Research Tool Helps Validate Efficacy of Functional Foods
Developed by researchers in Germany and Australia, the Fraunhofer-Monash workflow tool identifies bioactive compounds and degradation products in food from processing through consumption to assist in the formulation of healthier and safer products.
Article Content
The food supply chain, from primary production through industrial processing into products and ultimately, the preparation of foods, embodies a challenging array of variables for systematic study. A particular challenge is to track the fate of specific molecules through processing and cooking environments and then to follow in vivo metabolic processes.
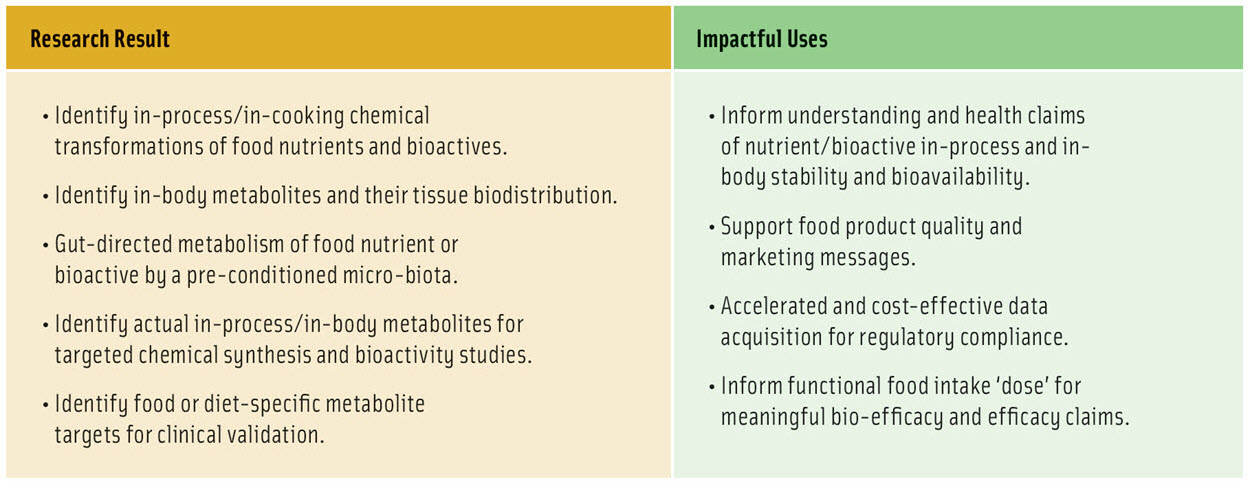
To overcome this challenge, we have developed a unique research-friendly workflow (i.e., Fraunhofer-Monash workflow) that starts with the radioisotope, 14C, placed within a target molecule and fed at low dose. Using ultra-high sensitivity LC-MS analysis, the 14C label permits the detection of degradation compounds produced either on the shelf (in-food), during processing or preparation for eating (in-process), and/or metabolites produced post ingestion (in-body). The identification of specific 14C-labeled metabolites creates powerful second and third horizons of enquiry that can inform processing conditions or formulation design. In particular, the workflow includes the option for chemical synthesis of metabolites, enabling deep insight into molecular nutrition and food safety.
A key application of the Fraunhofer-Monash workflow is for discovery and validation of molecules of interest in commercial “functional” foods, and to verify actual metabolites and degradation products for study of potential benefits and risks to consumers. These methods are now ready to support faster resolution of uncertainties in food bioactivity, safety and quality, so as to create healthier foods, and accelerate understanding of the complex food and health paradigm.
Food and Health
Authoritative organizations like FAO (Food and Agriculture Organization) and WHO (World Health Organization) clearly indicate that nutrition (compared to lifestyle, environment, etc.) exerts the major impact on the health status of individuals. Accordingly, the increased awareness in recent times of the leading role nutrition plays in well-being has led to health benefits being an important motivator for food choices. As a result, a major innovation focus of the food industry is in the development of functional food products with specific health-related properties, in recognition of the strong market demand and benefits for consumers.
This trend is accompanied by a similarly growing demand for food ingredients that comply with “clean” labeling and that product label health claims are supported by independent and sound scientific evidence. Currently, there are many bioactive or functional ingredients for which we have a basic understanding of their role in health and well-being. However, the research is typically focused on the parent compound (or chemical class) but does not account for degradation products formed during food processing or domestic preparation, nor metabolites generated in vivo.
