Formulating for Satiety with Hydrocolloids
To be effective in product applications, hydrocolloids must be hydrated in vivo. Here’s a look at methods to improve hydration.
One of the hottest trends in weight management is developing foods and beverages that provide satiety or hunger satisfaction (Tecklenburg, 2010). Two-thirds of consumers say satiety is an important factor in their food and beverage choices (Nicholson, 2010), and, according to research firm Mintel (2010), satiety is the next big thing in weight management. Satiety is not a panacea for obesity, and diet and exercise is still the best solution for long-term weight loss, but a little help is big business. Weight Watchers alone has global sales of nearly $1.4 billion (Weight Watchers, 2010), demonstrating that consumers are willing to pay for support to lose weight.
A wide range of ingredients is available for the creation of satiety products, but developing the products can be an elusive goal for food manufacturers. Technical problems can be challenging, demonstrating efficacy using human subjects is expensive, and regulatory and labeling claims can be tricky.
Hydrocolloids have been studied as a means to provide satiety and blunt glucose absorption for more than 30 years (Wong, 1973; Kay, 1978; Wilmhurst and Crawley, 1980). Early work using guar gum by Jenkins et al. (1980a, b, c) clearly showed lowered blood glucose response. Subsequent research demonstrated similar results with guar and other hydrocolloids (Wood et al., 1990; Landin et al., 1992; Flammang et al., 2006). Hydrocolloids provide viscosity, which delays gastric emptying and slows diffusion of glucose and other nutrients into the body through the small intestine. Presumably, the same mechanism is responsible for satiety effects.
A less significant and less studied mechanism is fermentation in the large intestine. Recent research has shown fermentation of nondigestible carbohydrates can also cause satiety (Delzenne et al., 2005; Cani et al., 2006; Willis et al., 2009). Although most research in this area has been conducted with inulin or resistant starch, hydrocolloids are also fermentable to varying extents. Some, such as pectin, are very fermentable, while others, such as hydroxypropyl methyl cellulose, are not.
Defining Satiety and Physiological Mechanisms
Appetite is a multi-phase process occurring several times a day in an individual; it influences humans to eat (intake energy) in distinct blocks of time (meals). Hunger is the primary component of appetite, and is defined as a motivational drive toward an energy source. When the body, through effector mechanisms, has satisfied the need for energy intake, we become satiated. The maintained feeling of fullness between meals is termed satiety.
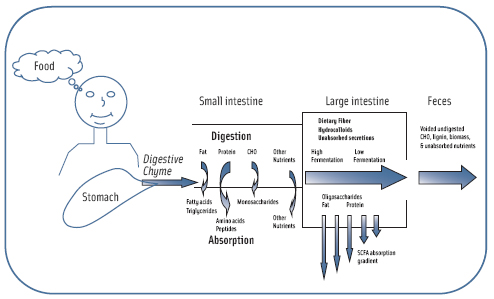
While hormonal regulation controls much of our digestive process, the peptide hormone ghrelin signals our brain to eat again to maintain energy homeostasis by the mere mention, smell, or sight of food (Figure 1). If our previous meal was nutrient dense, the body releases less ghrelin from the duodenum to signal it is time to eat again. Ghrelin secretion is closely linked to hunger sensations as it increases before a meal and decreases after a meal. Meals high in lipids seem to have little impact on ghrelin concentrations.
--- PAGE BREAK ---
Satiation may be regulated by the plasma peptide cholecystokinin (CCK). Once CCK has been released, it signals satiation by activating vagal afferent mechanosensitive nerve fibers in the stomach and duodenum. Once receptors are activated, the pyloric sphincter contracts and slows gastric emptying. This action further promotes gastric distension, which is also known to induce satiation. Proteins and carbohydrates, including fiber, very effectively stimulate CCK release and prolong the circulating levels longer than a meal rich in glucose or lactose.
Glucose-dependent insulinotropic polypeptide (GIP) is released within 5 minutes after nutrient ingestion begins and peaks 30–60 minutes postprandially, depending on meal size and composition. The role of GIP is to regulate pancreatic secretion including insulin and lipoprotein lipase release for carbohydrate and lipid metabolism, respectively. Dietary fats and carbohydrates are the stimuli for GIP release, and protein seems to have no effect. Gastric emptying is not affected by GIP.
