic compounds endogenous to foods are anti-angiogenic as a result of their inhibitory effects on both the migration of endothelial cells (ECs) and the integrity of EC monolayers.
Omega-3-rich fish oils can down-regulate hormonal activation of protein kinase C. Protein kinase C induces collagenase, which in turn plays an important role in angiogenesis and metastasis. These properties suggest that omega-3-rich fish oils may inhibit angiogenesis and slow tumor metastasis, a phenomenon observed in fish oil feeding studies using animal models of tumorigenesis.
Lipoxygenase promotes inflammation. Chronic inflammation leads to angiogenesis. Gamma linolenic acid inhibits lipoxygenase.
Conjugated linoleic acid (CLA) showed significant inhibitory effects against animal mammary carcinogenesis even at concentrations less than or equal to 1%, alone or in combination with 20% saturated or unsaturated fat, in the diet (Parodi, 2001).
Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide), a major lipophilic pungent and irritating ingredient of hot chili peppers, is one of the most potent anti-inflammatory bioactive compounds to date. The anti-angiogenic activity of capsaicin and its analog involves the inhibition of microsomal monooxygenases and cytochrome P450 II E1 isoforms, respectively. Capsaicin is a major component in several over-the-counter products used to treat inflammation associated with arthritis. Similarly, ergosterol from the lipid fraction of Agaricus blazei Murill has been shown to inhibit angiogenesis associated with neovascularization in sarcoma 180- and Lewis Lung carcinoma-bearing mice (Takaku et al., 2001).
Docosahexaenoic acid (DHA) at the 4% level in a 20%-fat diet containing 4% linoleic acid significantly inhibited the MDA-MB-231 human breast cancer cell proliferation and reduced microvessel counts and the growth of the solid tumors in athymic nude mice, but did not reduce tumor VEGF concentrations (Rose et al., 1999).
COX-2, an enzyme implicated in the pathogenesis of several inflammatory diseases and in cancer, especially the gastrointestinal type, is inhibited by ajoene (Dirsch and Vollmar, 2001). Diallyl disulfides from garlic and garlic oil are cytostatic to a wide range of human cancer cells.
• Isoprenoids. Isoprenoids are a diverse class of volatile phytochemicals present in minute concentrations in many fruits and vegetables and cereal grains. There are about 23,000 isoprenoid types in fruits and vegetables. Their actions are synergistic, and some, like farnesol and geraniol, are potent inhibitors of pancreatic tumor in animal models. They may be of great interest in functional foods because pancreatic cancer, which isoprenoids can inhibit, has a very poor prognosis. Other isoprenoids in edible fruits and vegetables include d-limonene, perillyl alcohol, perillaldehyde, carvacrol, thymol, β-ionone, geraniol, geranylgeraniol, perillyl amine, farnesol, dl-α-tocopherol, oryzanol-free tocotrienol-rich fraction of rice bran oil, and tocotrienols from palm oil, to name a few.
• Isothiocyanates and Indoles. Phenethyl isothiocyanate is effective against tobacco-induced lung cancer and is currently under development as a lung cancer inhibitor (Hecht, 2000). Glucoraphanin, the major glucosinolate, in broccoli is hydrolyzed by myrosinase to either sulforaphane or sulforaphane nitrile. Sulforaphane is more potent as a chemopreventive agent than sulforaphan nitrile; however, most commercial broccoli generate a sulforaphan nitrile from glucoraphanin. Indole melatonin administered orally at a level of 20 mg/day for at least 2 months to metastatic patients showed some improvement associated with reduced VEGF levels in the blood.
• Minerals. Selenium, as either Se-enriched garlic, sodium selenite, or Se-methylselenocysteine, reduced angiogenesis in a model of mammary carcinoma and decreased MMP-2 gelatinolytic activity of HUVEC in cultures and induced cell death by apoptosis (Jiang et al., 1999). Selenium in combination with saffron is more effective against angiogenesis than either compound alone (Abdullaev, 2001). Selenium has been listed as a promising chemopreventive agent for prostate cancer.
