Kinetic Models for Microbial Survival During Processing
World-renowned scientists participated in IFT’s second Research Summit to advance the understanding of microbial inactivation kinetics and models for non-log-linear survivor curves and identify needs for further research.
The Institute of Food Technologists convened its second Research Summit on January 14–16, 2003, in Orlando, Fla. Forty scientists met to advance the state of understanding of microbial inactivation kinetics and models for non-log-linear survivor curves, through an in-depth interchange of information and identification of needs for further research.
Seven leading experts provided plenary presentations that served as a baseline for effective, and sometimes impassioned, discussions. The recommendations from the conference communicate the views of the majority of participants. There were a few issues where the perspectives of a small number of the participants were so strongly held that consensus was not reached.
This article summarizes the plenary presentations and presents the summit conclusions and recommendations.
Mechanistic, Vitalistic, and Probabilistic Model Approaches
Arthur A. Teixeira, University of Florida, Gainesville, and Alfredo C. Rodriquez, Baxter Healthcare Corp., Round Lake, Ill.
Watson (1908) examined two main basic approaches—mechanistic and vitalistic—that were used to study the inactivation of bacterial spores early in the 20th century. Watson presented Chick’s model (Chick, 1908) mathematically as a first-order reaction (the Chick-Watson equation) describing the exponential decay commonly observed in microbial survivor curves. This model is the basis of the mechanistic approach and defines the inactivation of bacterial spores as a pseudo first-order molecular transformation. Temperature dependency of corresponding rate constants was found to be appropriately described by the Arrhenius equation.
The vitalistic approach was based on the assumption that exponential microbial decay could be explained by differences in resistance. Watson noted that for this argument to hold, most of the spores would have to be at the low extreme of resistance, instead of following the normal distribution that would be expected from natural biological variability. He concluded that the vitalistic approach could not be correct.
Kellerer (1987) stated that the vitalistic approach is naïve and ignores the rigorous stochastic basis for the inactivation transformation (the Maxwell-Boltzman distribution of speed of molecules or random radiation “hits” on DNA) by using the simplistic assumption that biological variability of resistance can explain the observed behavior correctly.
--- PAGE BREAK ---
Aiba and Toda (1967) developed a third approach, in which the life-span probability of spores was mathematically defined and used to develop expressions describing inactivation of single and clumped spores. This analysis postulated that for a population of spores (Ni in number and ti in life span), the probability Pr associated with the distribution of life span is:
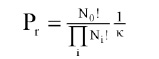
where K is a normalizing factor. Based on this probability function, Ni could be described as a function of the life span ti, provided that the initial number of spores N0 was large:
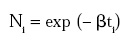
or, modifying the discrete equation into its continuous form (for large N0):
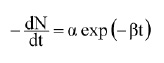
Aiba and Toda (1967) proved that α = N0 k and β = k, where k is the rate constant and N0 the initial number of microorganisms.
This probabilistic approach led to the same response equation as that of the mechanistic approach. This revelation leads to several important concepts: (1) Arbitrary use of frequency distribution equations, such as the Weibull distribution, is an extensive “jump” in logic that cannot be rigorously justified. (2) Clumping induces a delay that depends on the number of spores per clump and can be described using the probabilistic approach, but clumping will not lead to increments in the number of survivors such as those induced by spore activation. (3) The probabilistic approach established a direct relationship between the average life span and the rate constant used in the mechanistic approach, and both approaches lead to the same basic mathematical model.
• Scientific Basis for First-Order Kinetic Models. The nature of the inactivation transformation can be addressed in terms of Eyring’s transition-state theory and the Maxwell-Boltzman distribution of the speed of molecules from molecular thermodynamics. For a given configuration (mass and degrees of freedom), the fraction of molecules that have enough kinetic energy to overcome the energetic barrier (activation energy) for a transformation such as inactivation of spores depends mostly on temperature. Therefore, at a given temperature, the fraction of molecules that will reach the level of energy required for the transformation (e.g., inactivation) to happen is constant. The fraction of molecules that have enough energy to react will increase with temperature.