Unless linked with a specific food safety challenge, chemical transformation products formed through these multiple and complex pathways have been previously considered “too hard” to study within a holistic approach that seeks to understand both consumer benefit and risk. On one hand, consumers seek functionality in foods that enhance health, while on the other hand, they fear that “novel” formulations may contain additives that form products with unknown properties. A well-known example is acrylamide. After the accidental detection of this carcinogenic toxicant in processed foods, it took three years by available state-of-the-art methods to decipher and verify its chemical origin, and to develop strategies for its avoidance (Stadler et al. 2004). A full understanding of in-food/in-process chemical pathways and in-body product/metabolite interactions is now within reach using the Fraunhofer-Monash workflow.
Fraunhofer-Monash Workflow
In spite of the complex and divergent possibilities of chemical reactions that occur, the current approach is to simply compare the analytical recovery of the parent food ingredient or compound before and after processing. As such, intermediate products or side reactions have been previously ignored and treated as a “black box”, as most food products represent matrices with enormous complexity and potentially ongoing chemical reaction dynamics. This complexity makes it, up to now, impossible to decipher the fate of individual precursors or the generation of intermediate or final chemical products.
However, in the same way that biological heterogeneity and complexity has been tackled by the suite of “omics” research, technologies are also now available to address chemical heterogeneity, and chemical reaction dynamics in the context of food matrices and stressors. It is imperative that novel molecular research tools for interdisciplinary nutritional life sciences are now applied to understanding complex food systems. If we don’t, the void of understanding allows misinformation to reach consumers and fuel anxiety. In the absence of scientific facts, consumers may be susceptible to pseudoscience, falsehoods or even outright lies about the food supply. This new workflow—aiming to secure factual understanding about the chemical and biochemical fate of bioactive ingredients—addresses one of the two key factors in consumer trust.
In previous projects, Fraunhofer IME researchers have successfully demonstrated the significant influence of different food preparation steps on the chemical stability of pesticides. Several parameters, such as matrix composition, heating temperature, processing duration and individual chemical properties of substances, were shown to affect the degradation, volatilization and transfer processes during food processing (Goeckener, Kotthoff, and Buecking In Press). For example, investigations of in-food and in-process transformations of selected pesticides showed that the parent compound was degraded by ~70%, resulting in >10 degradation products, identified for the first time, which could not be assumed to be harmless (Goeckener, Kotthoff, and Buecking In Press). The application of the Fraunhofer-Monash workflow to food systems grew from this methodology, centrally involving 14C-radio-labeling of a target compound, and thereby enabling traceability of the fate and bioactivity of all (labeled) degradation products.
There are other examples of using 14C-labeling to track the metabolism and distribution of food compounds and their metabolites, using the pig model of human digestion. In particular, 14C-labeling of dietary fatty acids has traced their metabolism, oxidation rates, pharmacokinetics and bio-distribution in tissues (Odle, Benevenga, and Crenshaw 1992). Other studies of 14C-labeled food bioactives include triglycerides, vitamins, phytonutrients and toxins (Prelusky, Miller, and Trenholm 1996, Schweigert et al. 1995, Wang, Kearns, and Mock 2001, Heo et al. 2002), as limited by the ability to control the chemical localization of the 14C label, posing an increasing challenge for macronutrients.
Integrated Technology and Expertise
Representing a proven workflow for pesticides, in December 2017, the Fraunhofer 14C research team joined forces with Monash University and developed a unique assemblage of joint expertise in an ambitious study of the fates of food nutrients and bioactives: in-food, in-process and in-body. Complementing the state-of-the-art analytical science experts of Fraunhofer IME are experts from three Faculties of Monash University: Science, Medicine, Nursing and Health Sciences, and Pharmacy and Pharmaceutical Sciences, as well as the team from Monash Food Innovation. Members of the Monash team bring strong competence in synthetic chemistry, biological pathway analysis, clinical nutrition, health sciences and food marketing, reflecting the second and third horizons of research to evolve from primary identification of 14C metabolites.
