The Ultimate on Ultrafiltration
PROCESSING
Ultrafiltration (UF) is a selective separation step used to both concentrate and purify components of medium to high molecular weight, such as plant and dairy proteins, carbohydrates, and enzymes.
In 1850, Thomas Graham, a chemist in Glasgow, Scotland, demonstrated diffusion of gases across a semipermeable membrane, and in 1861, he reported his first dialysis experiments, which allowed separation of dissolved substances or removal of water from solutions through synthetic semipermeable membranes. The term “ultrafiltration” was first introduced in Germany by Von H. Benchold in 1907 to describe forcing solutions at several atmospheres through nitrocellulose membranes of graded pore-size structures.
A pressure-driven purification process that separates particulate matter from soluble compounds using an ultrafine membrane media, UF is an excellent technology for desalination pretreatment, reverse osmosis pretreatment, and wastewater reclamation, as well as for producing potable water.
In the dairy industry, ultrafiltration is used for a wide range of applications such as protein standardization of cheese milk, fresh cheese production, protein concentration, and decalcification of permeates, as well as lactose reduction of milk. Specifically, UF allows the smaller lactose, water, mineral, and vitamin molecules to pass through the membrane, while the larger protein and fat molecules (key components for making cheese) are retained and concentrated.
The global ultrafiltration membrane market was valued at $5.3 billion in 2019 and is expected to grow significantly during the years ahead, according to Market Study Report LLC. Dairy processing accounts for the largest share of membrane capacity worldwide.
In the beverage industry, UF is used to improve product yield and quality. UF systems remove proteins, suspended colloids, polyphenolic compounds, starch, pectin, and microorganisms from natural juices, providing a brilliantly clear juice permeate that is stable even after extended storage. UF is commonly used for product concentration (i.e., solvent removal) in bioprocessing; concentration of cell-free fermentation broths containing complex biological compounds such as monoclonal antibodies; and biomolecule/product recovery from very dilute solutions.
Food UF applications include concentration of proteins (enzymes, milk proteins, egg whites), starch, and pectin; clarification/stabilization of fruit juices and wine (removal of haze components); removal of cellular debris and bacteria from beer; removal of polysaccharides, proteins, and colloidal impurities in sugar refining; and sterile filtration of biologicals (removal of bacteria and viruses). Additional food applications of UF are detailed in Table 1.
