Aquaculture Drives Growth in Fish Production
FOOD SAFETY & QUALITY

Aquaculture is the production of marine and freshwater fish, shellfish, and plants under controlled conditions for human consumption and other purposes, such as sport fishing and restoring populations of endangered species. Also called fish farming, aquaculture has been practiced for more than 4,000 years, is one of the world’s fastest-growing forms of food production, and has become increasingly technologically developed over the past 40 years. In its report The State of World Fisheries and Aquaculture 2012, the Food and Agriculture Organization of the United Nations (FAO) said that global capture of fish and shellfish has leveled off since the mid 1990s and that aquaculture has been the engine driving growth in total fish production. Aquaculture’s contribution to world fish production for human consumption increased from 9% in 1980 to 47% in 2010.
Aquaculture is the only way to meet the world population’s growing demand for fish protein in the face of limited wild populations of fish, the FAO said. It also allows for better overall control of the product from hatching to slaughter and initial processing, which decreases overall costs and provides a more consistent, high-quality product in the retail market.
Regulating & Certifying Aquaculture Systems
There are a variety of aquaculture systems: inland ponds, indoor recirculating systems in closed containers, outdoor recirculating systems, cages or net pens suspended in a natural body of water outdoors or indoors, and flow-through raceway water systems using natural water flows. However, aquaculture systems
around the world differ widely and offer different degrees of safety and sustainability, so work is being done to set standards and certification for aquaculture systems.
Aquaculture in the United States is jointly regulated by a variety of federal agencies, including the U.S. Food and Drug Administration (FDA), the U.S. Dept. of Agriculture, the U.S. Environmental Protection Agency, the National Oceanic and Atmospheric Administration (NOAA), the U.S. Dept. of the Interior’s Fish and Wildlife
Service, and state and local agencies. The FDA must approve any drug used in an aquaculture operation and requires seafood processors to implement a Hazard Analysis and Critical Control Points program to control food safety hazards associated with aquaculture products. In addition, state agencies in cooperation with the FDA administer a shellfish certification program, the Interstate Shellfish Sanitation Conference. NOAA offers a voluntary fee-for-service inspection program that also provides product quality evaluation, grading, and certification.
The Joint Institute for Food Safety and Nutrition (JIFSAN), a multidisciplinary research, education, and outreach program established by the FDA and the University of Maryland, has developed a Good Aquaculture Practices training program and manual designed to foster efficient and responsible aquaculture production and help ensure product quality, safety, and environmental sustainability. In February 2013 the JIFSAN began offering an online training module for foreign aquaculture producers to help them comply with the FDA’s import regulations. The training module clarifies how the FDA regulates drugs for aquaculture. The FDA’s Center for Veterinary Medicine Office of New Animal Drug Evaluation works with government agencies and aquaculture associations to increase the number of safe and effective drugs that can be used by the aquaculture industry.
--- PAGE BREAK ---
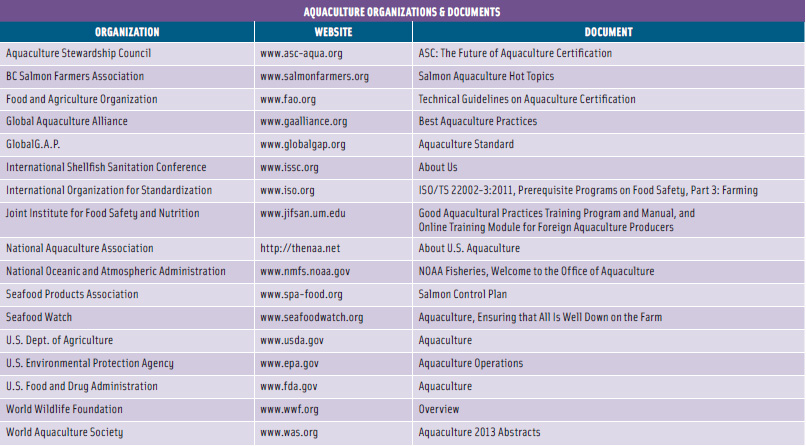
The FAO has issued minimum criteria for developing certification standards for animal health and welfare, food safety, environmental integrity, and socioeconomic aspects of aquaculture systems. Numerous other organizations have also addressed environmental and sustainability concerns and developed standards and certification guidelines (see table on p. 89).