--- PAGE BREAK ---
• Non-Log-Linear Survivor Curves. Activation of dormant spores and presence of subpopulations of differing resistance give rise to the “shoulders and tails” appearing as deviations from log-linearity in some survivor curves. Models including these concepts based on systems analysis of population dynamics were developed in the late 1980s and early ’90s (Rodriguez et al., 1988, 1992; Sapru et al. 1992, 1993) and verified experimentally under extreme-case conditions. These models may be described by a summation of first-order terms:
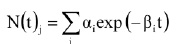
where N(t)j = density of population j (cfu/mL) as a function of time t; j may refer to subpopulations of active spores, dormant spores, or other spores with different resistance; αi is a parameter related to initial population density of the different subpopulations in the system; and βi is a parameter related to rate constants for the different transformations in the system.
The parameters needed for each term (αi,βi) can be estimated using nonlinear regression or the successive residuals method, similar to analysis of stress relaxation curves. In the simulation of curves where two or more subpopulations with different resistances to a lethal agent must be taken into consideration, each of the subpopulations is described by one of the exponential terms and the total effect by their summation.
High-temperature, short-time pasteurization or ultra-high-temperature sterilization processes operate in temperature ranges far above those in which survivor curves can be generated. Models derived from survivor curves for use in the design/specification of such processes should be deterministic models that are based on an understanding of the mechanisms responsible for the behavior patterns observed in the survivor curves. Empirical (curve-fitting) models generally should not be used for this purpose.
Alternate Kinetic Models for Microbial Survivor Curves, and Statistical Interpretations
M.A.J.S. van Boekel, Wageningen University, The Netherlands
The traditional first-order model for inactivation of microorganisms assumes occurrence of a linear survival curve. Most survival curves, however, appear not to be linear. Alternative empirical models were therefore considered. The Weibull-like model with two parameters:
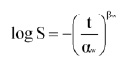
appeared promising because it can describe both concave-upward (βw < 1) and concave-downward (βw > 1) curves (van Boekel, 2002). The αw parameter has time units, and the βw parameter has a so-called shape factor. If βw = 1, the model reduces to the classical first-order (power-law) model:
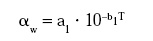
where a1 and b1 are new parameters indicating the temperature dependence of aw, or, alternatively:
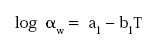
--- PAGE BREAK ---
This model was applied to 55 published cases for vegetative cell survival curves. In 53 cases, the curves were found to be nonlinear and could be described very well by the Weibull model. In 39 cases, βw > 1; in 14 cases, βw < 1; and only in 2 cases was a true linear curve (βw = 1) found. The temperature dependence of αw could be described by a power-law relationship, just as for the traditional D-value (resulting in a z-value). The temperature dependence of βw was not as straightforward—in 47 cases, βw seemed to be independent of temperature, while in 7 cases it was dependent, either increasing or decreasing with temperature.
For spore inactivation, the occurrence of so-called “activation shoulders,” complicates matters; i.e., the number of spores increases at the beginning of treatment. Models accounting for this phenomenon—the Shull and Sapru models (both based on first-order kinetics), the stochastic Peleg model, and the “double Weibull” model—were compared and evaluated by the Akaike criterion and a Bayesian posterior probability test. The two empirical models—Peleg and double Weibull—performed best.
Mathematical Models of Microbial Inactivation During Preservation Processes
Micha Peleg, University of Massachusetts, Amherst
According to general and food microbiology textbooks, microbial inactivation follows first-order kinetics. Hence, it can be characterized by a single rate constant k or its reciprocal, the D-value, considered a measure of resistance to an applied lethal agent. The temperature dependence of k or the D-value has been frequently described by the Arrhenius log-linear equation which produced the famous z-value used in sterility calculations. The efficacy of thermal sterilization has been traditionally determined in terms of an F-value, which is computed by combining the temperature “profile” with the above survival parameters.
