Irradiation and Food Safety
This Scientific Status Summary reviews recent activity surrounding food irradiation as a food safety measure and addresses the issues of concern for consumers, activists, and government.
Food irradiation is the process of exposing food to a controlled source of ionizing radiation for the purposes of reduction of microbial load, destruction of pathogens, extension of product shelf life, and/or disinfestation of produce. Irradiation has received approval for use in several food categories from the United States Food and Drug Administration (FDA) and has been proven as an effective food safety measure through more than 50 years of research. Yet, food irradiation continues to generate controversy, inhibiting broad acceptance and use.

In recent years, the U.S. food industry has made great advances toward improving the safety of our nation’s food supply. A recent report from the Centers for Disease Control and Prevention revealed the incidence of Escherichia coli O157:H7 infections are down 36% from 2002 to 2003 (CDC, 2004). Despite this, foodborne diseases continue to present unacceptable public health risks that have generated the need for still further improvements in food safety, a need that is stimulated by increased public awareness of food safety issues. The purpose of this Scientific Status Summary is to review recent activity surrounding food irradiation as a food safety measure and address the issues of concern for consumers, activists, and government in an effort to further greater understanding of this promising technology.
Importance of Food Safety
The presence of microbial pathogens on human foods is a serious global problem. Even in highly industrialized and developed countries like the United States, pathogen-contaminated foods and the resulting health and economic impacts are significant. According to CDC (2004), each year Americans suffer 76 million infections, 325,000 hospitalizations, and approximately 5,000 deaths due to pathogen-contaminated foods. These events carry an estimated annual healthcare cost totaling $7 billion (USDA/ERS, 2000). Consider also that more than 74 million lb of pathogen-contaminated meat and meat products were recalled between 2000 and 2003 (USDA/FSIS, 2004), and the need for pathogen reduction is clear.
Food pathogens enter the food supply through various extrinsic sources, such as fecally contaminated irrigation water supplies, farm workers, and food-processing plants. They may also enter via intrinsic routes, such as meat and meat products contaminated with pathogens (E. coli O157:H7, Salmonella, and Campylobacter spp.), in which case the pathogen source is the gastrointestinal tract of the slaughtered animal. A survey conducted by the U.S. Dept. of Agriculture’s Food Safety and Inspection Service (USDA/FSIS, 1998) revealed that more than 97% of young turkey carcasses were contaminated with one of five pathogens, including Campylobacter spp., Clostridium perfringens, E. coli O157:H7, Staphylococcus aureus, and Listeria monocytogenes. For the most part, these pathogens are either part of the animal’s normal microflora or are inevitable colonizers, and any amount of preharvest pathogen prevention strategies may not totally prevent contamination. The Hazard Analysis Critical Control Point (HACCP) system has been shown to greatly reduce the prevalence of pathogens (USDA/FSIS, 1999); however, improved processing technologies, such as irradiation in combination with HACCP, can further advance postharvest food safety.
Science of Irradiation
More than 50 years of research has gone into our understanding of the safe and effective operation of irradiation as a food safety measure—more than any other technology used in the industry today. Food irradiation employs controlled amounts of ionizing (having sufficient energy to create positive and negative charges) radiation to destroy bacteria, pathogens, and pests in food and agricultural products, greatly reducing the threat of foodborne disease. CDC experts estimate that irradiating half of all ground beef, poultry, pork, and processed meat would reduce food poisoning by one million cases and prevent 6,000 serious illnesses and 350 deaths (Tauxe, 2001).
--- PAGE BREAK ---
Ionizing radiation includes gamma rays (from radioactive isotopes cobalt-60 or cesium-137), beta rays generated by electron beam or “E-beam,” and X-rays. None of these irradiation sources has sufficient energy to be capable of inducing radioactivity; however, they do have enough energy to remove electrons from atoms to form ions or free radicals. The freed electrons collide with chemical bonds in the microbial DNA molecules, thereby breaking them and rendering the microbe dead. The amount of ionizing radiation absorbed is termed radiation absorbed dose and is measured in units of rads (1 rad=100 erg/g) or grays (1 Gy=100 rads), with 1 gray equal to 1 Joule/kg and 1,000 grays equal to 1 kiloGray (kGy). The level of microbe reduction is dependent on the dose absorbed by the target food (Olson, 1998). Gamma rays and X-rays are able to penetrate further into foods than beta rays; therefore, E-beam generators arranged to deliver electrons from one side can penetrate about 1.5 in in food; two-sided treatment can achieve maximum penetration, up to about 3.5 in (GAO, 2000).
Ionizing radiation can damage the nucleic acids and ultimately kill microbes by direct or indirect “hits.” In the case of an indirect hit, damage to the nucleic acids occurs when the radiation ionizes an adjacent molecule, which in turn reacts with the genetic material. Because water is the largest component of most foods and microbes, it is often the adjacent molecule that ends up producing a lethal product (Grecz et al., 1983). Ionizing radiation causes water molecules to lose an electron, producing H2O+ and an electron. These products react with other water molecules to produce a number of compounds, including hydrogen and hydroxyl radicals (OHÿ), molecular hydrogen, oxygen, and hydrogen peroxide (H2O2) (Arena, 1971). Hydroxyl radicals and hydrogen peroxide are very reactive and are known to interfere with the bonds between nucleic acids within a single strand or between opposite strands. Though biological systems do have a capacity to repair both single-stranded and double-stranded breaks of the DNA backbone (Bartek and Lukas, 2003), the damage occurring from ionizing radiation is random (Razskazovskiy et al., 2003) and extensive. Therefore, bacterial repair of radiation damage is a near impossibility.
The relative sensitivity of different microorganisms to ionizing radiation is based on their respective D10 values (which is the dose required to reduce the population by 90%). Lower D10 values indicate greater sensitivity of the organism in question. The data in Table 1 shows that minimal doses can achieve significant gains in food safety.