Retailers are increasingly requiring guarantees that the fish they purchase are produced sustainably and handled in ways that safeguard consumers’ health, respect the environment, and address various ethical and social concerns, such as child labor. For example, Walmart (www.corporate.walmart.com) requires all fresh and frozen, farmed and wildcaught seafood products it sells in the United States to be thirdparty certified as sustainable, Whole Foods Market (www.wholefoodsmarket.com) has established standards for farmed seafood, and Costco Wholesale Corp. (www.costco.com) has been working with the Marine Stewardship Council and the Aquaculture Stewardship Council to purchase its seafood only from sources that are certified as meeting environmental and sustainability guidelines.
Farming Genetically Engineered Atlantic Salmon
Salmon and shrimp represent the largest share of the global farmed seafood market, and salmon ranks third among all the seafood consumed in the United States, preceded by shrimp and canned tuna. Commercial catches of Atlantic salmon are prohibited in U.S. waters, so almost all of the Atlantic salmon sold in the United States is either wild fish caught in Europe or farmed fish. About two-thirds of the salmon consumed in the United States is farmed, and 94% of the Atlantic salmon consumed in America is imported, both farmed and wild caught.
To increase the availability of Atlantic salmon and reduce pressure on wild Atlantic salmon populations, AquaBounty Technologies (www.aquabounty.com) over the past 24 years has developed a genetically engineered, farmraised Atlantic salmon, AquAdvantage® Salmon, that the company says grows to market size (7 lb – 11 lb) in about half the time as other farmed Atlantic salmon. To ensure that it would have no adverse environmental effect on wild Atlantic salmon, the company advocates stocking its sterile, all-female salmon in contained land-based aquaculture production systems instead of the traditional production systems—floating net pens or cages in coastal waters—that are used for more than 99% of the world’s farmed salmon.
The genetically engineered salmon was initially developed in 1989 by inserting a Chinook salmon growth hormone gene into fertilized eggs (i.e., eyed eggs) of wild Atlantic salmon, followed by selection and breeding. The genetically engineered line has now been propagated for 10 generations. Because the FDA regulates genetically engineered animals under the new-animal-drug provisions of the Federal Food, Drug, and Cosmetic Act, AquaBounty Technologies submitted a new-animal-drug application in 1995 to the FDA for approval. After evaluating the technical and safety data, the FDA held a meeting of its outside experts, the Veterinary Medicine Advisory Committee, in September 2010 and reported its preliminary assessment that the genetically engineered salmon is indistinguishable from other Atlantic salmon, is safe to eat, and does not pose a threat to the environment under the proposed conditions of use.
The FDA published for public comment a draft environmental assessment (EA) and a preliminary finding of no significant impact (FONSI) in December 2012 and in February 2013 extended the deadline for comments until April 26, 2013. An FDA spokesman said that the FDA will then review all the comments and decide whether to prepare a final EA and FONSI or prepare an environmental impact statement. It is not possible to predict how long that process will take, he said.
Henry C. Clifford, Vice President of Marketing & Sales at AquaBounty Technologies, said that AquAdvantage Salmon is nutritionally and biologically equivalent to a conventional farmed Atlantic salmon except for the faster growth and that multiple precautions have been taken to prevent any adverse environmental impact from introduction of the salmon. The company’s business plan is to produce triploid, all-female populations in the company’s FDA-approved facility on Prince Edward Island, Canada, and ship eyed eggs to Panama, where the fish would be grown in inland tanks until they reach market size and are harvested. In both locations, the fish will be raised in land-based facilities with redundant biological and physical containment so they cannot escape or reproduce in the wild, thus avoiding the possibility of displacing wild salmon populations or harming genetic diversity.
According to the FAO, ocean net-pen farming of Atlantic salmon has long been controversial. The major areas of concern are local nutrient pollution into water systems from waste feed and feces, local chemical pollution from use of chemical treatments, potential spread of disease to wild fish by fish escaping from farms, and sustainability since salmon production relies on supplies of fishmeal and fish oil for feed production.