There are three problems with methods based on these models: (1) Substantial evidence indicates that microbial inactivation, usually is not a process which follows first-order kinetics. (2) Even if it were, neither of the above two temperature-dependence models would be a good choice because both give an inappropriate relative “weight” to low temperatures, where little activation takes place, at the expense of high temperatures, where most of the inactivation actually occurs. (3) The standard formula to calculate the F-value has a reference temperature. But since the F-value can be translated into a residual survival ratio, the latter will be independent of the reference temperature if the relationship between log D and temperature is linear over the whole pertinent temperature range.
Alternatively, consider the survival curve the cumulative form of the temporal distribution of lethal events. If the resistance of the individual organism or spore to the lethal agent is expressed by the time needed for inactivation, then the slope of the population’s survival curve has a rate dimension; hence the relation of the survival curve’s shape to the inactivation kinetics. Since different populations have different spectra of resistance under different conditions, the isothermal, isobaric, or constant-concentration semi-logarithmic survival curves have a variety of nonlinear shapes describable by a variety of models.
If the local slope of a survival curve under transient conditions is the slope of the isothermal, isobaric, or iso-concentration curve at the momentary temperature, pressure, or concentration, respectively, at a time corresponding to the momentary survival ratio, then this survival curve can be calculated under almost any temperature, pressure, or chemical agent concentration profile from survival parameters determined under constant agent intensity conditions. This requires the solution of a differential equation, which is constructed on the basis of the organism’s actual survival patterns, without assuming any mortality kinetic order.
--- PAGE BREAK ---
The concept’s validity has been demonstrated by comparing the actual survival patterns of Salmonella, Listeria, and Bacillus sporothermodurans subjected to various non-isothermal heat treatments to those patterns predicted using parameters derived from isothermal inactivation data. The isothermal survival curves for each could be described by the power-law model
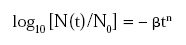
and the temperature dependence of the rate parameter b by the log logistic model:
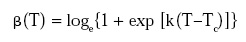
where the coefficients k and Tc are the of the survival parameters organism or spore. It has also been shown that if n is more or less constant at the pertinent temperature range, then these survival parameters can be estimated directly from survival data obtained during non-isothermal heat treatments.
Comparison of Alternative Models with the First-Order Model when Applied to Preservation Processes
Cynthia M. Stewart and Martin B. Cole, Food Science Australia, CSIRO, North Ryde, New South Wales
Microbiologists have been analyzing thermal inactivation data using the linear D-value and z-value models since Esty and Meyers published the first systematic study on thermal resistance of spores in the 1920s, even though visual inspection of the data when plotted often showed curvature (Peleg and Cole, 1998). Process engineers have been using the results of these analyses to establish safe food processes ever since.
This traditional approach to inactivation kinetics is based on the assumption that microorganisms or their spores die exponentially following first-order kinetics. It also assumes that all cells or spores have identical heat resistance. In no other area of biology are either of these two assumptions made (Anderson et al., 1996). This approach allows for simple, straightforward calculations and comparison of thermal process equivalencies. However, throughout the past 80 years, deviations in log-linear models have been repeatedly noted and the “phenomenon” of shoulders and tails has been hotly debated.
When semi-logarithmic survival curves are scrutinized, one can see that in fact they are not linear, but slightly curved. The vitalistic theory of inactivation explains these observations by considering shoulders and tails as due to underlying physiological reactions of the cells/spores to lethal conditions rather than as “artifacts” of experimentation. This theory, which is more in line with other areas of biological study, states that the individual microorganisms in a population do not have identical resistances to inactivation, that differences are permanent, and that microbial sensitivity to heat or any other means of inactivation is distributed (Anderson et al., 1996).
--- PAGE BREAK ---
Many examples in the scientific literature report evidence of biovariability in microbiology: growth from single heat-treated Salmonella cells show a distribution of lag times (Stephens et al., 1997); germination of single Clostridium botulinum spores shows distribution in time to germination (Billon et al., 1997); and flow cytometry experiments show variation in injury (Ueckert et al., 1997). Variability of organisms in a population is critical for species survival, allowing survival in changing conditions. Perhaps Darwin, in his Origin of the Species (1859), summarizes it best: “Survival of any life form requires a delicate balance between fidelity of replication and intergeneration variation.”