--- PAGE BREAK ---
• Proteins and Peptides. Bioactive proteins with demonstrated anti-angiogenic activity include protamine, the Bowman-Birk inhibitor (BBI), lactoferrin, lactalbumin, lysozyme, and shark and bovine cartilage. Protamine, a Gram-positive and Gram-negative antimicrobial protein rich in arginine residues, inhibited pro-MMP-1 activation by trypsin (Obubuafo et al., 2002). The inhibition of angiogenesis through the inhibition of ornithine decarboxylase, an enzyme associated with the onset and progression of a variety of cancers, including colon, prostate, and breast, by micromolar concentration of protamine, has been reported (Flamigni et al., 1985). Gastric mucosa was regenerated and ulcer healed when protamine sulfate was fed to rats suffering from acetic acid–induced gastric ulcer (Li and Cho, 1999).
BBI, an anti-carcinogenic water-soluble protein of 9,856 daltons occurring in soybean, legumes, and many monocotyledonous seeds, has been studied for three decades (Kennedy, 1998). Its anticarcinogenicity has been associated with its ability to inhibit chymotrypsin and chymases. However, studies in our laboratory have shown that BBI from rice or soybean may prevent angiogenesis by preventing serine proteinase–catalyzed activation of pro-MMP1, pro-MMP-2, and pro-MMP-9 (Antunes and Losso, 2001; Munene et al., 2002 ; Losso, 2002).
Oral administration of iron-depleted bovine lactoferrin (bLf) to rats inhibited VEGF-induced angiogenesis (Norrby et al., 2001). Administration of bLf or bovine lactoferricin (a peptic hydrolysate of lactoferrin) to mice bearing melanoma, colon carcinoma, or lymphoma inhibited angiogenesis and metastasis.
Lactalbumin inhibits metal-catalyzed activation of pro-MMPs and metal-catalyzed activity of active MMPs (Bawadi and Losso, 2002). Lysozyme chloride as an ointment formulation (Reflap) at 50 mg/g was less effective than prostacyclin analog at 1–100 Fg/g as an anti-angiogenic bioactive molecule (Yamamoto et al., 1996).
Bovine cartilage and shark cartilage are anti-angiogenic. The low incidence of tumors in shark has been related to the presence of anti-angiogenic compounds in shark cartilage. The anti-angiogenic compound in shark cartilage was reported to be a 10-kD proteoglycan containing keratan sulfate units (Liang and Wong, 2000). Shark cartilage preparations such as Marimastat, Prinomastat, and Neovastat are in clinical trials against small-cell lung cancer (Shepherd, 2001; Falardeau et al., 2001). Neovastat inhibits MMP-2, MMP-9, MMP-12, and VEGF, decreases lung metastases in the Lewis lung carcinoma model, and enhances the effect of cisplatin. Shark cartilage is bioavailable.
• Amino Acids. Arginine and glycine are the only two amino acids for which a direct link to angiogenesis has been established. Ingested arginine has the ability to reduce endothelial cell dysfunction in hypercholesterolemic patients, smokers, hypertensive individuals, diabetics, obese people, the elderly, coronary artery disease, ischemia/reperfusion, and congestive heart failure. Bioavailable arginine is deposited in endothelial cells, where it is converted to nitric oxide, which induces ulcer healing. L-arginine is a conditionally essential amino acid for adults under certain pathological conditions, such as trauma.
Glycine, a powerful antioxidant, in the diet inhibits endothelial cell proliferation and neovascularization, as demonstrated in C57BL/6 mice bearing B16 tumor cells (Rose et al., 1999).
• Carbohydrates and Carbohydrate Conjugates. Endostatin is a 184-aminoacid-residue, 20-kD proteoglycan from the C-terminal region of collagen XVIII found in vessel walls and basement membrane. It inhibits angiogenesis and endothelial cell migration in vitro and appears to be highly effective in murine models of carcinogenesis (Zatterstrom et al., 2000). pH-modified citrus pectin dose-dependently inhibited prostate cancer and melanoma metastasis in mice and reduced tumor by as much as 70% (Hayashi et al., 2000).
• Carotenoids and Retinoids. Cataracts, glaucoma, diabetes blindness, and age-related macular degeneration are the four main causes of visual disability among the aging and diabetic patients. Age-related macular degeneration is an angiogenic process whereby excessive new vessels are generated and vascular endothelial growth factor, hemorheology, and endothelial dysfunction are increased.
--- PAGE BREAK ---
Lutein, 3,3'-dihydroxy-α-carotene, has been identified and recognized by various interdisciplinary studies as one of the dietary strategies that can delay the onset of macular degeneration. Lutein and zeaxanthin are major xanthophylls detected in the eye. Elevated levels of lutein in the plasma have been associated with lower risk of macular degeneration.