A list of some of the leading manufacturers of ultrafiltration membranes and systems serving the dairy, food and beverage, and biopharmaceuticals industries can be found in Table 2.
Increased use in the dairy industry, improvements in membrane filtration technology, rising demand for premium products, and legislative restrictions regarding water safety and filtration are all factors driving market growth. Polymeric membranes have the largest global market share due to their relatively low prices and new market applications.
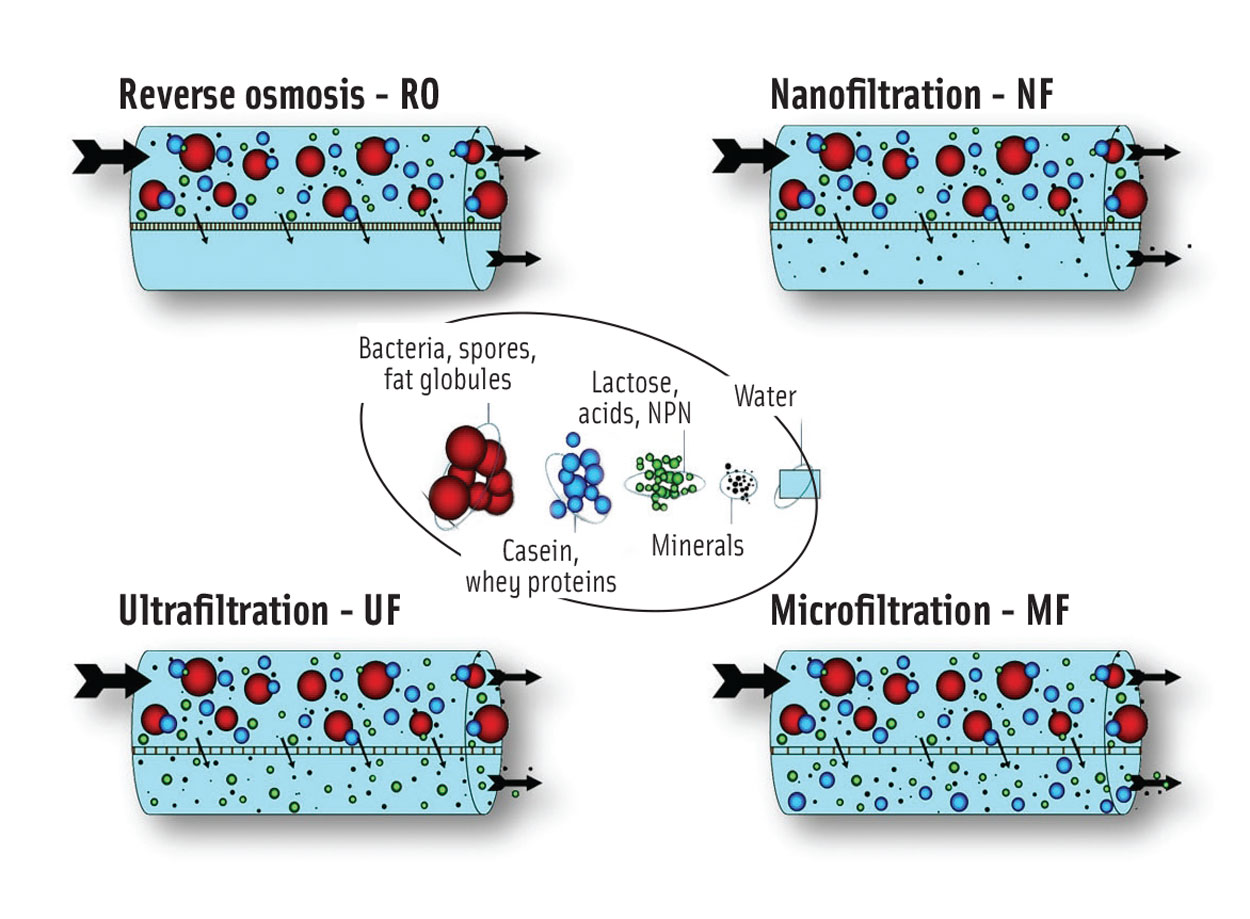
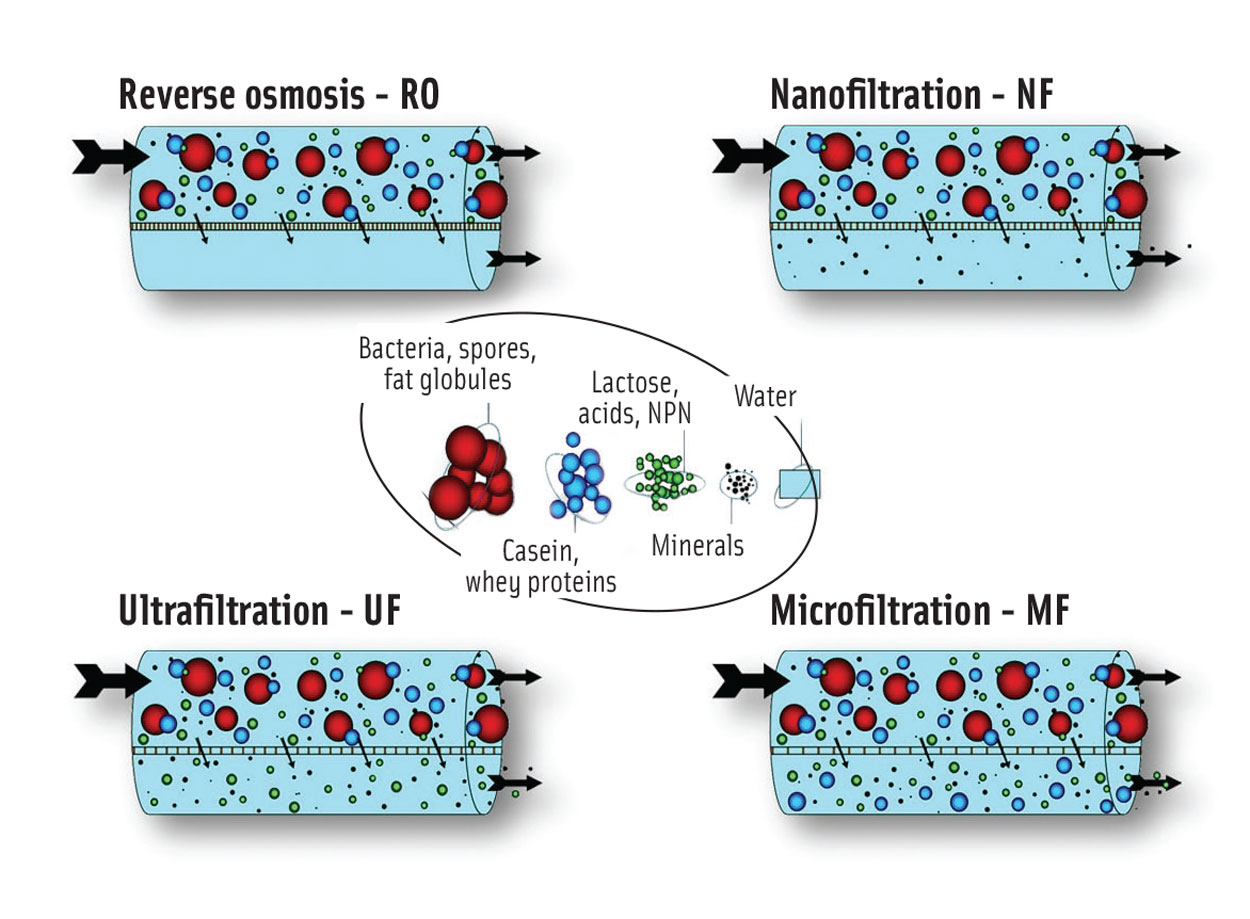
Within the dairy industry, three membrane filtration processes are used in addition to UF: microfiltration (MF), nanofiltration (NF), and reverse osmosis (RO). Figure 1 illustrates which milk and whey components can be concentrated by means of each process, depending on membrane density. RO is the tightest possible membrane process in liquid separation. It concentrates the total solids, and only water can pass through the membrane; all dissolved and suspended material is rejected. NF separates a range of minerals from a liquid, allowing only the fluid and certain monovalent ions to pass through the membrane. UF membranes separate the feed (e.g., skim milk) into two streams, allowing water, dissolved salts, lactose, and acids to pass through the filter in either direction, while retaining (and thereby concentrating) proteins and fat. MF uses the most open type of membrane, which is used to separate bacteria, spores, and fat globules from the stream, and for fractionation of skim milk.
UF is also used to recover valuable components for recycling, as in the case of oily wastewater from the animal/vegetable fats industries. Hydrophilic UF membranes (e.g., polyacrylonitrile [PAN] and polyethersulfone [PES]) are used for recovering oil from oily wastewaters instead of hydrophobic membranes (e.g., polyvinylidene fluoride [PVDF]) that would get fouled with oil, resulting in loss of flux. The oil droplets are completely retained by the UF membrane although the membrane is permeable to free (soluble) oil. UF permeate of a highly stable emulsified feed stream can be discharged to a municipal wastewater system. The recovered oil is less than 5% of the original waste volume.