Microbiological models are typically empirical models and should not be used outside the range of the factors used to create them, because there is no underlying principle on which to base extrapolation. When considering inactivation data, extrapolation may result in possible underprocessing or “fail-dangerous” processing, or overprocessing and in a lower-quality product. Interpolation, on the other hand, provides better understanding of data and processing. Evidence for thermal death of C. botulinum 213B indicates that extrapolation is problematic in non-log-linear as well as log-linear models.
Regulatory Framework of Microbial Survivor Curves
John W. Larkin, Food and Drug Administration, National Center for Food Safety and Technology, Summit-Argo, Ill.
Expression of the necessary treatment level required to render a food safe is not consistent across the regulated food supply. The different approaches to defining the treatment level, or sterility assurance level (likelihood of a potentially hazardous product demonstrating non-sterility before the end of its life cycle), can be grouped into three categories.
The first category comprises processes with a specific target end-point, or an expected probability of finding a nonsterile unit (PNSU). Within the PNSU concept, the final surviving level of microbial hazard defines product safety; thus, the processor must ensure that the initial level of contaminant is at or below the expected initial level. A typical target level used in the pharmaceutical and medical device industries is a PNSU of 10–6. Thus, the process is designed to consistently deliver a treatment such that only one out of every million products (units) produced would possibly be nonsterile.
The second category contains processes designed to consistently deliver a specific log reduction of the microbial hazard. The log-reduction process design assumes that the initial level of contaminant will never go above a predetermined maximum expected level and that to deliver a specific level of safety, a known number of log reductions of the organism of concern must be delivered by the process. The Food and Drug Administration used this category in its juice regulation (21 CFR 120) and its recommended 5-log reduction of Salmonella Enteritidis for in-the-shell pasteurization of eggs. The frequent assumption that FDA requires a 12-log reduction of C. botulinum for low-acid canned food is a misconception. The low-acid canned food regulations (21 CFR 108 and 113) require that the food product be commercially sterile.
The concept of commercial sterility is best thought of as a process of producing a food product “free from” the microorganisms capable of reproducing within the food during normal storage conditions. The process target of making the food product “free from” the organism of concern is the third category of process designs. FDA has used the expectation of the food product’s being free from the organism of concern for pasteurized liquid egg and low-acid canned foods. For canned food products, the “free from” element is tied to the possibility of the microorganism growing under normal storage conditions. Thus, products can be produced under the low-acid canned food regulations without a thermal treatment traditionally used to destroy spores if the spores remaining in the food product can be prevented from growing by other hurdles (e.g., salt, pH, and water activity).
Since the level of safety of a process is related to the possible survival and growth of any remaining organisms of concern, the ability to control delivery of the expected log reductions is critical. When a treatment exhibits a diminishing ability to reduce the organism of concern, either with dose or time (i.e., has a tail), the food processor should establish an acceptable range for all of the critical factors that are used to control the process. Thus, if a process deviation occurs, the processor will be able to make the necessary process correction and still be able to deliver the necessary log reductions. If the necessary correction in the critical factor starts to cause the treatment to enter into the treatment range exhibited by the tail, the effectiveness of the process starts to become questionable. Thus, with each critical control point within the process, the processor should also identify its acceptable range for which an adequate process can be designed.
--- PAGE BREAK ---
Future Directions in Food Safety Management
Bruce Tompkin, retired from ConAgra Refrigerated Foods, Downers Grove, Ill.
For decades, microbiological safety has been defined through the use of microbiological criteria. Regulatory agencies and the food industry applied microbiological testing as a means to assess food operations and food safety. Unfortunately, microbiological testing can be weak and unreliable in assessing lots of food and food operations, particularly when pathogen prevalence is low. For example, in an operation that produces contaminated food with a prevalence rate of 0.5%, there is a 61% probability of accepting a positive lot although 100 samples are analyzed. In addition, microbial testing only provides a snapshot and little confidence in whether an operation can consistently produce safe foods. Thus, a new approach is needed.