Microbial cells, whether pathogenic or comprising the normal microflora of foods, exhibit differences in their responses to ionizing radiation. The key factors that control the resistance of microbial cells to ionizing radiation are the size of the organism (the smaller the target organism, the more resistant it is to ionizing radiation), type of organism (i.e., cell-wall characteristics and gram positive or gram negative in nature), number and relative “age” of the cells in the food sample, and absence or presence of oxygen. The physical and chemical composition of the food also affects microbial responses to irradiation. For example, as the temperature of ground turkey is decreased from 30°C to –30°C (Table 1), the D10 value increases from 0.16 kGy to 0.29 kGy. D10 values change as the water in the product freezes, thereby decreasing the rate of migration of the ionization products, including free radicals, and requiring greater energy input to cause the collisions necessary to destroy the microbes (Thayer, 2004).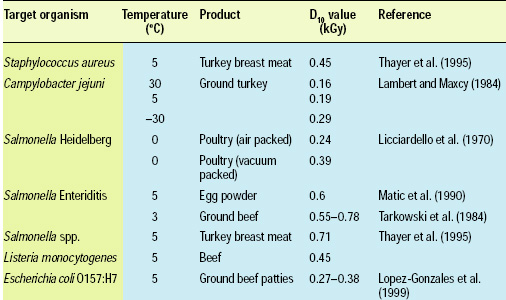
Effectiveness and Benefits of Irradiation
Aside from the obvious improvements in food safety through destruction of pathogens, irradiation provides other benefits. Some of these contributions include increasing shelf life of meats (Murano et al., 1998; Thayer, 1993) and fruits and vegetables (Thayer and Rajkowski, 1999); improving quality of fruits and vegetables (Thayer and Rajkowski, 1999); providing a suitable alternative to chemical treatments (e.g., methyl bromide and ethylene oxide), especially for decontamination of fruits and vegetables (Thayer and Rajkowski, 1999); and providing economic savings due to reduced incidence of illness. Despite these added benefits, this technology remains vastly underutilized in the food industry.
--- PAGE BREAK ---
Issues Confronting Widespread Use of Irradiation
Several extensive reviews of toxicological and other data by regulatory and health organizations, including Health Canada (2003), FDA (1986), Codex Alimentarius Commission (CAC, 1983), and European Commission’s Scientific Committee on Food (EC, 2003), have determined that food irradiated below 10 kGy is safe. More recently, the CAC (2003) revised slightly its General Standard for Irradiated Foods, stating that the maximum absorbed dose delivered to a food should not exceed 10 kGy, except when necessary to achieve a legitimate technological purpose.
In 1999, a joint study group of the U.N.’s Food and Agriculture Organization (FAO), International Atomic Energy Agency (IAEA), and World Health Organization (WHO) concluded that food irradiated to any dose appropriate to achieve the intended technological objective is both safe to consume and nutritionally adequate. The group also concluded that no upper limit on absorbed dose was necessary because use of irradiation would be limited to doses that do not detrimentally impact the sensory attributes, thus creating a practical cut-off at about 50–75 kGy (WHO, 1999). The group’s report included all pertinent animal feeding studies (82 in total), mutagenicity studies (47 in vitro), and food type and test species through 1997. Although 14 studies showed an effect, the cause was attributed in each case to a diet/nutrient deficiency, not irradiation. It is important to remember that these trials involved feeding diets containing significant amounts of food items (average 35%–100%) irradiated at very high doses, often to 59 kGy. There were eight possible effects of high-dose radiation observed in the mutagenicity studies. Two of the studies involved feeding irradiated oils, which apparently caused extensive oxidation and loss of carotenoids. The other six studies used irradiated simple sugar solutions (e.g., sucrose, fructose, glucose, etc.) that are now known to involve formation of mutagens by radiation-induced chemical mechanisms (Fan, 2003).
In 1976, the U.S. government contracted Raltech Scientific Services to carry out comprehensive nutritional, genetic, and toxicological studies of food irradiation. Mice, hamsters, rats, and rabbits were fed chicken (as 35–70% of their diet) that had been irradiated at a minimum absorbed dose of 46 kGy. Dogs, rats, and mice were also fed the irradiated chicken at 35% of their diet during multigenerational studies. They found no evidence of genetic toxicity or teratogenic effects in mice, hamsters, rats, or rabbits and no treatment-related abnormalities or changes in the multigenerational studies (Thayer et al., 1987).
• Radiolytic Products. During the last 25 years (since the advent of gas chromatography/mass spectrometry, GC/MS), numerous volatile compounds have been isolated from irradiated products. The vast majority (more than 70%) of the radiolytic volatile compounds found in irradiated foods are hydrocarbons, such as alkanes, alkenes, ketones, and aldehydes, that are commonly found in unprocessed and thermally processed foods (Hannisdal, 1993; Morehouse et al., 1991; Nawar et al., 1990) and are considered safe for human consumption.
Two groups of compounds have generated concern. They are benzene (and its derivatives) and alkylcyclobutanones (ACBs). The Federation of American Societies for Experimental Biology evaluated 65 compounds found in beef and noted that small amounts of benzene could be detected in both irradiated (15 ppb) beef (56 kGy) and non-irradiated (3 ppb) beef (Chinn, 1979). This expert committee concluded that such small amounts of benzene do not constitute a significant risk. Health Canada’s Bureau of Chemical Safety reached a similar conclusion upon evaluation of an application for irradiated ground beef (Health Canada, 2002). Health Canada estimated that approximately 3 ppb of benzene would be formed in beef irradiated at the typical dose ranges (1.5–4.5 kGy). This level of benzene was noted to be much lower than naturally occurring levels in haddock (200 ppb) and eggs (average 62 ppb) (McNeal et al., 1993). Thus, the risk of benzene exposure from irradiated foods is considered negligible.
ACBs were first identified in irradiated fats due to the pioneering work of LeTellier and Nawar (1972). When pure triglycerides containing C6, C8, C10, C12, C14, C16, and C18 fatty acids were subjected to irradiation (60 kGy in vacuum), 2-substituted ACBs (2-ACBs) were formed having the same number of carbon atoms as the acids from which they were derived. Thus, when the four major fatty acids present in most foods (palmitic, stearic, oleic, and linoleic acid) are irradiated, they are converted to their corresponding cyclobutanones, 2-dodecyl (2-DCB), 2-tetradecyl (2-TCB), 2-tetradecenyl (2-TDCB), and 2-tetradecadienyl cyclobutanone (2-TDeCB). As yet, these ACBs have not been found in raw or heat-processed foods and are considered unique radiolytic products (Crone et al., 1992a; Stevenson, 1994).