Industrial UF was developed primarily for the treatment of wastewater to remove particulate and macromolecular matter. Its applications now include water treatment, food processing, biotechnology, and chemical processing. UF is used for removing macromolecules, colloids, emulsified oil, endotoxins, pyrogens, viruses, and bacteria.
Basic Science
Many simple filtration processes use a dead-end technique with the flow of liquid to be filtered directed perpendicular to the filter surface. This is effective when the concentration of particles to be removed is low or the packing tendency of the filtered material does not produce a large pressure drop across the filter medium. Some common examples of dead-end filtration are home water filters, vacuum cleaners, and automobile oil filters. Typical industrial uses include the sterile filtration of water, beer, and wine.
In contrast, there are many process streams that have high concentrations of particles or macromolecules such as cells, proteins, and precipitates that will rapidly compact on the filter surface when operated in a dead-end mode. Consequently, the filtration rate drops quickly to an unacceptable level. In these instances, a cross-flow membrane system, as used in UF, provides the means to maintain stable filtration rates.
UF membrane separation falls between NF and MF with a pore size range of 0.01–0.1 µm. UF membranes are porous in nature, with an asymmetric structure and dense skin layer with a small pore size and low surface porosity that produces a higher hydrodynamic resistance than MF membranes. The thickness of the top layer is 1.0–3.0 µm. Since the osmotic pressure of micro-solutes is low or negligible and the pore is larger than RO and NF membranes, UF operating pressure is low and in the range of 2–5 bar. In the case of solutions containing particles or macromolecules, as in the case of food fluids, the flux declines drastically with time due to the formation of a gel layer. UF membrane separation depends on membrane pore size, solute-membrane interactions, and the shape and size of the solutes/macromolecules. Because of pore size distribution, the pore diameters of UF membranes should be at least one-half that of the smallest solute to be removed.
There is a correlation between pore size and water flux through a UF membrane, but direct proportionality between flux and applied pressure is only true for water. For typical aqueous solutions used in UF separations, flux drops off drastically due to concentration polarization and fouling. Further, due to the larger pores in the membrane skin, UF membranes have an order of magnitude higher flux than RO membranes.