The International Commission on Microbiological Specifications for Foods (ICMSF) and the Codex Committee on Food Hygiene are developing a more effective approach to food safety management. The approach involves a sequence of steps:
• Assemble epidemiologic and other relevant information indicating a need for improved control (i.e., a risk profile).
• Conduct a qualitative or quantitative risk assessment, as may be appropriate.
• Assess possible risk management options, including an appropriate level of protection.
• Establish a food safety objective (FSO).
• Confirm that the FSO is achievable through Good Manufacturing Practices/Good Hygienic Practices (GMP/GHP) and Hazard Analysis Critical Control Points (HACCP).
• Establish process/product requirements.
• Establish acceptance procedures.
This stepwise scheme can be used domestically to control food production within a country and internationally to assess the equivalency of food produced in other countries. The framework recognizes the importance of process and product design as the most effective means to ensure food safety.
Integral to this approach is the FSO, a new concept that offers significant advantages. It defines the maximum frequency or concentration of a microbiological hazard in a food at the time of consumption that will provide an appropriate level of protection. A hypothetical example of an FSO is that the level of Listeria monocytogenes in ready-to-eat foods must not exceed 100 cfu/g at the time they are consumed. In practice, FSOs are realized through the application of GMP/GHP and HACCP and may involve performance, process, product, and/or default criteria that specify the conditions of a process or product which are necessary to meet an FSO. Performance criteria specify the expected level of control at one or more steps in the food chain, such as ≥ 5 log10 reduction of enteric pathogens (i.e., salmonellae, Escherichia coli O157:H7) in citrus juice.
In practice, the expected level of control is achieved through the use of process and/or product criteria. Process criteria appear commonly as critical limits in HACCP plans. Product criteria—e.g., pH of 4.6 or below for high-acid shelf-stable canned foods—relate to attributes of the food that must be controlled and may involve chemical, physical, organoleptic, and/or microbiological values. Because some food operators do not have the resources to develop the scientific information needed to support the selection of their processing conditions, they must rely on guidance provided by an agency or other recognized authority. Thus, agencies often issue conservative, default values as reliable, minimum requirements in guidance material and regulations.
--- PAGE BREAK ---
Impact of Changes in Microbial Survivor Models on Industry
Virginia N. Scott, National Food Processors Association, Washington, D.C., and Dane Bernard, Keystone Foods, West Conshohocken, Pa.
A number of limitations to developing microbial inactivation models exist, and variability and uncertainty may be large. Modelers should assure that the methods used were adequate to produce data of sufficient quality to support model development and validation.
Ability to accurately measure the delivery of the inactivation parameters is critical in determining microbial inactivation. For thermal processes, measurement of both time and temperature delivered to the microbial population is critical in minimizing uncertainty. When evaluating the adequacy of data from a particular study, variability of the estimated heat resistance resulting from variations among strains and cells within the population and cells in different growth phases (exponential, stationary, or the spore state) should be considered.
Estimated heat resistance is also affected by growth medium and temperature, heating medium, and ability to recover injured cells and spores. Heating and cooling lags and how they are measured also have an impact on data accuracy. Inactivation parameters often will need to be extrapolated to the processing temperature or parameter of interest, adding uncertainty to any prediction. While inactivation by agents other than heat may be affected by different factors, it is likely that the parameters will similarly have effects on variability of results. When determining an appropriate modeling approach, it is important to understand sources of natural and experimental variation that will affect the ability to estimate inactivation rates.
When considering modeling alternatives, widely adopted conventions, based on application of the log-linear model, should be noted. These conventions include use of microorganisms with standardized resistance and use of conventional log reductions (5 D, 12 D). Adoption and use of these conventions has standardized calculations and provided benchmarks that facilitate comparability between studies and between process calculations derived from the studies. Models differing from the log-linear model will likely need to be accompanied by conventions that are adopted by the processing authority community.
Classical thermal death time studies assume log-linear inactivation kinetics. Nonlinear survivor curves can occur and complicate the determination of D-value, especially when count-reduction data are used in constructing thermal death curves. Using an end-point method as opposed to a count-reduction method, however, can minimize nonlinear survivor issues and provide more consistency in applying the log-linear model.