Crocin, crocetin, and picrocrocin—all carotenoids from saffron—significantly inhibited the growth of a variety of cancer cells at micromolar concentration (Abdullaev, 2002). Although carotenoids in general enhance intercellular communication—a key event in carcinogenesis regulation—data on β-carotene and chemoprevention have been controversial on a number of occasions.
Angiogenesis also plays a role in the pathogenesis of leukemia. Retinoic acid can effectively block vascular permeabilization induced by VPF/VEGF and VPF/ VEGF-induced angiogenesis in the chorioallantoic membrane (CAM) assay (Pal et al., 2000).
What Needs to Be Done
The future of anti-angiogenic functional foods looks promising. Many substances known as antinutrients two or three decades ago, such as phytic acid, BBI, and saponins, are now being promoted as chemopreventive agents when used in moderation. Other compounds of interest, such as lipoic acid, beta-glucan, alkylglycerols, and astaxanthin, should be investigated.
Science-based publications in refereed journals and the lay press and clinical trials about the contribution of functional foods at the molecular level for disease prevention will definitely dispel doubt about the role of food in disease prevention. Food scientists and nutritionists have the daunting task of communicating well to convince medical schools and pharmacology programs that functional foods are for the common good.
Fotsis et al. (1998) wrote that consumption of a plant-based diet has the potential to prevent angiogenesis and the progression and growth of solid tumors. However, over-refining functional foods to replace the unfamiliar taste associated with bioactive compounds may not provide the same purported health-enhancing properties of these foods. We may have to adjust our taste buds to the bitterness of functional foods and not remove them to please our sweet-loving taste buds.
Our love for sweet may thwart efforts to incorporate very effective functional foods such as saponins in our diet. For instance, fenugreek is healthful mostly because of the bitter compounds it contains. Sensory evaluation techniques used today will not bring foods that are enriched but odd-tasting to the market, because most of these compounds are not appealing to the palate and do not pass a consumer acceptance test based on “like” vs “dislike” criteria, the latter unfortunately being the litmus test.
To bring some of the functional food ingredients into successful food products, the food industry may have to work on several fronts:
Establish safety and dose–response relationship of functional foods. Once the safety of functional foods has been established, it will be easy for the industry to legally introduce products. It is important to establish dose–response relationships in the context of the food matrix being consumed, since most of these compounds have potentially beneficial as well as potentially toxic effects, depending on the dose and synergistic or antagonistic interactions with other food components.
Provide adequate processing and storage conditions. Black tea is less efficient than green tea against angiogenesis because catechins are lost during thermal processing of black tea. Food fortification with safe bioactive compounds will be necessary for consumers to obtain a sufficient dose of the active components for anti-angiogenesis activity, since otherwise a huge amount of food might have to be consumed. And the conditions of processing and storage are crucial in ensuring maintenance of the bioactivity of the compounds.
--- PAGE BREAK ---
Address children. Children, not just the Baby Boomers, are the future of the functional food industry. Training children as early as possible to enjoy the taste of functional foods will create a niche of loyal consumers that the industry can count on for many years to come. Responsible parents can help introduce odd-tasting foods to their children before the children’s taste buds are irreversibly conquered by sweet and greasy foods. It is well established that eating is a culture and people do not change their eating habits overnight. However, once kids have enjoyed odd-tasting foods, they will remember them.
Reformulate food with bioactive ingredients. This front encompasses Baby Boomers, the elderly, and any consumer who dislikes the taste of phytochemicals but whose health requirements involve taking innumerable pills. For these groups, processing technologies that help camouflage the odd taste of some bioactive compounds can provide individuals with needed health benefits. Encapsulation techniques such as multiple emulsions and cyclodextrin are classical examples of techniques that have helped the pharmaceutical industry reach the children’s market with success. However, adding functional foods to a bad product will only damage the credibility of functional foods.
Remove potential angiogenesis-promoting compounds from food. Angiogenin and osteopontin are good examples of compounds in the human diet whose potential health effects need to be investigated.
Provide government funding. Government—the Food and Drug Administration, U.S. Dept. of Agriculture, National Institutes of Health, and National Cancer Institute—should provide support to food scientists working on functional foods rather than to scientists who promote the use of food components as drugs. Research on angiogenesis-promoting compounds should be encouraged and funded by the federal government.