--- PAGE BREAK ---
• Mutagenic/Genotoxic Studies. Current discussions on the potential mutagenicity of irradiated foods have centered on the work of Burnouf et al. (2002), which has shown that the radiolytic compounds originating from 2-ACB fatty acids appear to induce DNA damage under unique experimental conditions. Cell cultures were evaluated for toxicity when exposed to concentrations of up to 400 μMolar. Cytotoxicity was observed in some cultures at 50 μM, with most ACBs exhibiting toxicity at 100 μM. Genotoxicity of the cultures was measured with the DNA Computerized Molecular Evaluation of Toxicity (Comet) assay after a 30-min exposure and no significant differences were found. DNA strand breaks in two different cell lines were also measured with ACB concentrations of up to 40 ppm (90 for 2-TCB). The effects seem to appear at 10 ppm for some ACBs, though cell death that starts at 25 ppm may confound the data and call it into question. With one of the cell lines there appeared to be fewer DNA breaks than controls at low levels of ACBs (10 ppm). The breaks increased with concentration; however, there was extensive cell death at the higher levels (Delincée and Pool-Zobel, 1998).
Raul et al. (2002) induced colon cancer in rats with azoxymethane (AOM) injection and then fed either 2-TDCB or 2-TDeCB (50 ppm) in drinking water ad libitum. Tumor development was followed for 27 weeks post injection. The total number of colon preneoplastic lesions was the same for all treatments, indicating that the cyclobutanones did not increase colon lesions. The only significant differences were that the treated animals developed larger lesions and a greater number of larger tumors. These findings are suspect, however, because each treatment group contained only six animals, which is probably insufficient to draw conclusions, and there was not a dose-dependent response. Also, the control animals, which received ACB in the drinking water, did not develop lesions unless they had been preinjected with AOM.
Another study measured the recovery of dietary 2-TCB and 2-TDCB included in the daily feed of rats (Horvatovich et al., 2002). After four months of feeding, no 2-TDCB was recovered. Small amounts of 2-TDCB were recovered in the feces (1%), and only a trace amount was found in the adipose tissue (0.3 ppm). The authors concluded that the lack of recovery was a concern. If the cyclobutanones were catabolized via some oxidative mechanism to some type of water-soluble lactone, then it would have been quickly eliminated or metabolized for energy production. Thus, metabolism, a desirable outcome of consumption, may suggest a lack of toxicity.
Health Canada (2003) released an evaluation of the ACB genotoxic data, expressing the opinion, similar to that of the EC’s Scientific Committee on Food (EC, 2002), that the Comet test was inappropriate because it does not perform well for weak agents, of which the 2-ACBs would qualify, and is not “validated or adequately standardized.” They also found that the concentrations of 2-DCB tested were very high compared to human consumption levels. Based on the levels of 2-DCB measured by Burnouf et al. (2002) in chicken (0.342 mg/g lipid/kGy) and ground beef (0.409 mg/g lipid/kGy) and the average consumption by Canadians, Health Canada calculated that the amount of 2-DCB ingested via chicken and via ground beef would be 8,500 and 10,000 times lower, respectively, than the lowest dose deemed to elicit a Comet response. Other researchers have reported lower 2-DCB levels in irradiated beef, pork, and chicken—0.2 mg (per g of lipid) when processed at 1 kGy and 1–1.2 mg (per g of lipid) at 5 kGy (Stevenson, 1994), which would further dilute the value of the Comet assay.
The EC’s Scientific Committee on Food released a revision of its 1986 opinion on food irradiation that addressed the ACB toxicity concerns (EC, 2003). It was the consensus of the committee that the genotoxicity of ACBs had not been established because there was no mutagenic effect in the Ames test or in standard cell lines. Burnouf et al. (2002) and Gadgil and Smith (2004) have evaluated cyclobutanones for their mutagenic potential using the classical Ames test. No mutagenicity was observed either with or without liver microsomal activation. Sommers and Schiestl (2004) evaluated the 2-ACBs for mutagenicity with the E. coli TRP assay and for a DNA-strand-induced recombination and were unable to find any 2-ACB effects.
Gadgil and Smith (2004) also found that 2-DCB was of low toxicity in the Microtox assay with Vibrio fischeri cells. Their results indicated that 2-DCB is similar in toxicity to the food additive cyclohexanone (which has Generally Recognized as Safe status), and was 10-fold less toxic than t-2 nonenal, a normal constituent of cooked ground beef and an approved food additive (GRAS status flavorant), indicating that 2-DCB has very low toxicity and does not warrant concern.
--- PAGE BREAK ---
• Vitamin and Nutrient Losses. In general, macronutrient (protein, lipid, and carbohydrate) quality does not suffer due to irradiation (Thayer, 1990; Thayer et al., 1987; WHO, 1999), and minerals have also been shown to remain stable (Diehl, 1995).
There is a fair amount of concern over the effect of irradiation on other micronutrients, especially vitamins. In most studies, vitamins have been shown to retain substantial levels of activity post irradiation. Vitamins A, C, and E are more sensitive and are thereby reduced at higher doses of irradiation, even though these losses are often similar to those occurring with thermal processing. Vitamin E is the most sensitive of the fat-soluble vitamins with significant losses (50%) occurring when irradiated in the presence of oxygen. When oxygen was excluded or vacuum packaging was used, the losses were less than 10% (Josephson et al., 1975). Significant losses were shown to occur in cream cheese (vitamin A)when air was not excluded (Diehl, 1979) and in fruits and vegetables (vitamin C) treated with high doses. However, these findings are irrelevant because high-dose radiation is not used for such products.
Thiamine (vitamin B1) has been shown to be the most vulnerable to radiation and is therefore used to demonstrate “worst-case” results (WHO, 1994). Significant losses can occur in irradiated meat products (Fox et al., 1995; Graham et al., 1998; Thayer, 1990). However, the extent of such losses is dependent on processing conditions (temperature and dose) and can be minimized using packaging techniques (Fox et al., 1997). Meats, with the exception of pork, do not make major dietary contributions to B1 intake (Subar et al., 1998). Therefore, FDA and Health Canada have determined that even with high irradiation doses, thiamine intake would still be above its recommended dietary allowance. FDA has concluded that the effects of irradiation processing on nutrient quality are similar to those of conventional food-processing methods.
• Sensory Changes. Foods such as milk, certain cheeses, eggs, and some fruits and vegetables are not likely candidates for irradiation because of the potential for undesirable off-odors, flavors, and texture changes (WHO, 1999). The bulk of sensory work has focused on muscle foods, because most of the emphasis for this technology has been on these foods (Molins, 2001).