The log-linear model is widely used, relatively simple, and understood by those who use it. Applications of this model have been successful in providing information used in calculating processes that provide a margin of public health protection. Inoculated packs and the absence of failures support the model. The paramount purpose of microbial inactivation data and calculations deriving from whatever model is applied is development of a calculated process that is safe, robust in its design, and flexible in approach such that deviations can be evaluated for safety impact. Alternatives to the log-linear model will likely be expected to be adaptable to provide for these same levels of protection.
It is important to ask whether an alternative model can be readily applied by industry and is needed. It seems illogical to expect that industry would redo all processes relying on classically derived D-, z-, and F-values, based on new, unfamiliar models.
Food manufacturers should be open to application of alternative models, especially as new inactivation technology becomes available. We encourage those advocating alternative models to assure that the models provide appropriate levels of public health protection, simplicity of use, and flexibility in application. Above all, alternatives must be thoroughly validated by practitioners of process technology.
--- PAGE BREAK ---
Recommendations for Further Research
Following reflection on and debate of key points of the plenary presentations, the participants discussed research needed to elucidate and advance understanding of issues in several areas and reached consensus on the following research needs:
Survivor Curves
• Appropriate survivor-curve models should be developed and validated for all preservation technologies.
• Future publications of microbial inactivation parameters should include reference to raw data obtained during survivor-curve measurements. Discussants highlighted the desirability of maintaining raw data in data banks readily accessible by the scientific community, as is done for genomic data.
• Publications containing survivor-curve parameters should include a detailed description of methodology for data collection and analysis, including limits of detection.
• Publications should include error estimates for experimental data and uncertainty on individual parameter estimates.
Agent Intensity Rate Parameters
• A preferred universal protocol for measuring agent intensity parameters, including steps for analysis of data and reporting of results, should be developed.
• Future research efforts should include evaluation of a common model for quantifying the influence of agent intensity on microbial inactivation rate.
Statistical Variability and Uncertainty
• Future research on microbial inactivation kinetics should include measures of variability and uncertainty (population distributions need not be normal).
• Procedures for process calculations should avoid compounded conservative steps to overcome biological and process variability; however, the incorporation of a safety factor may be considered as a concluding step.
• Statistical methods to account for variability in measurements and analysis should be incorporated into process calculation procedures and validated.
• Variability within microbial populations should be assessed and expressed as appropriate statistical distributions.
Research
• Data on the influence of preservation processes on recovery of microorganisms—with attention to injury, activation, repair, germination, and growth as a function of heat, pressure, and other stresses—should be assembled and incorporated into future process models.
--- PAGE BREAK ---
• Research on low initial microbial populations (<10), and the influence on survivor-curve and preservation-process models should be initiated.
• Process models incorporating uncertainty should be developed.
• Increased research attention to methodologies for rapid measurement of microbial populations is needed to reduce time and labor for collection of microbial inactivation data. Automated benchtop instrumentation for thermal death time studies is needed for conducting more studies and doing so more rapidly.
• Future research dealing with microbial inactivation should include testing of appropriate survivor curve models. Examples for consideration include:
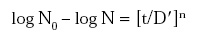
where D' = time constant and n = a constant to account for deviation from first-order
or
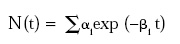
where αi = initial populations of different subpopulations and βi = rate constants for different subpopulations.
• Research should define indicator microorganisms (surrogates) for validation of new preservation technologies.
• Significant research funding is needed to demonstrate the efficacy of systems and models for new preservation technologies.
Implications
Advances in modeling of survivor curves, measuring agent intensity parameters, and dealing with statistical variability and uncertainty will impact food safety regulation and food manufacturing in several ways:
• Food Safety Regulation. In the new reality of safety assurance, FSOs are replacing Fo, creating a need for translating between Fo and FSOs. Thus, target microorganisms and target lethality must be specified. As a result: • The performance of food preservation processes should be communicated in terms of the number of log cycles of reduction that the process is expected to deliver for the microorganism of concern, rather than the D value. • Future process models must account for the agent (pressure, temperature, electric fields, etc.) used for microorganism inactivation and the potential for multiple agent parameters to achieve the desired performance. • Protocols for validating preservation processes should be standardized. • Education programs must be developed to ensure that process authorities understand HACCP as applied to new preservation technologies. • Education programs on assessing new preservation technologies must be developed for process-authority regulatory personnel.