--- PAGE BREAK ---
The application for AquAdvantage Salmon currently under review by the FDA is for only one grow-out site in Panama, Clifford said. Inspected and approved by the FDA, the site is more than 100 km inland from the ocean. It contains 21 containment barriers confining the salmon to the aquaculture system. A natural (ecological) thermal barrier of lethally high ambient-temperature water downstream would kill the salmon or prevent their migration to the ocean if they managed to escape the containment barriers. In addition, the salmon are sterile and all female, so they cannot establish reproductively active, self-sustaining populations in the environment or breed with other fish if they do manage to escape. It could be argued, Clifford said, that growing AquAdvantage Salmon under these FDA-mandated conditions of use is more sustainable and environmentally friendly than the traditional sea-cage method for farming salmon.
Ideally, Clifford said, the company would like to begin producing AquAdvantage Salmon at the site in Panama and export edible products back to the United States. The FDA approval would facilitate the importation, sale, and consumption of the salmon in the United States, but the company must first comply with Panamanian regulatory requirements for the commercial production, consumption, and export of the salmon from Panama. This would be the case for any country where the company intends to produce AquAdvantage Salmon, he said. If the company receives approval for the salmon new-animal-drug application and identifies other suitable production sites (including in the United States) or qualified producers willing to grow the salmon, the company will submit supplemental applications for those sites. The supplemental applications would undergo the same scrutiny as the first application. The sites must be inspected and pre-approved by the FDA and local authorities as well as adhere to the same strict conditions of use. If an FDA approval is granted in 2013, Clifford said, the first shipments of AquAdvantage Salmon could appear in U.S. stores by 2014.
IFT Symposium Covers Aquaculture
Speaking at the symposium “Safety Issues and Concerns Impacting Aquaculture Products” at the 2012 Annual Meeting of the Institute of Food Technologists, Brett Koonse, Consumer Safety Officer and aquaculture expert in the FDA’s Center for Food Safety and Applied Nutrition, discussed safety concerns about aquaculture products, including antibiotic resistance, pathogens, the environment, and social issues. He said that Good Aquaculture Practices cover hatchery, grow-out, nursery, and feed; farm location and density; personnel/workers; disinfection; clean ice and containers; animals on farm; and feed source and control. He said that the United States has a prevention-with-verification program in place that is proactive, not reactive.
Steven Wilson, Chief Quality Officer at the National Oceanic and Atmospheric Administration (NOAA), said that NOAA’s Seafood Inspection Program has been actively inspecting seafood firms and products across the globe since 1948. He said that all of the U.S. processing plants are extremely clean and process oriented, so there are no problems with export products. However, there are concerns regarding imports, including food safety, child labor, and other issues. He said the United States is requiring a level of detail in export certificates from foreign producers that shows how people were involved in the process “from boat to throat.” NOAA has been working for 20 years to get more production domestically, so there is less reliance on imports. He added that with the passage of the Food Safety Modernization Act, the FDA is moving toward a cooperative, prevention type of program.
Juan Silva, Professor in the Dept. of Food Science, Nutrition, and Health Promotion at Mississippi State University, said that catfish are the largest aquaculture product in the United States, generating more than 46% of the value of aquaculture production in the United States but added that there is a need to assess the potential risks associated with domestic and imported catfish and pangasius. Issues that have been identified with farmed seafood are use of unapproved drugs and Salmonella contamination, Silva said. Other issues are feed, filth, antibiotics, water quality, and diseases. He said that adequate sanitation will take care of filth, allergens can be controlled by proper labeling and minimal use of antibiotics, water quality can be controlled through education and infrastructure funding, and diseases can be controlled through proper management. What’s needed, he said, is surveillance at the source, research and development, understanding of risks, dealing with misbranding and mislabeling, oversight, testing, and education.
Christopher Sommers, Research Leader in Food Safety and Intervention Technologies at the U.S. Dept. of Agriculture’s Agricultural Research Service, discussed how advanced thermal and non-thermal processing technologies can be used to improve the safety and shelf life of aquacultured seafood.
 Neil H. Mermelstein,
Neil H. Mermelstein,a Fellow of IFT, is Editor Emeritus of Food Technology
[email protected]