Membrane Fouling
Fouling refers to the irreversible alteration in membrane properties, resulting from several interactions of feed stream components and membranes. In food application, a membrane is usually fouled by biofoulants such as proteins and polysaccharides. Mechanisms of membrane fouling by protein suspensions involve the following: a) the phenomenon of concentration polarization followed by the formation of a gel layer; b) adsorption of solutes on the membrane surface and inside the pore structure; and c) deposition and pore blocking of protein aggregates due to denaturation. All of these lead to the blockage of the membrane and thereby reduce its flux. The fact is that, in addition to the decline in flux, the retention of protein generally increases with time; this is an advantage in UF applications where high protein retention is required.
Membrane fouling, on the other hand, is more complicated in that it is considered as a group of physical, chemical, and biological effects leading to irreversible loss of membrane permeability. Concentration polarization effect usually takes place in less than a minute, whereas fouling takes place over the length of the processing period. Biofouling is another general problem with many membrane processes and involves all biologically active organisms, mainly bacteria and (in some cases) fungi. Biofouling is a dynamic process and involves the formation and growth of a biofilm attached to the membrane. The biofilm may reduce the water flux and even totally prevent water passage.
Fouling Control
Although fouling is an inevitable issue in membrane technology, techniques to control and minimize the effect and extent of fouling are emerging to ensure that membrane technology is competitive with other technologies. Completely avoiding fouling may not be possible, but its impact can be reduced by a variety of techniques, including choice of membrane, module, process configuration, membrane cleaning, and pretreatment.
For an installed plant, the options for fouling abatement become more limited. They are more focused on the physical and chemical methods that are used at various stages of the UF system. During pretreatment, prefiltration can be used as a physical method to reduce fouling; precipitation, coagulation/flocculation, disinfectants, anti-scalants, and absorption can also be used as methods to reduce fouling. During design, vibrating or rotating membranes or turbulence promotors can be added to reduce fouling physically. Pulsed/reverse-flow and electric fields may also be employed. Chemical methods to reduce fouling in UF include membrane surface modification and the selection of specific membrane material. During operation, reducing trans-membrane pressure, maintaining high cross-flow, and periodic hydraulic or mechanical cleaning reduce fouling, as does selection of cleaning chemicals and frequency of cleaning.
Ultrafiltration Equipment
The key to the design of a UF cross-flow system is selecting a membrane geometry that suits the physical characteristics of the process fluid. UF cross-flow membranes can be provided in tubular, flat sheet, spiral wound, and hollow fiber configurations, each of which provides certain advantages for specific process needs. Membrane materials are usually synthetic polymers, ceramic, or stainless steel, depending on processing conditions and durability.
The UF polymeric membranes are manufactured by a phase-inversion process. The most widely used polymer is polysulpone (PS), but increasingly other polymers are being used, such as cellulose acetate (CA), PES, PAN, and PVDF polymers. Typically, CA-based membranes have a higher flux at equivalent rejections than PS membranes. Although CA membranes are less prone to fouling, PS membranes are necessary for many applications because of their higher stability. In the food industry, where steam sterilization is required, PES is used instead of PS. Polymers commonly used for dairy, juice, and beverage applications include PS, CA, cellulose triacetate, PES, PAN, PVDF, and polypropylene. Among the newer membranes in use are polyimide polymeric (PI) membranes. PI membranes are promising for UF because of their resistance to many organic solvents, such as hexane, benzene, methanol, acetic acid, acetone, ethyl ether, ethoxy, ethanol, and chlorinated hydrocarbons.
Ultrafiltration Forecast
In the past decade, fouling (27%), modeling (17%), and wastewater (12%) were the dominant research topics on UF technology and accounted for more than half of the 4,547 scientific articles published within this specified period. Topics like UF membrane fabrication and modification, food processing, and hybrid membrane process have revealed a growing trend in terms of current interest in UF applications.
Cross-flow membrane UF technology is quickly gaining worldwide acceptance as an important manufacturing step in many of the process lines in the food, dairy, pharmaceutical, biotechnological, chemical, and starch and sweetener industries. The ability to produce very specific separations at low or ambient temperatures with no phase change can, in many applications, make membrane UF a much more cost-effective solution than more conventional methods such as rotary vacuum filtration or filter presses.
UF technology also will play an important role during the current COVID-19 pandemic, as it is effective for virus removal in the production of therapeutic proteins and vaccines, as well as for antibiotics recovery.
REFERENCES
Mohammad, A.W., C. Y. Ng, Y.P. Lim, et al. 2012. “Ultrafiltration in food processing industry: Review on application, membrane fouling, and fouling control. Food Bioprocess Technol. 5: 1143–1156.
Urosevic, T., D. Povrenovic, P. Vukosavljevic, et al. 2017. “Recent developments in microfiltration and ultrafiltration of fruit juices.” Food Bioprod. Process. 106: 147–161.