Gastric motility, after the stomach and duodenum, is stimulated by nutrients moving further down the tract and is a signal for other neurohormonal and digestive enzyme release. Digestive enzymes further hydrolyze food that is undergoing digestion (digesta) and enable the host to absorb nutrients for homeostasis. As partially digested food and gastric secretions (chyme) begin reaching the distal ileum, peptide tyrosine-tyrosine (PYY) and glucagon-like peptide (GLP-1) are released by intestinal enteroendocrine cells and have a role in further reducing gastric motility. Furthermore, it seems GIP secretion is connected to GLP-1 secretion, but the mechanism controlling this co-secretion is unknown. Carbohydrate metabolism is facilitated by release of pancreatic insulin by GLP-1 and GIP. These incretin hormones also work with PYY to reduce secretion and motility in the stomach and proximal small intestine to protect the gut from over-secretion. This so called ileal brake signals the completion of the intestinal phase of digestion and has an important role in satiety.
The impact of ingestion of dietary fiber, including hydrocolloids, on gastric motility and gastric emptying is well studied. Viscous fibers were for many years reported to be responsible for reduced gastric motility, but several studies have confounded these findings and suggested gastric emptying may be more controlled by nutrient content of the intestinal lumen fluid than luminal viscosity. The challenge in comparing these reported studies is that different methodologies are used to study gastric emptying, as well as different meal types. In general, dietary fiber has several factors that contribute to satiation.
--- PAGE BREAK ---
Food that is high in fiber is generally chewier and requires increased mastication to develop a mass or bolus that can be safely swallowed. This extended time and effort sends signals to the brain, which begins communicating with the stomach to activate a cascade of contractual events involved with gastric motility, specifically relaxation of muscular levels. Receptive relaxation allows food to enter the stomach while simultaneously initiating release of stomach acids and muscular contractions to mix the food bolus for absorption and digestion. The pyloric valve remains in a contracted state until secretin, motilin, GIP, and CCK at appropriate concentrations signal digesta to enter the duodenum. Recent theory is that fiber containing “gel rafts” floats atop the stomach chyme and primes incretin hormones by particulating over an extended time, providing a continual, slow release of nutrients to the duodenum and down regulating ghrelin.
Recall that increased ghrelin concentrations signal our brain that we are hungry. Nutrient composition of the meal will influence timing of this sequence of events, ultimately impacting gastric emptying rate. Chewing further up or down regulates the neurohormonal sequence, specifically CCK, GIP, GLP-1, and PYY, to begin signaling the host when satiation has occurred. Dietary fibers also absorb large quantities of liquid, resulting in increased stomach distension and triggering of vagal nerve signals for satiation.
Fibers and hydrocolloids that are nondigestible in the upper regions of the gastrointestinal tract have a further role in satiety when they reach the large intestine. Normal colonic microflora will ferment some fibers, yielding increased concentrations of volatile fatty acids, specifically butyric acid, resulting in reduced gastric emptying and gastrointestinal secretion by up regulating PYY and GLP-1. This “colonic brake” feedback mechanism occurs both in dogs (Wen et al., 1995) and humans (Nightingale et al., 1996). Furthermore, butyric acid is readily absorbed across the brush border barrier into the circulatory system and may influence neurohormonal regulation further up the intestinal tract. Anecdotal evidence suggests elevated concentrations of butyric acid in the bloodstream may influence satiety by a yet-to-be identified mechanism.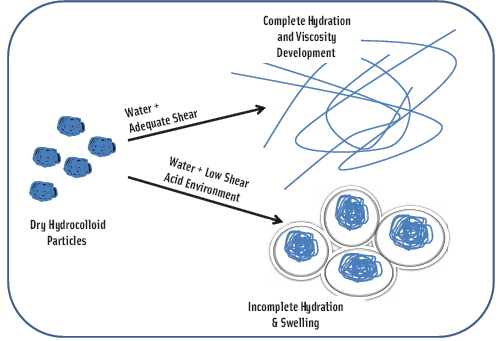
Do Hydrocolloids Actually Work to Create Satiety?