Two groups have evaluated ground beef under various conditions of radiation dose (0–4.5 kGy), temperature (–25°C to room temperature), and packaging (Murano et al., 1998; Vickers and Wang, 2002). These researchers have shown that irradiation causes no significant differences in the flavor, texture, or color of beef irradiated at less than 3 kGy (Murano et al., 1998; Vickers and Wang, 2002).
Luchsinger et al. (1996) evaluated acceptance of fresh or frozen irradiated boneless pork chops at 1.5, 2.5, and 3.85 kGy using a trained panel and consumers (n=108). They found no significant differences in acceptance, meatiness, freshness, or juiciness in products irradiated at 2.5 kGy or below.
However, some researchers have shown that poultry and pork are sensitive to flavor and color (pinking) changes (Houser et al., 2003; Nam and Ahn, 2002). Several studies have been published recently to address this issue. Process techniques (packaging and antioxidants) that improve these meat characteristics are being evaluated (Bagorogoza and Bowers, 2001; Nam et al., 2004) and, in at least one instance, consumers have shown preference for the pink color (Lee et al., 2003).
Although there have been fewer studies with fruits and vegetables, the use of low-dose irradiation as a countermeasure to quarantine (due to pest infestation) and/or for extension of shelf life is promising. Follet and Sanxter (2002) studied the tropical fruits and found papayas, rambutans, and Kau oranges were acceptable when treated with a quarantine level of 0.75 kGy (minimum dose required is 0.25 kGy). They also found Chompoo and Biew Kiew fruit to be more acceptable when treated with 0.40 kGy than with the currently used hot-water immersion. Due to restrictions on chemical treatments and the increasing demand of imported products, application of low-dose irradiation has become an active area of research.
--- PAGE BREAK ---
Concerns expressed by Anti-Irradiation Groups
• Misuse to Avoid Plant Sanitation. A common concern stated by those opposed to food irradiation is that it would be used as an alternative to proper food-processing plant sanitation and cleanliness practices. A similar argument was used to dissuade implementation of milk pasteurization in the early 1900s. Today, milk pasteurization is a commonly used practice proven to have prevented countless illnesses due to milkborne salmonellosis (Satin, 1996). Heavily contaminated food requires higher doses that would have a negative impact on the acceptability of the product. Using food irradiation to overcome inadequate sanitation practices, or irradiating only selected lots or batches of food (having documented pathogen presence) with radiation doses, would be counter productive and serve as a death knell to this food processing technology. Food irradiation is intended as the final step of a comprehensive HACCP program.
• Environmental Concerns. There are lingering concerns among opponents to food irradiation regarding the environmental safety of irradiation facilities. Issues surrounding use, safety, and exposure to radioactive materials are often promoted as a concern relative to food irradiation, while similar concerns have not been major issues pertaining to the use of irradiation to sterilize medical equipment and other healthcare products (Derr, 1993). Regulation of irradiation facilities is dependent on the source used. Gamma facilities have specific characteristics to protect workers (regulated by the Occupational Safety and Health Administration) and the surrounding environment from the radioactive isotopes and for storing the isotope material under water when not in use, which are regulated by the U.S. Nuclear Regulatory Commission. Cobalt-60, the isotope used in such facilities, requires 16–21 years to decay to approximately 6–12% of its initial activity level, at which time it is shipped back to the manufacturer in hardened steel shipping canisters to be regenerated and reused. Unlike gamma facilities, E-beam and X-ray do not employ radioactive sources and thereby avoid such issues. They do contain a significant amount of electrical circuitry, cooling systems, worker safety systems, and ozone attenuation capabilities (Olson, 1998). These facilities are regulated by FDA and by state agencies that regulate other medical, dental, and industrial devices.
Production of ground-level ozone from E-beam facilities has also been cited as a concern. Ozone is produced when the accelerated electrons come into contact with air and is routinely exhausted when interior levels reach maximum continuous exposure levels. It must be emphasized that there are state and federal rules governing ozone emissions by industrial facilities. The National Ambient Air Quality Standards have set the upper emission limit at 0.12 ppm/hr (40 CFR 50.9). Thus, E-beam irradiation facilities are not permitted to operate if ozone emissions exceed this limit.
State of the Technology
• Regulatory Summary. FDA evaluates irradiation as a food additive on the logic that it affects the characteristics of the food or becomes a component in the food; however nothing is physically added to the food. Other processes such as baking, frying, boiling, etc., cause chemical changes in the food and they are not considered additives, but processes. Regardless, the United States currently has the most widespread approvals for the use of irradiation for food (Table 2).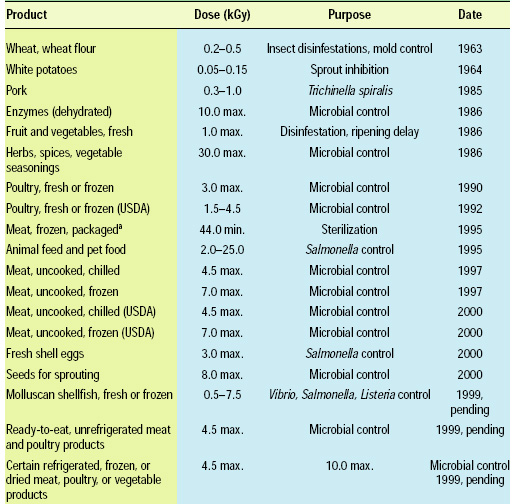
FDA labeling requirements call for inclusion of the radura, which is the symbol developed to signify a food having been irradiated. Also, the words “treated with radiation” or “treated by irradiation” must be printed on the package, unless the word “irradiated” is part of the product name (21 CFR 179.26).
In Canada and Europe, approvals are more limited. Canada has issued approvals for use on potatoes, onions, spices, dehydrated seasonings, wheat, and flour (Health Canada, 1989). The addition of poultry, beef, shrimp, prawns, and mangoes to the Health Canada approved list has been in the approval process since 2002 and was expected to receive approval in the first half of 2004 (Dalpe, 2004). Until 1999, use in Europe varied from country to country; however, due to concerns among the EU member states, the European Parliament has issued directives to establish a community list. The current list contains only dried aromatic herbs, spices, and vegetable seasonings (EC, 1999). EC (2003) issued a report reconfirming its resistance to expansion of this list. A lack of breadth of the human clinical studies database was cited as the reason behind this decision.