--- PAGE BREAK ---
• Food Manufacturing. Further research in process modeling is expected to have implications for food manufacturing as well: • Process validation by processors should include microbial inactivation data to substantiate model performance and establishment of critical factors and critical control points. • The food industry and academic institutions should collaborate in the development of protocols for validation of new preservation technologies.
Summit Resolution
A measure of the success of this research summit is the resolution that the participants developed in concluding their deliberations:
“Since there is significant evidence that microbial survivor curves can be described by non-log-linear kinetic expressions, the scientific and technical community should recognize alternative models and parameters for description and communication of the survival of microbial populations when exposed to various lethal agents.”
Definitions
Summit participants agreed on the following definitions:
Model—an expression (or combination of expressions) that describes the relationship among two or more parameters.
Preservation process model—a series of expressions that predict the probability of microbial survivors from a defined preservation process.
Mathematical model—a mathematical expression describing the relationships between dependent variables (“responses”) and independent (“controllable”) variables.
Mechanistic model—a mathematical model based on some presumed molecular or physical mechanisms, i.e., a mono- or bimolecular reaction, or an enzyme-catalyzed reaction, or heat/mass transfer.
Empirical model—a purely mathematical description of observed data without any presumed mechanism (e.g., polynomials, power-law models).
Deterministic model—a mathematical model that always gives the same output when provided with the same input (no uncertainty).
Stochastic or probabilistic model—a mathematical model that gives a variable output when provided with the same input; variability is a part of the model.
--- PAGE BREAK ---
SUMMIT PROGRAM COMMITTEE
Dennis R. Heldman (organizer and moderator), Rutgers U.; Gustavo Barbosa-Cánovas, Washington State U.; Dane Bernard, Keystone Foods, LLC; Frank Busta, U. of Minnesota; Ashim Datta, Cornell U.; Michael P. Davidson, U. of Tennessee; C. Patrick Dunne, U.S. Army Natick Soldier Center; Dallas Hoover, U. of Delaware; John Larkin, Food and Drug Administration; Huub Lelieveld, Unilever PLC, The Netherlands; Charles Sizer, National Center for Food Safety and Technology; Cynthia Stewart, CSIRO/Food Science Australia; Arthur Teixeira, U. of Florida.
SUMMIT PARTICIPANTS
Gustavo V. Barbosa-Cánovas, Washington, State U.; Dane Bernard, Keystone Foods LLC; Ashim Datta, Cornell U.; Pilar De Massaguer, State U. of Campinas–UNICAMP, Brazil; Patrick Dunne, U.S. Army Natick Soldier Center; John Floros, Pennsylvania State U.; Grahame W. Gould, formerly Unilever Research, U.K.; Renée Goodrich, U. of Florida, John Hanlin, General Mills, Inc.; Kai Lai Grace Ho, Praxair, Inc.; Hans Hoogland, Unilever Research, The Netherlands; Mukund Karwe, Rutgers U.; Stephen Knabel, Pennsylvania State U.; Tatiana Koutchma, National Center for Food Safety and Technology; John Larkin, Food and Drug Administration; David Legan, Kraft Food; Bradley Marks, Michigan State U.; Arthur Miller, Food and Drug Administration; Michael Peck, Institute of Food Research, U.K.; Micha Peleg, U. of Massachusetts; Hosahalli Ramaswamy, National Center for Food Safety and Technology; Alfredo Rodriguez, Baxter Healthcare Corp.; Allan Russell, Cardiff U., U.K.; Sudhir Sastry, Ohio State U.; Don Schaffner, Rutgers U.; Virginia Scott, National Food Processors Association; Dennis Seman, Oscar Mayer Kraft; Thomas Shellhammer, Oregon State U.; Charles Sizer, National Center for Food Safety and Technology; Pieter ter Steeg, Unilever, The Netherlands; Cynthia Stewart, CSIRO/Food Science Australia; Barry Swanson, Washington State U.; Arthur Teixeira, U. of Florida; Ewen Todd, Michigan State U.; R. Bruce Tompkin, retired, ConAgra Refrigerated Foods; Arjan J. van Asselt, NIZO Food Research, The Netherlands; M.A.J.S. “Tiny” van Boekel, Wageningen U., The Netherlands; Elizabeth Webb, Brown Citrus Systems, Inc.; Richard C. Whiting, Food and Drug Administration; Ahmed Yousef, Ohio State U.