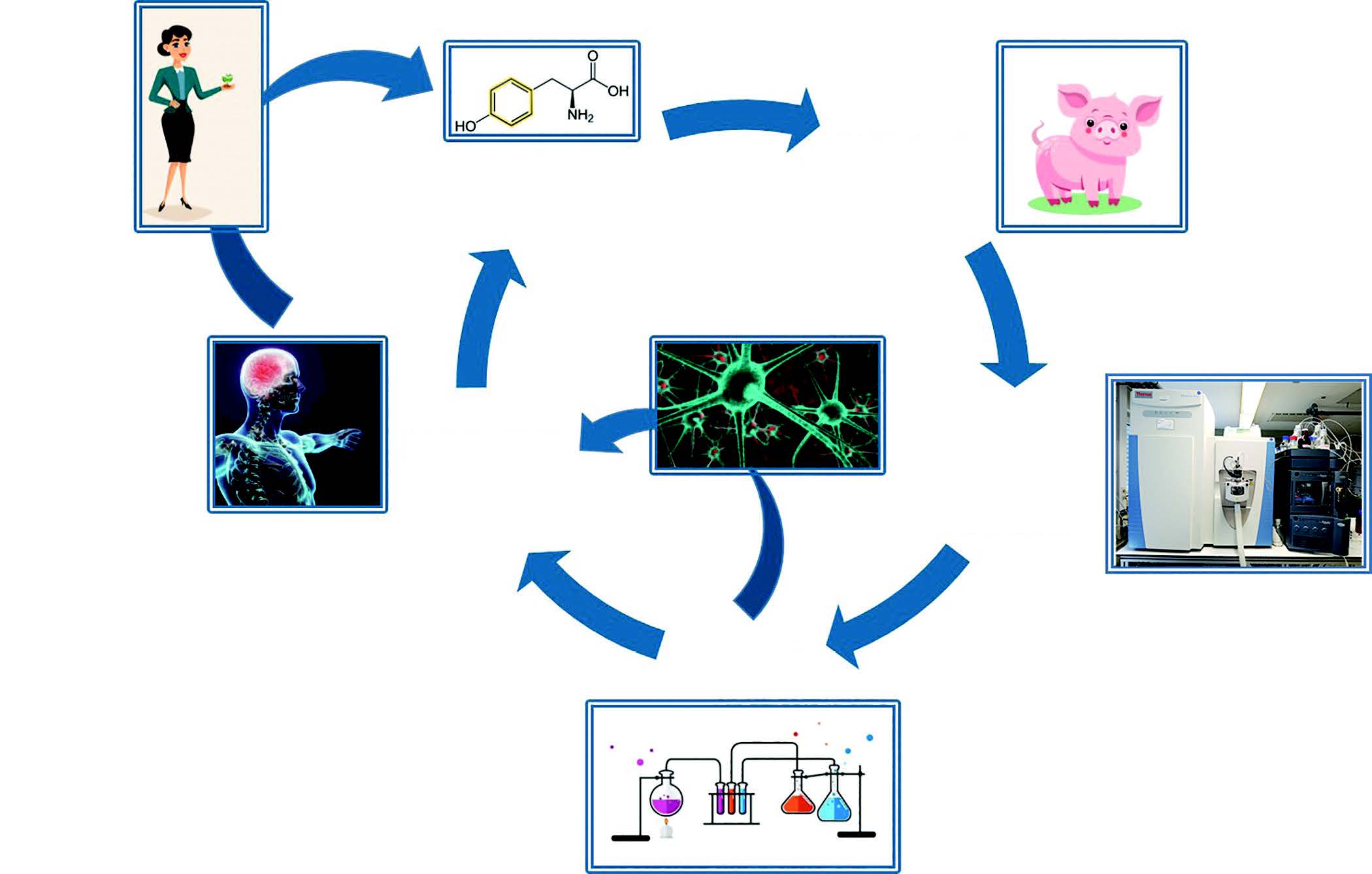
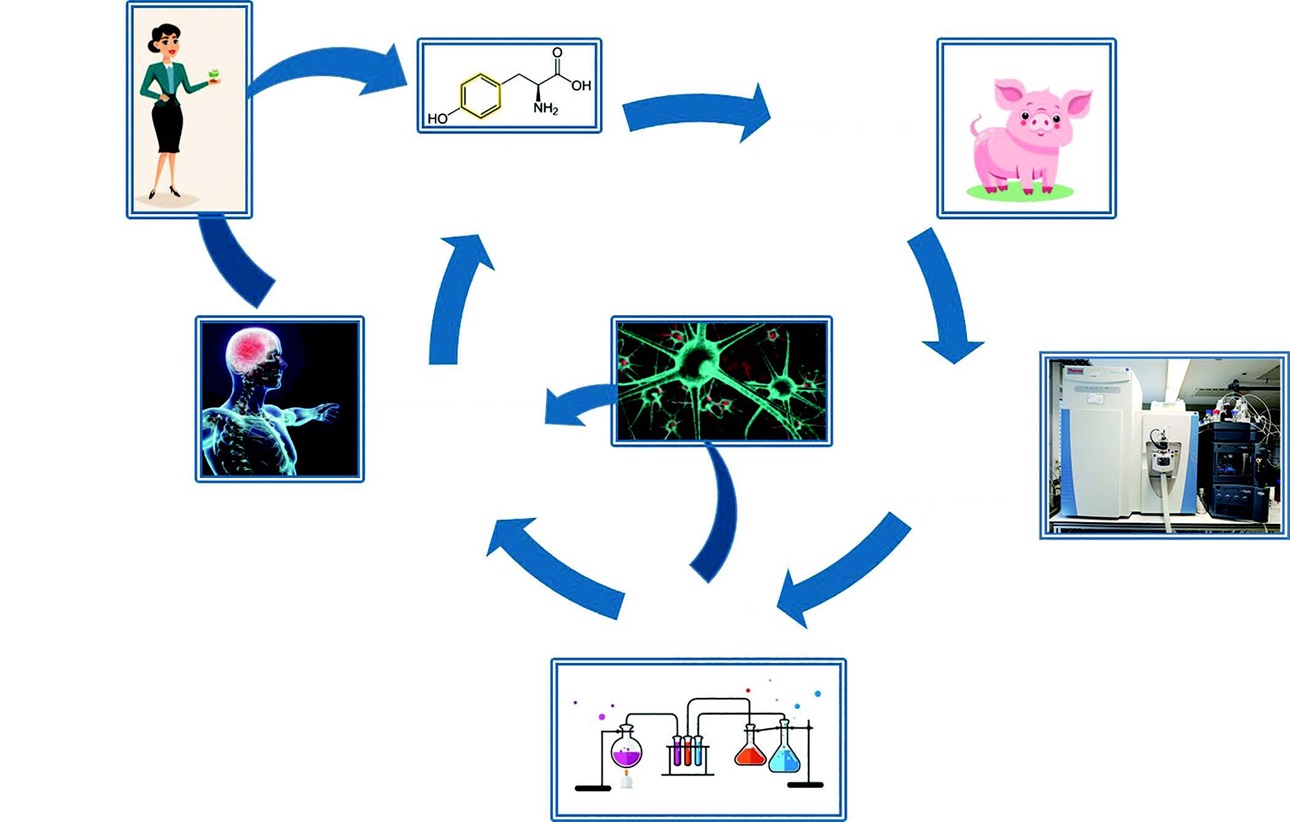
The extended workflow will validate detection of confirmed metabolites in commercial food products and in humans, and undertake synthesis of metabolites for conducting cell and animal studies of safety and efficacy using cellular and animal models of lifestyle diseases (Figure 1). The research findings will drive stakeholders to optimize industrial food processing conditions and develop new products in which the fate of bioactive compounds can be engineered to produce targeted health outcomes, and avoid risk-associated chemical and biochemical pathways.
The primary project workflow involves in-food, in-process and in-body monitoring of a 14C-food bioactive. Studies modeling in-food and in-process chemical degradation will model industrial conditions relevant to the target bioactive and food vehicle. Studies modeling in-body metabolism will be conducted in pigs, the closest animal model of human digestion. Chemical analysis of 14C-metabolites and degradation products will be conducted by ultra-high-performance liquid chromatography coupled with high resolution mass spectrometry (accurate-mass Quadrupole-Orbitrap MS systems), with capacity for rapid, highly selective detection of low-level 14C-compounds in complex matrices. The identification of 14C-labeled chemical products and metabolites will be subsequently validated by screening for matching compounds in commercial food products and in human studies, respectively. In order to get this exciting Fraunhofer-Monash workflow for food quality substantiation underway, two model compounds were chosen: the essential fatty acid, alpha-linolenic acid (ALA) and the neurotransmitter pre-cursor, tyrosine.