Using hydrocolloids for satiety is not a new concept, so why aren’t there an abundance of satiety products on the market featuring hydrocolloids? The main issues are that research results are inconsistent as far as efficacy and high hydrocolloid concentration is necessary to cause satiety, which has detrimental effects on organoleptic aspects as well as processing.
Inconsistencies in the literature may be attributable to variability in the state of hydrocolloid hydration when ingested and subsequent fate in vivo. There are three ways to ingest hydrocolloids: dry, semi-hydrated, and fully hydrated. Examples of these include cookies or bars (dry); powders to mix into water or other liquid (semi-hydrated); or beverages (fully hydrated).
To obtain maximum viscosity, hydrocolloids must be fully hydrated as colloidal suspensions. This is not an issue with beverages, but it is a significant concern with semi-hydrated and dry delivery systems because the shear rate of muscle contractions in the stomach, antrum, and pyloric valve may not be sufficient to fully hydrate dry hydrocolloids. Hydration without enough shear is incomplete, and the hydrocolloid simply swells and creates what is commonly known in the food industry as “fish eyes” (Figure 2).
Hydrocolloids also do not hydrate well in an acid environment. Maximum potential viscosity cannot be achieved under these circumstances. Given the variable conditions in individual stomachs as far as pH and shear, it is not surprising that literature results are so varied for satiety with the use of hydrocolloids. Some show no effect even at high levels while others demonstrate a statistically significant effect at much lower levels. Much of the existing research does not describe test protocols sufficiently to assess whether the hydrocolloid was properly hydrated or what the potential viscosity may have been in vivo.
--- PAGE BREAK ---
Some research protocols used partially or semihydrated hydrocolloids in the form of a powder added to a solution and briefly stirred or shaken (Tiwary, 1997). The preparations are ingested as quickly as possible to avoid too much thickening, thus alleviating palatability issues. As with dry hydrocolloids, it is questionable whether full hydration can be achieved in vivo with a semi-hydrated form.
In fully hydrated forms, such as in beverages, hydrocolloid concentration may be diluted by stomach fluids, which decreases viscosity in vivo and reduces efficacy. Stomach volume is between 1–1.5 liters (Dressman, 1991), so a 3–20-fold dilution may occur, depending on the beverage volume. Some approaches have been used with satiety beverages to create interaction between hydrocolloids in vivo with ions or reactions with stomach acid to create additional viscosity or gels in vivo.
Beverages tend to be more successful in creating satiety in the reported literature because the hydrocolloid is fully hydrated and functional, despite the dilution effect in the stomach. Dry products are less successful, probably because the state of hydration in vivo can be quite variable and is unknown in most cases.
In products containing dry or semi-hydrated hydrocolloids, it is critical to understand hydration extent before and after ingestion. If the hypothesis of satiety creation through viscosity is correct, maximizing viscosity of a particular hydrocolloid in vivo at the lowest concentration should be the ultimate goal. Lower concentrations are desirable because they reduce organoleptic and processing concerns as well as reducing cost.
To date, little research has been conducted to understand how much viscosity in vivo is actually necessary to induce satiety. Most studies pick an arbitrary amount of hydrocolloid, and very rarely does the research protocol actually measure viscosity before ingestion. Measurement of viscosity after ingestion is almost never done, except by nasal gastric intubation, an invasive and uncomfortable procedure. MRI work has been done by Marciani et al. (2000, 2001) and Hoad et al. (2004, 2009) to follow digestion of viscous solutions, bars, and alginate beads in vivo and shows promise as a technique to study what actually happens in vivo in a noninvasive manner.
Hydrocolloid Selection for Satiety
Hydrocolloids are not equal in their physical properties. There are basically two types, with some variation within each type. Neutral hydrocolloids including guar, locust bean gum, konjac, and xanthan simply hydrate to a fully extended form, creating viscosity through polymer entanglement. Synergistic combinations are possible, but the hydrocolloids must be in solution to make use of the interaction, and some are pH dependent.