--- PAGE BREAK ---
• Market Status. Despite its promise, irradiation is not a major factor in today’s food-processing environment. According to a report released by the U.S. General Accounting Office, as of January 2000, irradiated fruits and vegetables and fresh and frozen uncooked poultry accounted for only 0.002% of annual U.S. consumption in each of their respective categories. Irradiation to preserve spices and botanicals is the largest area of application and is estimated at 9.5% of annual U.S. consumption (GAO, 2000). The report states that at that time, irradiated beef and pork products were not available commercially. Since then, irradiated beef has been placed in supermarkets; however, sales have staggered at least partly due to inconsistency in availability. In January 2004, a major irradiation company, SureBeam Corp., filed for Chapter 7 bankruptcy, prompting several grocery stores and a major fast-food chain to suspend sale of irradiated ground beef. Two other irradiation facilities, Food Technology Services, Mulberry, Fla., and CFC Logistics, Quakertown, Pa., have picked up much of that business.
• Consumer Acceptance. Over the years, polls have revealed acceptability rates ranging from 45% to more than 90%, depending on the food type and method of presentation (Fox, 2002). Nayga et al. (2004) published a report stating that consumers would purchase irradiated foods and are “willing to pay” premiums ranging from $0.05 to $0.50/lb., depending on their level of concern and awareness and the provision of sufficient background information. These findings emphasize the importance of educating the public on the controversy, the technology, and the benefits of irradiation, especially since the public has been shown to be more receptive to the negative argument (Fox, 2002; Hayes et al., 2002).
• National Nutrition Programs. As a result of the 2002 Farm Bill, which directs USDA to utilize any and all approved food safety technologies for food purchased through the National Nutrition Programs, irradiated ground beef became an option for school lunches in January 2004. The product comes at a premium ($0.13–0.20/lb), and the decision to use it resides with each individual district. A letter from USDA Under Secretary of Nutrition and Consumer Services Eric Bost to school superintendents encouraged them to inform parents and children of the decision to include irradiated meat; however, USDA cannot require such action (USDA, 2003). There has been backlash to the provision of irradiated ground beef, with some districts (e.g., Washington, D.C., Berkeley, Calif., and Los Angeles, Calif.) quickly moving to prohibit its use. However, as of July 2004, 200 of the 26,000 school districts decided to purchase irradiated ground beef (Eustice, 2004).
Research Needs
• Pathogen Reduction Protocols. Standardization of pathogen reduction protocols is a much needed area of research. Currently there is no required “kill” such as that established for E. coli O157:H7 in juices (i.e., a 5-log10 reduction). Such standards are needed to establish global continuity and enable trade.
• Inactivation Kinetics of Foodborne Viruses. Enteric viruses (Noroviruses and Rotavirus) are responsible for a significant number of food-borne illnesses in the United States (Mead et al., 1999), but are generally assumed to be unaffected by radiation. Recent studies suggest that, depending on the sample matrix, viruses can become sensitive to E-beam radiation at levels significantly lower than those produced with cobalt-60 irradiation (Pillai and Espinosa, 2003). Studies are needed to identify the conditions that can eliminate viral pathogens in ready-to-eat (RTE) foods and minimally-processed fruits and vegetables.
• Radiosensitization. Studies show that certain chemical components, when added extraneously, can significantly reduce the D10 value of a particular pathogen. The precise mechanisms that are involved in this radiosensitization of microbial pathogens need to be further elucidated. A better understanding of the factors controlling the sensitization of microbial pathogens can allow for the incorporation of specific “sensitizing” molecules directly to the food, the matrix, or the packaging materials to attain or prevent a desired level of nucleic acid damage.
--- PAGE BREAK ---
• Microbial Stress Conditions and Radiation Sensitivity. Recent studies have shown that the physiological state of the cell is critically important when evaluating its radiation resistance. Buchanan et al. (1999) reported that different strains of the same pathogen can exhibit significant differences in radiation sensitivity, presumably a reflection of their physiological status. Microbial cells in the starvation mode can also exhibit increased resistance to radiation. Since starved or moribund cells have a significantly reduced number of DNA replication forks, the potential targets for DNA damage are subsequently reduced. Stress-induced proteins and other cellular components such as lipid and protein-rich foods may either protect the cells, or enhance DNA repair under optimal conditions. Studies have also shown that carbon monoxide in MAP and hydrogen peroxide treatments can also protect microorganisms from ionizing radiation to varying degrees.
The precise mechanism of protection or repair needs to be elucidated so that appropriate strategies (e.g., microbial hurdle techniques) can be adopted when irradiating such foods. A number of other stress factors, such as osmotic stress, heat stress, and alkali stress, can also enhance radiation resistance. Thus, when D10 values are established for specific foods, the possibility that these factors (in addition to the physical state of the food matrix) may influence the behavior of pathogens and indigenous organisms must be taken into consideration.
• Organoleptic Attributes. There is an urgent need for standardization to evaluate sensory changes or organoleptic attributes of irradiated products as they relate to radiation sources, irradiation conditions, dosimetry, and product profiles. Without such standardization, it would be difficult to compare and analyze irradiation results. There is also a need to objectively characterize and quantify adverse or positive changes in these attributes analytically.
• Multi-Component Foods. Once federal approvals for RTE foods are obtained, there will be a significant set of opportunities to use food irradiation for multi-component foods, such as RTE meals. The issues of dosimetry, pathogen reduction, and sensory will be extremely significant in these types of foods because of the anticipated differences in the food matrix, potential varying pathogen loads, types of pathogens that could be encountered, and the critical need to retain the sensory attributes of the packaged meals.
• Product Packaging. Research is needed on the next generation of packaging materials to retard negative sensory attributes or enhance desirable ones. The combination of modified-atmosphere packaging (MAP) and irradiation has been reported to enhance desirable changes and improve safety of sausage (Ahn et al., 2002), ground beef (Kusmider et al., 2002), turkey (Bagorogoza and Bowers, 2001), fresh-cut iceberg lettuce (Fan and Sokorai, 2002), and romaine lettuce (Prakash et al., 2000). The use of antimicrobial coatings (Vachon et al., 2003) and antioxidant additions (Lee et al., 2003) also provide avenues that could potentially extend the usage of irradiation. The development of packaging materials that can visually denote an irradiated product or dose range, or detect adverse changes in a product would also be beneficial.
A Safe and Effective Process
An overwhelming body of evidence spanning a period of more than 50 years supports the FDA determination that food irradiation can be used without posing a human health hazard and that furthermore, its use will improve the microbial safety of the food supply. This technology has been proven beneficial for not only controlling pathogens, but also increasing shelf life and maintaining food quality. Irradiation to ensure food safety is to be implemented as part of an overall HACCP plan and is not meant to replace existing control measures.