Understanding Metabolism of Alpha-Linolenic Acid
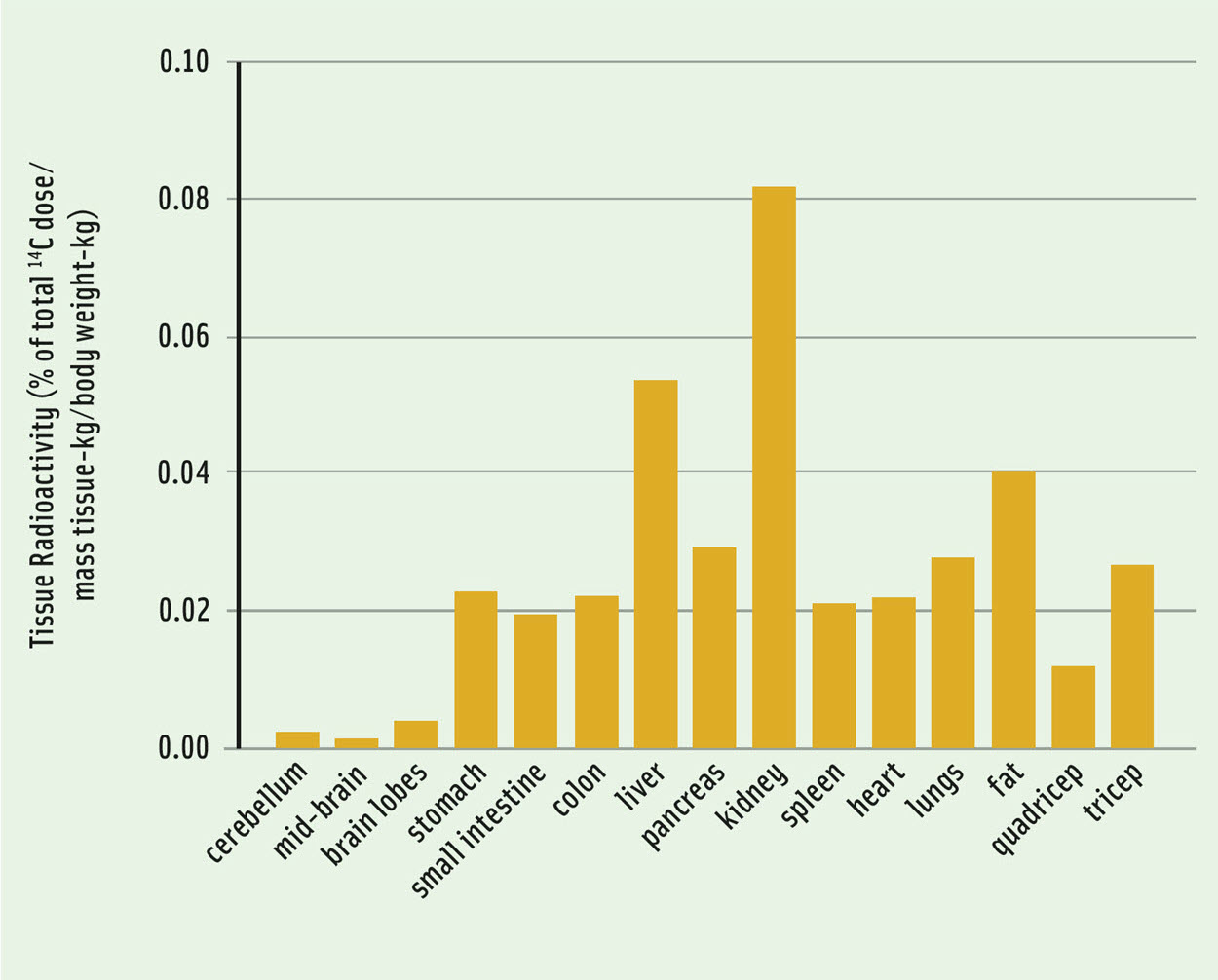
Omega-3 fatty acids drive many important health benefits for the body and brain and are often added as a functional ingredient, promoting both healthiness and value addition in the food. The essential omega-3 fatty acid, ALA, was selected as a model bioactive for study in the Fraunhofer-Monash workflow, and we report some preliminary data demonstrating the biodistribution of 14C-ALA in pigs determined three days after 14C-ALA intake (Figure 2). The ALA peaked in the plasma approximately 6 hr, and in the feces approximately 24 hr after intake. Three days after intake, trace levels of 14C-ALA were detected in the digestas of the small intestine and colon but there was no residual radioactivity in the stomach digesta. The highest tissue enrichments of ALA were measured in liver, kidney and fat tissues (Figure 2). In the future, we aim to report the omega-3/omega-6 distribution in body tissues and their metabolites.