Government can also take the initiative in providing functional foods to school lunch programs as a scheme to help children inculcate the habit of adjusting their palate to health-enhancing foods. This would greatly help in the proliferation of functional foods and attainment of wider acceptability. Government can also assist in introducing functional foods into high school and college curricula, and assist in clinical trials—an area where the food industry has been lacking in leadership.
Future Prospects
As indicated above, many physiologically active compounds in functional foods have the ability to inhibit the enzymatic process of angiogenesis. Proteins (BBI, protamine, lactoferrin, lysozyme, shark cartilage), lipids (CLA, fish oil, garlic oil), carbohydrates (endostatin, pHmodified pectin), vitamins (E, D), phenolics (resveratrol, curcumin, EGCG), carotenoids (lutein and beta-carotene), amino acids (glycine, arginine), mineral (selenium), terpenes (ursolic and oleanolic acids), and other minor constituents (phytic acid, isoprenoids) are classical examples.
Functional foods are an interdisciplinary area that should involve people from all walks of life because we all need food. Medical schools, practicing physicians, hospital foodservices, nurses, pharmacists, and other health care professionals have a responsibility to be educated about the benefits of functional foods. Once the effectiveness and safety of functional foods have been proven at the clinical trial level, the inhibition of angiogenesis may form the basis for developing health claims. However, merchants of functional foods will have to demonstrate specific efficacy for each compound being advertised. It is also important to educate consumers with respect to the interpretation of health claims.
Once a consumer base has been established and individuals feel the benefit of eating well, everybody, including medical doctors, will appreciate the benefits of functional foods. Then the industry may move into using knowledge from ethnobotany, ethnopharmacology, and Quantitative-Structure-Activity Relationship (QSAR) to offer designer foods to specific groups according to needs. It will be imperative that future food and nutrition scientists be trained in proteomics, genomics, and nutrigenomics.
An opportunity really exists for the food industry to lead and help prolong healthy life expectancy for all of us. Hippocrates was right when he said, Let food be thy medicine.
The author thanks Eunice Li-Chan at Food, Health, and Nutrition, University of British Columbia, Vancouver, BC, Canada, for her critical review of the manuscript; his graduate students (Tania Antunes, Rishipal Bansode, Hiba Bawadi, Armen Khachatryan, Cate Munene, Annie Obubuafo, and Jeho Shin); and Henry Njapau (Food and Drug Administration) for their input into this article.
The author is Assistant Professor, Dept. of Food Science, Louisiana State University Agricultural Center, Baton Rouge, LA 70803.
References
Abdullaev, F.I. 2002. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.). Exp. Biol. Med. 227: 20-25.
Antunes, T.M.S. and Losso, J.N. 2001. In vitro inhibition of matrix metalloproteinase-2 (MMP-1) by rice proteins. Presented at Brunner Protein Symp., Michigan State Univ., Lansing, April 24-25.
Bansode, H. and Losso, J.N. 2002. Inhibition of MMP-1, MMP-2, and MMP-9 by phytic acid. Unpublished manuscript, Louisiana State Univ., Baton Rouge.
Bawadi, H.A. and Losso, J.N. 2002. Interaction of metalloproteinase-2 and -9 (MMP-2 and MMP-9) with bovine alpha-lactalbumin. Unpublished manuscript, Louisiana State Univ., Baton Rouge.
Dawson, M.I., Chao, W.R., Hobbs, P.D., and Zhang, X.K. 1998. Effects of trans-retinoic acid, 9-cis-retinoic acid, 1-alpha,25-(dihydroxy)vitamin D3 and a novel apoptosis-inducing retinoid on breast cancer and endothelial cell growth. Cancer Lett. 133: 1-8.
Dirsch, V.M. and Vollmar, A.M. 2001. Ajoene, a natural product with non-steroidal anti-inflammatory drug (NSAID)-like properties? Biochem Pharmacol. 61: 587-593.
Dorai, T., Cao, Y.C., Dorai, B., Buttyan, R., and Katz, A.E. 2001. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate 47: 293-303.
Falardeau, P., Champagne, P., Poyet, P., Hariton, C., and Dupont, E. 2001 Neovastat, a naturally occurring multifunctional antiangiogenic drug, in phase III clinical trials. Semin. Oncol. 28: 620-625.
Flamigni, F., Guarnieri, C., Marmiroli, S., and Caldarera, C.M. 1985. Inhibition of rat heart ornithine decarboxylase by basic polypeptides. Biochem. J. 229: 807-810.
Folkman, J. and Ingber, D. 1992. Inhibition of angiogenesis. Semin. Cancer Biol. 3: 89-96.