Recent attention has focused on the formation of unique radiolytic products because initial reports revealed the possibility of associated carcinogenicity. However, Burnouf et al. (2002) warned against applying their findings directly, did not find positive results for the Ames test, and used only pure ACBs in quantities much greater than those measured in actual foods. Since the release of that report, several researchers have refuted the findings of Burnouf et al. and indicated that levels in irradiated foods do not warrant a public health concern. Food irradiation is a safe and effective process that can be used to improve the safety of our food supply.
by J. Scott Smith, Ph.D., and Suresh Pillai, Ph.D.
Author Smith is Professor of Food Chemistry, Dept. of Animal Science & Industry, Kansas State University, 208 Call Hall, Manhattan, KS 66506, and author Pillai is Professor of Food Safety & Environmental Microbiology and Director of the National Center for Electron Beam Food Research, Texas A&M University, 418D Kleberg Center, College Station, TX 77843-2472. Both are Professional Members of IFT. Send reprint requests to Marcia Bruxvoort at 312-782-8424 or [email protected].
References
Ahn, H.J., Kim, J.H., Jo, C., Lee, C.H., and Byun, M.W. 2002. Reduction of carcinogenic N-nitrosamines and residual nitrite in model system sausage by irradiation. J. Food Sci. 67: 1370-1373.
Arena, V. 1971. “Ionizing Radiation and Life.” Mos by, St. Louis, Mo.
Bagorogoza, K. and Bowers, M. 2001. The effect of irradiation and modified atmosphere packaging on the quality of intact chill-stored turkey breast. J. Food Sci. 66: 367-372.
Bartek, J. and Lukas, J. 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3: 421-429.
Boyd, D.R., Crone, A.V.J., Hamilton, J.T.G., Hand, M.V., Stevenson, M.H., and Stevenson, P.J. 1991. Synthesis, characterization, and potential use of 2-dodecylcyclo-butanone as a marker for irradiated chicken. J. Agric. Food. Chem. 39: 789-792.
Buchanan, R.L., Edelson, S.G., and Boyd, G. 1999. Effects of pH and acid resistance on the radiation resistance of enterohemorrhagic Escherichia coli. J. Food Prot. 62: 219-228.
Burnouf, D., Delincée, H., Hartwig, A., Marchioni, E., Miesch, M., Raul, F., and Werner, D. 2002. Toxikologische Untersuchung zur Risikobewertung beim Verzehr von bestrahlten fetthaltigen Lebensmitteln. (Eine französisch-deutsche Studie im Grenzraum Oberrhein). Schlussbericht INTERREG II, Projekt No 3.171) Eds. Eric Marchioni and Henry Delincée. Bundesforschungsanstalt für Ernährung Karlsruhe. www.bfa-ernaehrung.de/Bfe-Deutsch/Information/e-docs/bfer0202.pdf.
CAC. 1983. Codex General Standard for Irradiated Foods. CODEX STAN 106-1983, joint FAO/WHO Food Standards Programme. United Nations Food and Agriculture Organization/World Health Organization/Codex Alimentarius Commission, Rome, Italy.
CAC. 2003. Revised Codex General Standard for Irradiated Foods. CODEX STAN 106-1983, Rev. 1-2003. Codex Alimentarius Commission, Rome, Italy. ftp://ftp.fao.org/codex/standard/en/CXS_106e_1.pdf.
CDC. 2004. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—Selected sites, United States, 2003. Morb. Mortal. Weekly Rep. 53(16): 338-343.
Chinn, H.I. 1979. Evaluation of the health aspects of certain compounds found in irradiated beef. Chapter I: Further toxicological considerations of volatile products, pp 1-29. Life Sciences Research Office, Federation of American Societies for Experimental Biology, Bethesda, Md.
Crone, A.V.J., Hamilton, J.T.G., and Stevenson, M.H. 1992a. Detection of 2-dodecylcyclo-butanones in radiation sterilized chicken meat stored for several years. Intl. J. Food Sci. Technol. 27: 691-696.
Crone, A.V.J., Hamilton, J.T.G., and Stevenson, M.H. 1992b. Effects of storage and cooking on the dose response of 2-dodecylcyclobutanone, a potential marker for irradiated chicken. J. Sci. Food Agric. 58: 249-252.
Dalpe, C. 2004. Personal Communication of the Associate Director, Food Regulatory Program, Bureau of Food Regulatory and Interagency Affairs, Food Directorate, Health Products and Food Branch, July 27. Health Canada, Ottawa.
Delincée, H. and Pool-Zobel, B.L. 1998. Genotoxic properties of 2-dodecylcyclo-butanone, a compound formed on irradiation of food containing fat. Rad. Phys. Chem. 52(1): 39-42.
Derr, D.D. 1993. International regulatory status and harmonization of food irradiation. J. Food Prot. 56: 882-886.
Diehl, J.F. 1979. Vitamin A in bestrahiten Lebensmittel (Vitamin A in irradiated foodstuffs). Zeitschrift fur Lebensmittel-Untersuchung und-Forschung 168: 29-31.
Diehl, J.F. 1995. “Safety of Irradiated Foods,” 2nd edition. Marcel Dekker, New York.
EC. 1999. Directive1999/3/EC on the establishment of a community list of foods and food ingredients treated with ionising radiation. European Commission, Brussels, Belgium.
EC. 2002. Statement of the Scientific Committee on Food on a report on 2-alkylcyclo-butanones. European Commission, Brussels, Belgium. www.iaea.or.at/icgfi/documents/out135_en.pdf.
EC. 2003. Revision of the opinion of the Scientific Committee on Food on the irradiation of food. European Commission, Brussels. http://europa.eu.int/comm/food/fs/sc/scf/out193_en.pdf.
Eustice, R.F. 2004. Marketing and consumer acceptance of irradiated foods. Presented at the Institute of Food Technologists’ Annual Meeting, Las Vegas, Nev. Abstract 36-4.
Fan, X. 2003. Ionizing radiation induces formation of malonaldehyde, formaldehyde, and acetaldehyde from carbohydrates and organic acid. J. Agric. Food Chem. 51: 5946-5949.
Fan, X. and Sokorai, K.J. 2002. Sensorial and chemical quality of gamma-irradiated fresh-cut iceberg lettuce in modified atmosphere packages. J Food Prot. 65: 1760-1765.