Charged hydrocolloids such as alginate, pectin, carrageenan, and gellan gum also develop maximum viscosity with full hydration in water, but may develop additional viscosity through association with mono- and divalent ions and, in some cases, hydrogen ions. An increase in viscosity occurs with a low amount of ions through formation of junction zones. With higher amounts of ions and a greater amount of junction zones, gels may form. Alginate and low methoxy pectin are good examples. These hydrocolloids increase in viscosity with low concentrations of calcium ions, and when the concentration of calcium ions is high enough, more junction zones occur, and gelation takes place. Novel systems to form junction zones with charged hydrocolloids in vivo have been the subject of much patent activity in recent years (Wolf et al., 2007, 2008; Aimutis et. al., 2007; Boers et al., 2010).
--- PAGE BREAK ---
Mitigating Sliminess and Viscosity
The level of hydrocolloid required to induce viscosity for satiety purposes is much higher than that used for stabilization in food products. Levels of hydrocolloids used for satiety typically range from 5 g to 20 g. If one considers the average weight of a cookie, which is 30 g, reference amount customarily consumed (RACC), or a bar, 40 g, RACC, it is apparent that the high level necessary may cause difficulties in actual food product processing as well as organoleptic concerns. In liquid products such as beverages, high levels result in thick, slimy solutions that are difficult to swallow. In dry products, the hydrocolloids attempt to hydrate while being chewed and swallowed, producing an eraser-like bolus and slimy, sticky tooth-packing. Production issues may include difficulty in pumping, rolling or sheeting, and cutting because the hydrocolloids will gradually take up all of the water, leaving a dry, sandy product or a sticky, viscous mass. Baking times will be affected because high levels of hydrocolloid take up water, and it is difficult to “bake off” excess water.
Getting around these issues is not easy, but there are approaches to alleviate some of these difficulties. Incorporating hydrocolloids into dry foods is a double-edged sword. To obtain maximum functionality in vivo, quick hydration is very desirable, but that very property makes the organolepetic experience in the mouth a gruesome event, unlikely to result in repeat sales. The ideal hydrocolloid product would hydrate slowly in the mouth to reduce sliminess, yet quickly go into solution in the stomach for maximum viscosity development.
This can be partially achieved through processing techniques such as extrusion. Extruding hydrocolloids into dry cereal products such as crisps (Williams et al., 2004; Mattis, 2007; Aimutis et al., 2007) or with sugar-like materials (Paeschke and Aimutis, 2008) serves to disperse the hydrocolloid in a dry carbohydrate matrix. The glassy state in the mouth impedes hydration to some extent, while the dispersion within a matrix aids in dissociation in the stomach and small intestine. In the case of extrusion of hydrocolloids with sugar-like materials, any sugar-like material that forms a glassy state, such as sucrose, polyols, polydextrose, inulin, etc., may be used. The resulting pellets are ground into particle sizes amenable to food formulations.
To demonstrate the different dissolution characteristics of hydrocolloids encased in a glassy matrix, dissolution characteristics of extruded and ground carbohydrate glasses with hydrocolloids were tested with a Parr Physica Rheometer using a vane spindle or starch cell. Figure 3 is a comparison of dissolution of an alginate pectin (A/P) blend (75%) extruded with inulin, isomalt, and polydextrose (25%). The raw material A/P blend hydrates much faster than any of the extruded material, while the A/P blend extruded with carbohydrates hydrates more slowly, conditions favorable for improved organoleptics. The A/P blend extruded alone (100%) hydrates more slowly than any of the A/P blends extruded with carbohydrates. All of the extruded products were ground to equivalent particle size distributions before testing, so the effect of faster dissolution with carbohydrates is not due to a particle size effect. The amounts of carbohydrate glass forming ingredients may be altered, but given the large amount of hydrocolloid necessary in a food formulation, minimizing the amount is necessary for flexibility in product development.