Fotsis, T., Pepper, M.S., Montesano, R., Aktas, E., Breit, S., Schweigerer, L., Rasku, S., Wahala, K., and Aldercreutz, H. 1998. Phytoestrogens and inhibition of angiogenesis. Baillieres Clin. Endocrinol. Metabol. 12: 649-666.
Garbisa, S., Sartor, L., Biggin, S., Salvato, B., Benelli, R., and Albini, A. 2001. Tumor gelatinases and invasion inhibited by the green tea flavonol epigallocatechin-3-gallate. Cancer 91: 822-832.
Hayashi, A., Gillen, A.C., and Lott, J.R. 2000. Effects of daily oral administration of quercetin chalcone and modified citrus pectin. Altern. Med. Rev. 5: 546-552.
Heasman, M. and Mellentin, J. 2001. “The Functional Foods Revolution.” Earthscan Publications Ltd., London and Sterling, Va.
Hecht, SS. 2000. Inhibition of carcinogenesis by isothiocyanates. Drug Metab. Rev. 32: 395-411.
Hiromatsu, Y., Sato, M., Yamada, K., and Nonaka, K. 1992. Inhibitory effects of nicotinamide on recombinant human interferon-gamma-induced intercellular adhesion molecule-1 (ICAM-1) and HLA-DR antigen expression on cultured human endothelial cells. Immunol Lett. 31: 35-39.
Horsman, M.R., Khalil, A.A., Chaplin, D.J., and Overgaard, J. 1995. The ability of nicotinamide to inhibit the growth of a C3H mouse mammary carcinoma. Acta Oncol. 34: 443-446.
Igura, K, Ohta, T, Kuroda, Y., and Kaji, K. 2001. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 171: 11-16.
Jiang, C., Jiang, W., Ip, C., Ganther, H., and Lu, J. 1999. Selenium-induced inhibition of angiogenesis in mammary cancer at chemopreventive levels of intake. Molec. Carcinog. 26: 213-225.
Joussen, A.M., Rohrschneider, K., Reichling, J., Kirchhof, B., and Kruse, F.E. 2000. Treatment of corneal neovascularization with dietary isoflavonoids and flavonoids. Exp Eye Res. 71: 483-487.
Kennedy, A.R. 1998. Chemopreventive agents: Protease inhibitors. Pharmacol. Ther. 78: 167-209.
Lee, A. and Langer, R. 1983. Shark cartilage contains inhibitors of tumor angiogenesis. Science 221: 1185-1187.
Liang, J.H. and Wong, K.P. 2000. The characterization of angiogenesis inhibitor from shark cartilage. Adv. Exp. Med. Biol. 476: 209-223.
Li, Y. and Cho, C.H. 1999. The ulcer healing effect of protamine sulphate in rat stomach. Aliment Pharmacol. Ther. 13: 1351-1362.
Losso, J.N. 2002. Inhibition of pro-matrix metalloprotease-9 (pro-MMP-9) activation by the soybean anticarcinogen Bowman-Birk inhibitor: Antiangiogenic activity of BBI. Presented at Ann. Mtg., Am. Chem. Soc., Orlando, Fla., April 7-11.
Mantell, D.J., Owens, P.E, Bundred, N.J, Mawer, E.B., and Canfield, A.E. 2000. 1-alpha, 25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ. Res. 87: 214-220.
Munene, C.N., Bansode, R.R., and Losso, J.N. 2002. Targeting chronic diseases with functional food: The contribution of soybean BBI. Unpublished manuscript, Louisiana State Univ., Baton Rouge.
Norrby, K., Mattsby-Baltzer, I., Innocenti, M., and Tuneberg, S. 2001. Orally administered bovine lactoferrin systemically inhibits VEGF(165)-mediated angiogenesis in the rat. Intl. J. Cancer. 91: 236-240.
Obubuafo, A.K., Bansode, R.R., Moody, M.W., McMillin, K.W., and Losso, J.N. 2002. Inhibitory effect of protamine on metalloproteinase MMP-1. Unpublished manuscript, Louisiana State Univ., Baton Rouge.
Pal, S., Iruela-Arispe, M. L., Harvey, V.S., Zeng, H., Nagy, J. A., Dvorak, H. .F, Mukhopadhyay, D. Retinoic acid selectively inhibits the vascular permeabilizing effect of VPF/VEGF, an early step in the angiogenic cascade. Microvasc Res. 2000, 60: 112-120.