FDA. 1986. Irradiation in the production, processing, and handling of food. Fed. Reg. 51(75): 3376-13399.
Follett, P.A and Sanxter, S.S. 2002. Longan quality after hot-water immersion and X-ray irradiation quarantine treatments. Hort. Sci. 37: 571-574.
Fox, J.A. 2002. Influence on purchase of irradiated foods. Food Technol. 56(11): 34-37.
Fox, J.B., Lakritz, L., Hampson, J., Richardson, R., Ward, K., and Thayer, D.W. 1995. Gamma irradiation effects on thiamin and riboflavin in beef, lamb, pork, and turkey. J. Food Sci. 60: 596-598, 603.
Fox, J.B., Lakritz, L., and Thayer, D.W. 1997. Thiamin, riboflavin, and a-tocopherol retention in processed and stored irradiated pork. J. Food Sci. 62: 1022-1025.
Gadgil, P. and Smith, J. S. 2004. Mutagenicity and acute toxicity evaluation of 2-dodecylcyclobutanone. J. Food Sci. 69 (in press).
GAO. 2000. Food Irradiation: Available Research Indicates that Benefits Outweigh Risks. GAO/RCED-00-217. U.S. General Accounting Office, Washington, D.C.
Graham, W.D., Stevenson, M.H., and Stewart, E.M. 1998. Effect of irradiation dose and irradiation temperature on the thiamin content of raw and cooked chicken breast meat. J. Sci. Food Ag. 78(4): 559-564.
Grecz, N., Rowley, D.B., and Matsuyama, A. 1983. The action of radiation on bacteria and viruses. In “Preservation of Foods by Ionizing Radiation,” Vol. 2. CRC Press, Boca Raton, Fla.
Hannisdal, A. 1993. Analysis of lipid-derived volatiles (alkanes and alkenes) in irradiated foods (salmon and chicken). In “Proc. Workshop on Recent Advantage on Detection of Irradiated Food, BCR Information, Chemical Analysis, Report EUR 14135 EN,” ed. M. Leonardi, J. J. Raffi, J. J. Belliardo, pp. 295-302. Commision of the European Communities, Brussels.
Hayes, D.J., Fox, J.A., and Shogren, J.F. 2002. Experts and advocates: How information affects the demand for food irradiation. Food Policy 27(2): 185-193.
Health Canada. 1989. Food & Drug Act. Division 26, pp. 383-384. Ottawa.
Health Canada. 2002. Irradiation of ground beef: Summary of submission process. October 29. Food Directorate, Food Products and Health Branch, Ottawa. www.hc-sc.gc.ca/food-aliment/fpi-ipa/e_gbeef_submission.pdf.
Health Canada. 2003. Evaluation of the significance of 2-dodecylcyclobutanone and other alkylcyclobutanones. Ottawa. www.hc-sc.gc.ca/food-aliment/fpi-ipa/e_cyclobutanone.html.
Horvatovich, P., Raul, F., Miesch, M., Burnouf, D., Delincée, H., Hartwig, A., Werner, D., and Marchioni, E. 2002. Detection of 2-alkylcyclobutanones, markers for irradiated food in adipose tissues of animals fed with these substances. J. Food Prot. 65: 1610-1613.
Houser, T.A., Sebranek, J.G., and Lonergan, S.M. 2003. Effects of irradiation on properties of cured ham. J. Food Sci. 68: 2362-2365.
Josephson E.S., Thomas M.H., and Calhoun, W.K. 1975. Effects of treatments of foods with ionizing radiation. In “Nutritional Evaluation of Food Processing,” ed. R.S. Harris and E. Karmas, pp. 393-411. AVI, Westport, Conn.
Kusmider, E.A., Sebranek, J.G., Lonergan, S.M., and Honeyman, M.S. 2002. Effects of carbon monoxide packaging on color and lipid stability of irradiated ground beef. J. Food Sci. 67:3463-3468.
Lambert, J.D. and Maxcy, R.B. 1984. Effect of gamma radiation on Campylobacter jejuni. J. Food Sci. 49: 665-667.
Lee, E.J., Love, J., and Ahn, D.U. 2003. Effect of antioxidants on consumer acceptance of irradiated turkey meat. J. Food Sci. 68: 1659-1663.
Letellier, P.R. and Nawar, W.W. 1972. 2-Alkylcyclobutanones from the radiolysis of triglycerides. Lipids 7(1): 75-6.
Licciardello, J.J., Nickerson, J.T.R., and Goldblith, S.A. 1970. Inactivation of Salmonella in poultry with gamma radiation. Poultry Sci. 49: 663-675.
Lopez-Gonzalez, V., Murano, P.S., Brennan, R.E., and Murano, E.A. 1999. Influence of various commercial packaging conditions on survival of Escherichia coli O157:H7 to irradiation by electron beam versus gamma rays. J. Food Prot. 62: 10-15.
Luchsinger, S.E., Kropf, D.H., Garcia Zepeda, G.M., Chambers, E., Hollingsworth, M.E., Hunt, M.C., Marsden, J.L., Kastner, C.L., and Kuecker, W.G. 1996. Sensory analysis and consumer acceptance of irradiated boneless pork chops. J. Food Sci. 61: 1261-1266.
Matic, S., Minokovic, V., Katusin-Razem, B., and Razem, D. 1990. The eradication of Salmonella in egg powder by gamma irradiation. J. Food Prot. 53: 111-114.
McNeal, T.P., Nyman, P.J., Diachenko, G.W., and Hollifield, H.C. 1993. Survey of benzene in foods by using headspace concentration techniques and capillary gas chromatography. J. AOAC Intl. 76(6): 1213-9.
Mead, P.S., Slutsker, L., Dietz, V., McCaig, L.F., Bresee, J.S., Shapiro, C., Griffin, P.M., and Tauxe, R.V. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5(5): 607-625.
Molins, R.A. 2001. Irradiation of meats and poultry. In “Food Irradiation: Principles and Applications,” ed. R.A. Molins, p. 469. John Wiley & Sons, Hoboken, N.J.
Morehouse, K.M., Ku, Y., Abrecht, H.L., and Yang, G.C. 1991. Gas chromatographic and electron spin resonance investigations of gamma irradiated frog legs. Radiat. Phys. Chem. 38:62-68.
Murano, P.S., Murano, E.A., and Olson, D.G. 1998. Irradiated ground beef: Sensory and quality changes during storage under various packaging conditions. J. Food Sci. 63: 548 –551.