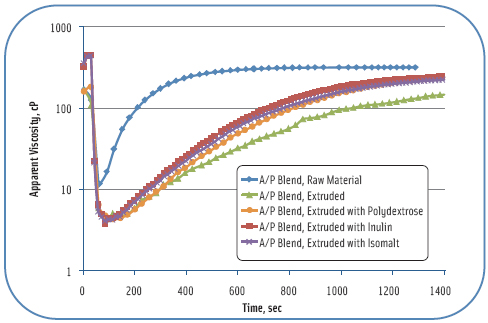
The glassy state of the encapsulating carbohydrate alone does not alleviate all organoleptic issues, but it has a very positive effect on the slimy nature of high levels of hydrocolloids during chewing. Other processing methods and ingredients may be used to alter hydration rates and have not been fully explored. The key is to delay hydration for a certain time period to get past the chewing and swallowing stage, yet increase hydration and dispersion in the stomach and small intestine to obtain maximum viscosity in vivo. Because efficacy and success of hydrocolloids as satiety ingredients depend on viscosity, it’s essential to consider the state of hydration in the gastrointestinal tract.
Teri M. Paeschke, a Member of IFT, is Principal Scientist with Global Food Research, Cargill Inc., 2301 Crosby Road, Wayzata, MN 55391 ([email protected]). William R. Aimutis, a Professional Member of IFT, is Director of Global Food Research, North America, Cargill Inc. ([email protected]).
This article is based on a paper presented during the symposium “Designing Macronutrient Ingredients for Enhanced Satiety” at the IFT Annual Meeting in Chicago, Ill., July 17–21, 2010.
References
Aimutis, W., Paeschke, T., Sun, N., Johnson, S.D., Sweeney, J.F., Patist, A. Vander Pol, T.J., and Finocchiaro, E.T. 2007. Fiber satiety compositions. U.S. patent application 20070082029.
Boers, H.M., Strom, A.H.E., and Wiseman, S.A. 2010. Liquid satiety enhancing composition. U.S. Patent Application 20100009932.
Cani, P.D., Joly, E., Horsmans, Y., and Delzenne, N.M. 2006. Oligofructose promotes satiety in healthy human: a pilot study. Eur. J. Clin. Nutr. 60: 567-572.
Delzenne, N.M., Cani, P.D., Daubioul, C., and Neyrinck, A.M. 2005. Impact of inulin and oligofructose on gastrointestinal peptides. Br. J. Nutr. 93, Suppl. 1: S157-161.
Dressman, J.B. and Yamada, K. 1991. Animal models for oral drug absorption. In Pharmacological Bioequivalence, ed. P.G. Welling, F.L.S. Tse, and S.V. Dighe, pp. 235-266. Marcel Dekker Inc., New York, N.Y.
Flammang, A.M., Kendall, D.F., Baurmgartner, C.J., Slagle, T.D., and Choe, Y.S. 2006. Effect of a viscous fiber bar on postprandial glycemia in subjects with type 2 diabetes. J. Am. Coll. Nutr. 25(5): 409-411.
Hoad, C., Rayment, P., Cox, E., Wright, P., Butler, M., Spiller, R., and Gowland, P. 2009. Investigation of alginate beads for gastro-intestinal functionality, Part 2: In vivo characterisation. Food Hydrocolloid 23(3): 833-839.
Hoad, C., Rayment, P., Spiller, R.C., Marciani, L., de Celis, A.B., Traynor, C., Mela, D.J., Peters, H.P., and Gowland, P.A. 2004. In vivo imaging of intragastric gelation and its effect on satiety in humans. J. Nutr. 134(9): 2293-2300.
Jenkins, D.J.A., Wolever, T.M.S., Nineham, R., Sarson, D.L., Bloom, S.R., Ahern, J., Albertii, K.G., and Hockaday, T.D.R. 1980a. Improved glucose tolerance four hours after taking guar with glucose. Diabetologia 19: 21-24.
Jenkins, D.J.A., Wolever, T.M.S., Bacon, S., Nineham, R., Lees, R., Rowden, R., Love, M., and Hockaday, T.D. 1980b. Diabetic diets: high carbohydrate combined with high fiber. Am. J. Clin. Nutr. 33: 1729-1733.
Jenkins, D.J.A. Wolever, T.M.S., Taylor, R.H., Reynolds, D., and Hokaday, T.D. 1980c. Diabetic glucose control, lipids, and trace elements on longterm guar. Br. Med. J. 280: 1353-1354.
Kay, R.M. 1978. Food form, postprandial glycemia and satiety. Am. J. Clin. Nutr. 31(5): 738-741.