Understanding the Food-Brain-Mood Paradigm
We will also use the Fraunhofer-Monash workflow to address the complex and challenging issue of food-derived metabolites that can reach and affect the brain. Neurons are specialized cells that propagate signals across the central nervous system (CNS) involving a variety of chemical signals (neurotransmitters) to regulate body functions. Tyrosine is an aromatic amino acid that is used to make two neurotransmitters: dopamine and noradrenaline, that have profound effects on mood, reward behavior, wakefulness and motor activity. 14C-tyrosine was also selected as a model compound which was fed to pigs in an encapsulated form that directed the 14C-tyrosine to the gut, so as to promote its exposure to bacterial fermentation and colonic absorption of metabolites. Preliminary results have indicated that gut-localized fermentation of 14C-tyrosine produced metabolites that were absorbed into circulation and detected in the brain. In the future, we aim to report the chemical nature of metabolites of tyrosine detected in the brain and other organs, demonstrating the potential for dietary proteins to reach and affect the brain.
What’s Next?
When we have identified the major 14C-metabolites of ALA and tyrosine using high resolution LC-MS, the next steps are to undertake chemical synthesis of unique chemical metabolites (Figure 1). Synthetic metabolites of both ALA and tyrosine will then be used for mechanistic studies in models of cell-based signaling and functioning of the brain and immune system. For example, the model system to be employed for tyrosine metabolite studies is a mixed culture of pluripotent stem cell-derived dopamine-containing neurons and astrocytes, which help regulate neuron activity and survival, and optionally, to also include microglia; the immune regulators of the CNS. This co-culture cellular model enables investigation of the role of diet in modulating chronic neuro-inflammation, especially that associated with neuro-degenerative diseases of aging such as Alzheimer’s and Parkinson’s diseases.
In parallel with mechanistic pathway studies, we will seek to detect evidence of the metabolites produced in pigs, also in humans; with potential to discover associative relationships of target metabolites against established biomarkers of wellness, disease risk (e.g., oxidative stress, inflammation), and also mood and cognitive symptoms. The Fraunhofer-Monash workflow thereby includes research arms that generate mechanistic support for bio-efficacy and toxicity risk, supported by clinical evidence, including capacity to study the very challenging food-brain-mood paradigm.
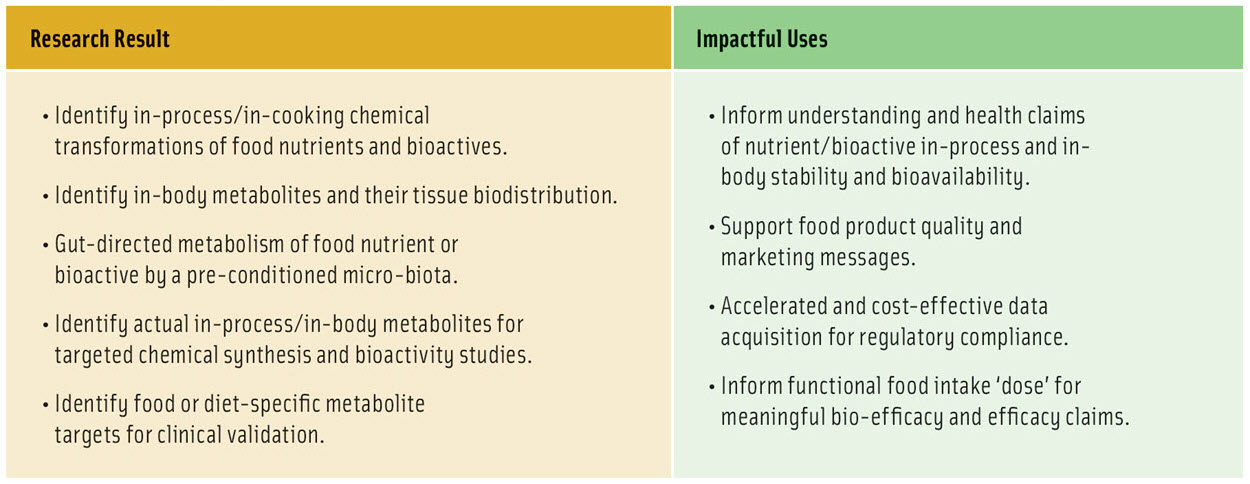
As for advances in other global challenges, the power of the Fraunhofer-Monash workflow is the strategic, unique and efficient integration of cross-disciplinary expertise and technology. This workflow is organized for the goal of building factual understanding that can be translated by industry into consumer health benefits, by marketing products that they trust (Table 1, Figure 1). The workflow supports the food industry to achieve this goal by unprecedented efficiency of identifying food degradation products and metabolites, that can ultimately lead to managing delivery of health benefits and avoiding toxicity risks. This convergence of research and data will accelerate understanding of the conditions needed to chemically stabilize and preserve the target bioactivity of foods. The research will support deep understanding of “loss-of-function” and “gain-of-function” scenarios, and permit strategies that promote target health benefits and manage risks.