Nam, K.C. and Ahn, D.U. 2002. Mechanisms of pink color formation in irradiated precooked turkey breast meat. J. Food Sci. 67: 600-607.
Nam, K.C., Min, B.R., Lee, S.C., Cordray, J., Ahn, D.U. 2004. Prevention of pinking, off-odor, and lipid oxidation in irradiated pork loin using double packaging. J. Food Sci. 69: 214-219.
Nawar, W.W., Zhu, R., and Yoo, Y.J. 1990. Radiolytic products of lipids as markers for the detection of irradiated foods. In “Food Irradiation and the Chemist,” ed. D.E. Johnston and M.H. Stevenson, pp. 13-24. Special Publication 86, Royal Society of Chemistry, Cambridge, UK.
Nayga Jr., R.M., Poghosyan, A., and Nichols, J. 2004. Will consumers accept irradiated food products? Intl. J. Consumer Studies. 28(2): 178-185.
Olson, D.G. 1998. Irradiation of food. Food Technol. 52(1): 56-62.
Pillai, S.D. and Espinosa, I.Y. 2003. E-beam inactivation of RNA and DNA containing viruses. Abstract. Annual Meeting of the American Society of Microbiology. Washington, D.C.
Prakash, A., Guner, A.R., Caporaso, F., and Foley, D.M. 2000. Effect of low-dose gamma irradiation on the shelf life and quality characteristics of cut romaine lettuce packaged under modified atmosphere. J. Food Sci. 65: 549-553.
Razskazovskiy, Y., Debije, M.G., Howerton, S.B., Williams, L.D., and Bernhard, W.A. 2003. Strand breaks in X-irradiated crystalline DNA: Alternating CG oligomers. Rad. Resistance Res. 160: 334-339.
Raul, F., Gosse, F., Delincée, H., Hartwig, A., Marchioni, E., Miesch, M., Werner, D., and Burnouf, D. 2002. Foodborne radiolytic compounds (2-alkycylobutanones) may promote experimental colon carcinogenesis. Nutr. Cancer. 44(2): 189-191.
Satin, M. 1996. “Food Irradiation: A Guidebook.” 2nd ed. CRC Press, Boca Raton, Fla.
Sommers, C.H. and Schiestl, R.H. 2004. 2-Dodecylcyclobutanone does not induce mutations in the Salmonella mutagenicity test or intrachromosomal recombinations in Sacccharomyces cerevisiae. J. Food Prot. 67: 1293-1298.
Stevenson, M.H. 1994. Identification of irradiated foods. Food Technol. 48(5): 141-144.
Subar, A.F., Krebs-Smith, S.M., Cook, A., and Kahle, L. 1998. Dietary sources of nutrients among U.S. adults, 1989-1991. J. Am. Diet. Assoc. 98: 537-547.
Tarkowski, J.A., Stoffer, S.C.C., Beumer, R.R., and Kampelmacher, E.H. 1984. Low-dose gamma irradiation of raw meat: Bacteriological and sensory quality effects in artificially contaminated samples. Intl. J. Food Microbiol. 1: 13-23.
Tauxe, R.V. 2001. Food safety and irradiation: Protecting the public from foodborne infections. Emerg. Infect. Dis. 7(3): 516-521. www.cdc.gov/ncidod/eid/vol7no3_supp/pdf/tauxe.pdf.
Thayer, D.W. 1990. Food irradiation: Benefits and concerns. J. Food Qual. 13: 147-169.
Thayer, D.W. 1993. Extending shelf life of poultry and red meat by irradiation processing. J. Food Prot. 56: 831-833, 846.
Thayer, D.W. 2004. Irradiation of food—Helping to ensure food safety. N. Engl. J. Med. 350(18): 1811-1812.
Thayer, D.W., Christopher, J.P., Campbell, L.A., Ronning, D.C., Dahlgren, R.R., Thomson, G.M., and Wierbicki, E. 1987. Toxicology studies of irradiation-sterilized chicken. J. Food Prot. 50:278-288.
Thayer, D.W., Boyd, G., Fox. Jr., J.B., Lakritz, L., and Hampson, J.W. 1995. Variations in radiation sensitivity of foodborne pathogens associated with the suspending meat. J. Food Sci. 60: 63-67.
Thayer, D.W. and Rajkowski, K.T. 1999. Developments in irradiation of fruits and vegetables. Food Technol. 53(11): 62-65.
USDA. 2003. Questions and answers on irradiated ground beef. Release No. qa0172.03. U.S. Dept. of Agriculture, Washington, D.C. www.usda.gov/news/releases/2003/05/qa0172.htm.
USDA/ERS. 2000. Economics of foodborne disease: Feature. U.S. Dept. of Agriculture Economic Research Service, Washington, D.C. www.ers.usda.gov/briefing/FoodborneDisease/features.htm.
USDA/FSIS. 1998. Nationwide young turkey microbiological baseline data collection program, August 1996–July 1997. U.S. Dept. of Agriculture Food Safety and Inspection Service, Washington, D.C. www.fsis.usda.gov/OPHS/baseline/yngturk.pdf
USDA/FSIS. 1999. HACCP implementation: First year Salmonella test results. U.S. Dept. of Agriculture Food Safety and Inspection Service, Washington, D.C. www.fsis.usda.gov/Frame/FrameRedirect.asp?main=/ophs/haccp/salmdata.htm.
USDA/FSIS. 2004. FSIS Recalls: Closed Federal Cases. U.S. Dept. of Agriculture Food Safety and Inspection Service, Washington, D.C. www.fsis.usda.gov/Fsis_Recalls/Closed_Federal_Cases_2004/index.asp.
Vachon, C., D’Aprano, G., Lacroix, M., and Letendre, M. 2003. Effect of edible coating process and irradiation treatment of strawberry Fragaria spp. on storage-keeping quality. J. Food Sci. 68: 608-612.
Vickers, Z.M. and Wang, J. 2002. Liking of ground beef patties is not affected by irradiation. J. Food Sci. 67: 380-383.
WHO. 1994. Safety and nutritional adequacy of irradiated food. World Health Organization, Geneva, Switzerland.
WHO. 1999. High-dose irradiation: Wholesomeness of food irradiated with doses above 10 kGy. Report of a joint FAO/IAEA/WHO study group. WHO technical report series 890. World Health Organization, Geneva, Switzerland.