Landin, K., Holm, G., Tengborn, L., and Smith, U. 1992. Guar gum improves sensitivity, blood lipids, blood pressure, and fibrinolysis in healthy men. Amer. J. Clin. Nutr. 56: 1061-1065.
Marciani, L., Gowland, P.A., Spiller, R.C., Manoj, P., Moore, R.J., Young, P., Al-Sahab, S., Bush, D., Wright, J. and Fillery-Travis, A.J. 2000. Gastric response to increased meal viscosity assessed by echoplanar magnetic resonance imaging in humans. J. Nutr. 130(1): 122-127.
Marciani, L., Gowland, P.A., Spiller, R.C., Manoj, P., Moore, R.J., Young, P. and Fillery-Travis, A.J. 2001. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am. J. Physiol-Gastr. L. 280(6): 1227-1233.
Mattis, R.D. 2007. Effects of a combination fiber system on appetite and energy intake in overweight humans. Physiol. Behav. 90: 705-711.
Mintel. 2010. Mintel announces IFT 2010 taste test winners. Press release, Aug. 9. Mintel Intl., Chicago, Ill. www.mintel.com.
Nightingale, J.M., Kamm, M.A., van der Sijp, J.R., Ghatie, M.A., Bloom, S.R., and Lennard-Jones, J.E. 1996. Gastrointestinal hormones in short bowel syndrome. Peptide YY may be the ‘colonic brake’ to gastric emptying. Gut 39(2): 267-272.
Nicholson, V. 2010. Desire for satiety. Dairy Management Inc., Rosemont, Ill. http://www.innovatewithdairy.com/Pages/Home.aspx. Accessed Feb. 8, 2011.
Paeschke, T. and Aimutis, W. 2008. Controlled hydration of hydrocolloids. U.S. patent 20080085354.
Tecklenburg, E. 2010. Food flavor and ingredient outlook 2010. Packaged Facts, Rockville, Md.
Tiwary, C.M., Ward, J.A., and Jackson, B.A. 1997. Effect of pectin on satiety in healthy U.S. Army adults. J. Am. Coll. Nutr. 16(5):423-8.
Weight Watchers. 2010. Annual report. http://phx.corporateir.net/External.File?item=UGFyZW50SUQ9Mzc1OTgyfENoaWxkSUQ9Mzc0NDkyfFR5cGU9MQ==&t=1. Accessed Feb. 7, 2011.
Wen, J., Phillips, S.F., Sarr, M.G., Kost, L.J., and Holst, J.J. 1995. PYY and GLP-1 contribute to feedback inhibition from the canine ileum and colon. Am. J. Physiol.-Gastr. L. 269(6): G945-G952.
Williams, J.A., Laie, C., Corwin, H., Ma, Y., Maki, K.C., Garleb, K.A., and Wolf, B.A. 2004. Inclusion of guar gum and alginate into a crispy bar improves postprandial glycemia in humans. J. Nutr. 134: 886-889.
Willis, H.J., Eldridge, A.L., Beiseigel, J., Thomas, W., and Slavin, J.L. 2009. Greater satiety response with resistant starch and corn bran in human subjects. Nutr. Res. 29(2): 100-105.
Wilmhurst, P. and Crawley, J.C.W. 1980. The measurement of gastric transit time in obese subjects using 24Na and the effects of energy content and guar gum on gastric emptying and satiety. B. J. Nutr. 44: 1-6.
Wolf, B.W., Blidner, B.B., Garleb, K.A., Laie, C., and Schenz, T.W. 2007. Dual induced viscosity fiber system and uses thereof. U.S. patent 7,183,266.
Wolf, B.W., Blidner, B.B., Garleb, K.A., and Laie, C. 2008. Acid controlled induced viscosity fiber system and uses thereof. U.S. patent 7,422,763.
Wong, G.O. 1973. Method of controlling human appetite. U.S. patent 3843786.
Wood, P.J., Braaten, J.T., Scott, F.W., Riedel, D., and Poste, L.M. 1990. Comparisons of viscous properties of oat and guar gum and the effects of these and oat bran on glycemic index. J. Agric. Food Chem. 38: 753-757.