Parodi, C.W. 2001. Cow’s milk components with anti-cancer potentials. Austral. J. Dairy Technol. 56: 65-73.
Pretlow, T.P., O’Rirdan, M.A., and Somich, G.A. 1992. Aberrant crypts correlate with tumor incidence in F344 rats treated with azoxymethane and phytate. Carcinogenesis 13: 1509-1512.
Rose, D.P. and Connolly, J.M. 1999. Antiangiogenicity of docosahexaenoic acid and its role in the suppression of breast cancer cell growth in nude mice. Intl. J. Oncol. 15: 1011-1015.
Rose, M.L, Madren, J., Bunzendahl, H., and Thurman, R.G. 1999. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis 20: 793-798.
Seeram, N. P., Momin, R.A., Nair, M.G., and Bourquin, L.D. 2001. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 8: 362-369.
Shao, Z.M., Wu, J., Shen, Z.Z., and Barsky, S.H. 1998. Genistein inhibits both constitutive and EGF-stimulated invasion in ER-negative human breast carcinoma cell lines. Anticancer Res. 18(3A): 1435-1439.
Shepherd, F.A. 2001. Angiogenesis inhibitors in the treatment of lung cancer. Lung Cancer 34(Suppl 3): 81-89.
Shibata, S. 2001. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J. Korean Med. Sci., Suppl: S28-37.
Shklar, G. and Schwartz, J.L. 1996. Vitamin E inhibits experimental carcinogenesis and tumour angiogenesis. Eur. J. Cancer B Oral Oncol. 32B: 114-119.
Sung, M.K., Lautens, M., and Thompson, L.U. 1998. Mammalian lignans inhibit the growth of estrogen-independent human colon tumor cells. Anticancer Res. 18: 1405-1408.
Takaku, T., Kimura, Y., and Okuda, H. 2001. Isolation of an antitumor compound from Agaricus blazei Murill and its mechanism of action. J. Nutr. 131: 1409-1413.
Thibout, D., Kraemer, M., Di Benedetto, M., Saffar, L., Gattegno, L., Derbin, C., and Crepin, M. 1999. Sodium phenylacetate (NaPa) induces modifications of the proliferation, the adhesion and the cell cycle of tumoral epithelial breast cells. Anticancer Res. 19: 2121-2126.
Thompson, L. U. , Robb, P., Serraino, M., and Cheung, F. 1991. Mammalian lignan production from various foods. Nutr Cancer 16: 43-52.
Troll, W. 1993. Prevention of cancer by vitamin B3 (nicotamide and nicotinic acid): A protease inhibitor available in pure form. In “Protease Inhibitors as Cancer Chemopreventive Agents.” ed. W. Troll and A.R. Kennedy, pp. 177-189. Plenum Press, New York.
Webb, T. 2000. Green tea experiments in lab, clinic yield mixed results. J. Natl. Cancer Inst. 92: 1038-1039.
Wernert, N., Stanjek, A., Kiriakidis, S., Hugel, A., Jha, H.C., Mazitschek, R., and Giannis, A. 1999. Inhibition of angiogenesis in vivo by ets-1 antisense oligonucleotides—Inhibition of Ets-1 transcription factor expression by the antibiotic fumagillin. Angew. Chem. Intl. Ed. 38: 3228-3231.
Yamamoto, T., Horikawa, N., Komuro, Y., and Hara, Y. 1996. Effect of topical application of a stable prostacyclin analogue, SM-10902 on wound healing in diabetic mice. Eur. J. Pharmacol. 302: 53-60.
Yoo, Y-C., Watanabe, S., Watanabe, R., Hata, K., Shimazaki, K-i., and Azuma, I. 1997. Bovine lactoferrin and lactoferricin, a peptide derived from bovine lactoferrin, inhibit tumor metastasis in mice. Japan J. Cancer Res. 88: 184-190.
Zatterstrom, U.K, Felbor, U., Fukai, N., and Olsen, B.R. 2000. Collagen XVIII/endostatin structure and functional role in angiogenesis. Cell. Struct. Funct. 25: 97-101.
Zhou, J.R., Mukherjee, P., Gugger, E.T., Tanaka, T., Blackburn, G. L., and Clinton, S. K. 1998. Inhibition of murine bladder tumorigenesis by soy isoflavones via alterations in the cell cycle, apoptosis, and angiogenesis. Cancer Res. 58: 5231-5238.