by Dennis R. Heldman and Rosetta L. Newsome
Author Heldman, a Fellow and Professional Member of IFT, is Professor, Food Process Engineering, Dept. of Food Science, Rutgers, The State University of New Jersey, 65 Dudley Rd., New Brunswick, NJ 08901. Author Newsome is Director, Dept. of Science and Communications, Institute of Food Technologists, 525 W. Van Buren St., Suite 1000, Chicago, IL 60607. Send reprint requests to author Newsome.
References
Aiba, S. and Toda, K. 1967. Thermal death rate of bacterial spores. Process Biochem. (Feb.): 35-40.
Anderson, W.A., McClure, P.J., Baird-Parker, A.C., and Cole, M.B. 1996. The application of a log-logistic model to describe the thermal inactivation of Clostridium botulinum 213B at temperatures below 121.1°C. J. Appl. Bacteriol. 80: 283-290.
Billon, C.M.P., McKirgan, C.J., McClure, P.J., and Adair, C. 1997. The effect of temperature on the germination of single spores of Clostridium botulinum 62A. J. Appl. Microbiol. 82: 48-56.
Chick, H. 1908. An investigation into the laws of disinfection. J. Hygiene 8: 92-158.
Kellerer, A.M. 1987. Models of cellular radiation action. In “Kinetics of Nonhomogeneous Processes,” ed. R.G. Freeman, pp. 305-375. John Wiley & Sons, New York.
Peleg, M. and Cole, M.B. 1998. Reinterpretation of microbial survival curves. Crit. Rev. Food Sci. 38: 353-380.
Rodriguez, A.C., Teixeira, A.A., Smerage, G.H., and Busta, F.F. 1988. Kinetic effects of lethal temperatures on population dynamics of bacterial spores. Trans. ASAE 31: 1594-1601, 1606.
Rodriguez, A.C., Teixeira, A.A., Smerage, G.H., and Lindsay, J.A. 1992. Population model of bacterial spores for validation of dynamic thermal processes. J. Food Proc. Eng. 15(1): 1-30.
Sapru, V., Teixeira, A.A., Smerage, G.H., and Lindsay, J.A. 1992. Predicting thermophilic spore population dynamics for UHT sterilization processes. J. Food Sci. 57: 1248-1257.
Sapru, V., Smerage, G.H., Teixeira, A.A., and Lindsay, J.A. 1993. Comparison of predictive models for thermophilic bacterial spore response to sterilization heat treatments. J. Food Sci. 58: 223-228.
Stephens, P.J., Joynson, J.A., Davies, K.W., Holbrook, R., Lappin-Scott, H.M., and Humphrey, T.J. 1997. The use of an automated growth analyser to measure recovery times of single heat-injured Salmonella cells. J. Appl. Microbiol. 83: 445-455.
Ueckert, J.E., Nebe von-Caron, G., Bos, A.P., and ter Steeg, P.F. 1997. Flow cytometric analysis of Lactobacillus plantarum to monitor lag times, cell division and injury. Lett. Appl. Microbiol. 25: 295-299.
van Boekel, M.A.J.S. 2002. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Intl. J. Food Microbiol. 74: 139-159.
Watson, H.E. 1908. A note on the variation of the rate of disinfection with change in the concentration of the disinfectant. J. Hygiene 8: 536.