About the Authors
Mark Buecking, PhD, is head of department of Environmental and Food Analysis at Fraunhofer Institute for Molecular Biology and Applied Ecology IME, Germany ([email protected]), and adjunct associate professor at Monash University, Australia. Bernd Goeckener is with the Fraunhofer Institute for Molecular Biology and Applied Ecology IME, Germany. John M. Haynes, PhD, is senior lecturer in the Monash Institute of Pharmaceutical Sciences, Monash University, Australia. Patricia Leitner is with the Fraunhofer Institute for Molecular Biology and Applied Ecology IME, Germany. Margaret Murray, PhD, a member of IFT, is research fellow in the School of Chemistry, Monash University, Australia. Gary Williamson, PhD, is professor in the department of Nutrition and Dietetics, Monash University, Australia. Louise Bennett, PhD, a member of IFT, is professor in the School of Chemistry, Monash University, Australia (louise.bennett1@ monash.edu).
Acknowledgment
The work of Lisa Kaminskas, PhD, (University of Queensland) in conducting radioactivity analysis is gratefully acknowl-edged, and also the contributions of additional members of the research team: Maxine Bonham, PhD, Aimee Dordevic, PhD, Anne Gibbon, Andrea Robinson, PhD, and Sophie Selby-Pham, PhD, all from Monash University.References
Goeckener, B., M. Kotthoff, and M. Buecking. In Press. “New Processing Induced Degradation Routes of Prochloraz in Rapeseed Oil.” J. Agri. and Food Chem.
Heo, K. N., X. Lin, I. K. Han, and J. Odle. 2002. “Medium-chain fatty acids but not L-carnitine accelerate the kinetics of C-14 triacylglycerol utilization by colostrum-deprived newborn pigs.” J. Nutr. 132 (7): 1989-1994.
Odle, J., N. J. Benevenga, and T. D. Crenshaw. 1992. “Evaluation of 1-C-14 -medium chain fatty acid oxidation by neonatal piglets using continuous-infusion radiotracer kinetic methodology.” J. Nutr. 122 (11): 2183-2189.
Prelusky, D. B., J. D. Miller, and H. L. Trenholm. 1996. “Disposition of C-14-derived residues in tissues of pigs fed radiolabelled fumonisin B-1.” Food Add. and Cont. 13 (2): 155-162.
Schweigert, F. J., I. Rosival, W. A. Rambeck, and J. Gropp. 1995. “Plasma transport and tissue distribution of C-14 beta-carotene and H-3 retinol administered orally to pigs.” Intl. J. Vit. and Nutr. Res. 65 (2): 95-100.
Stadler, R. H., F. Robert, S. Riediker, N. Varga, T. Davidek, S. Devaud, T. Goldmann, J. Hau, and I. Blank. 2004. “In-depth mechanistic study on the formation of acrylamide and other vinylogous compounds by the Maillard reaction.” J. Agri. and Food Chem. 52 (17): 5550-5558. doi: 10.1021/jf0495486.
Wang, K. S., G. L. Kearns, and D. M. Mock. 2001. “The clear-ance and metabolism of biotin administered intravenously to pigs in tracer and physiologic amounts is much more rapid than previously appreciated.” J. Nutr. 131 (4): 1271-1278.