Allergenicity and Food Processing
PROCESSING
Food allergy is complicated. It is defined as ‘‘an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food.’’
Allergies are immunologically mediated reactions that affect organs such as the skin, gut, and airways. The prevalence of allergic conditions is increasing at an alarming rate. A U.S. survey repeated on three occasions compared the prevalence of peanut allergy in children from 1997 to 2008, showing that the rate more than tripled from 0.4% to 1.4% (Sicherer et al. 2010). Food allergy is currently reported to affect nearly 5% of adults and 8% of children (Sicherer and Sampson 2018).
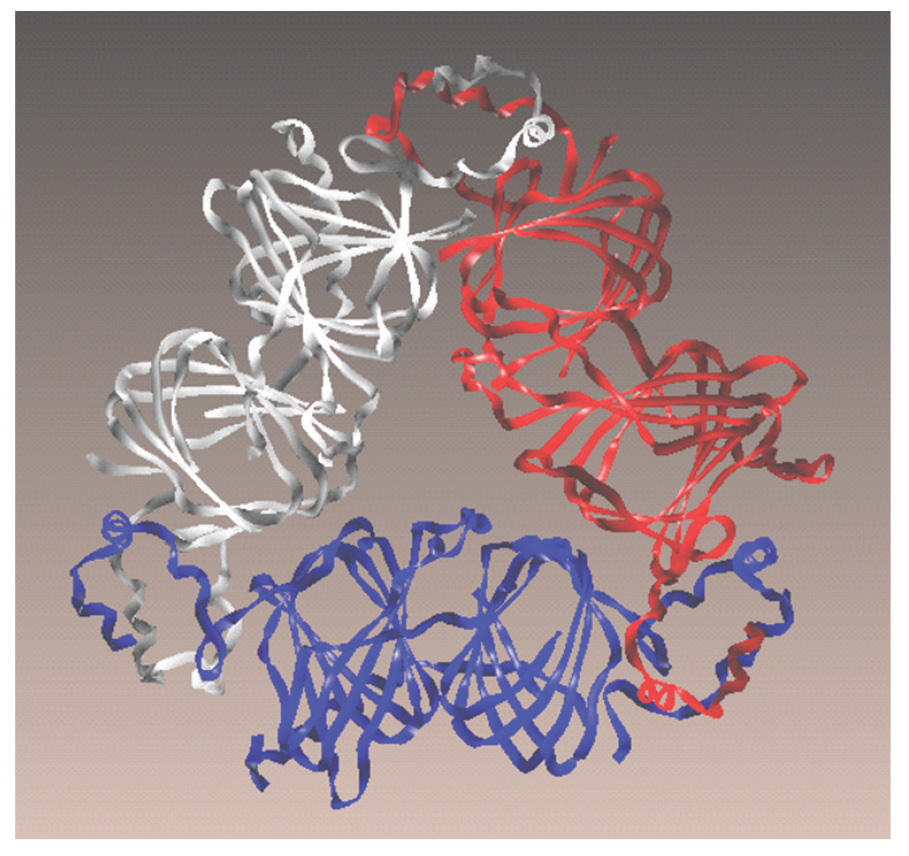
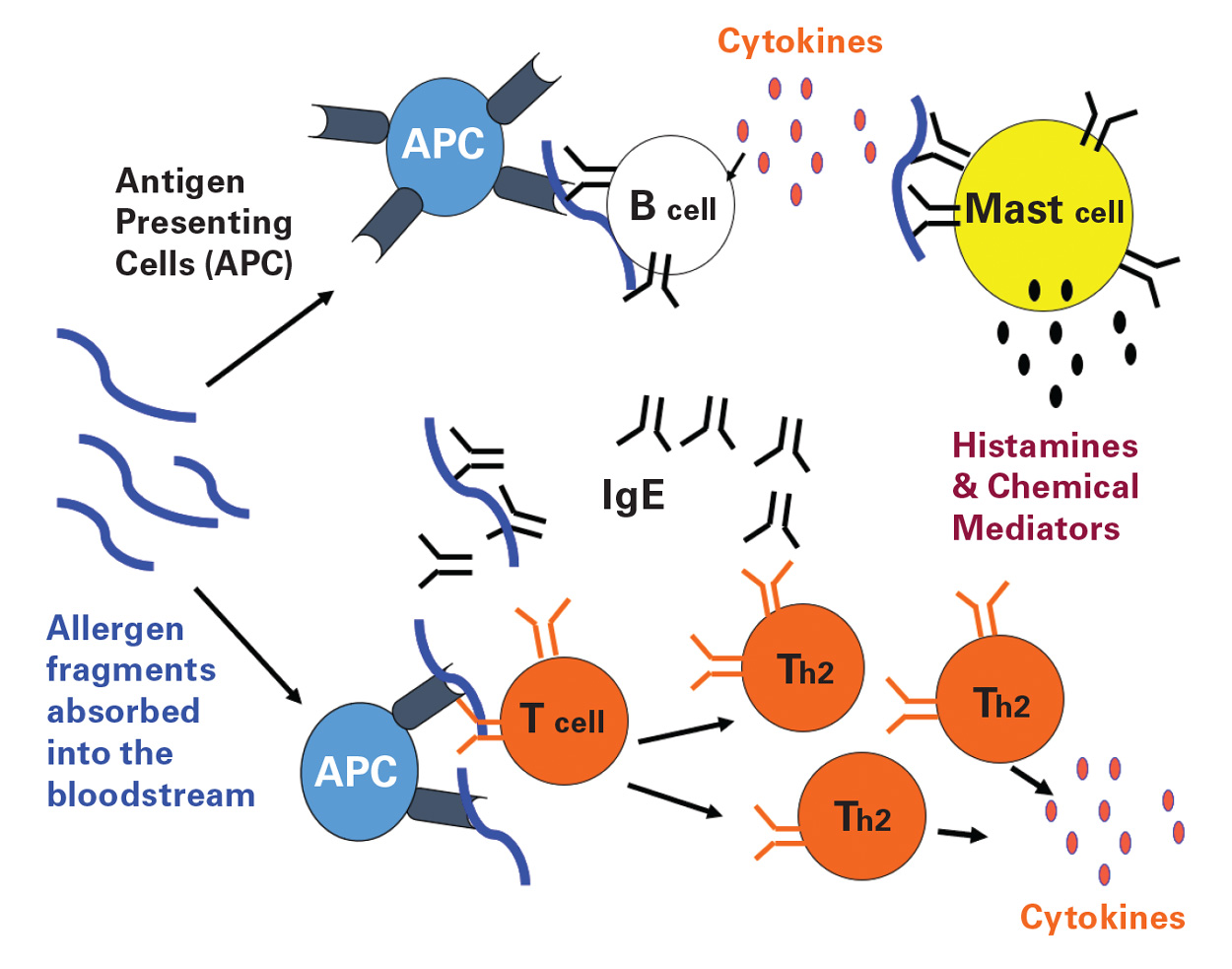
Potentially any food can cause food allergy, but there are eight foods (milk, egg, soy, wheat, fish, shellfish, peanuts, and tree nuts) that most commonly cause food allergies in the United States and are required to be labeled by the Food Allergen Labeling and Consumer Protection Act (FALCPA) of 2004. Immunoglobulin E mediated food allergies are classified as Type I or immediate hypersensitivity. (Immunoglobulin E [IgE] is the primary mediator of food allergic reactions). It is known that IgE-mediated mast cell degranulation, which results in the release of chemical mediators such as histamine, is a strategy that evolved to eliminate respiratory or gastrointestinal pathogens. However, few reasons have been offered to explain why certain foods elicit immunological responses similar to those elicited by multicellular metazoan parasites that reside in the respiratory and gastrointestinal systems while other foods are tolerated and nonallergenic. Whereas many of the structural characteristics, homology, and cross reactivity of food allergens have been explained, specific immunological and biophysical properties of the allergens that contribute to IgE antibody formation are not fully understood.
Some of the immunological properties of allergenic proteins include their ability to stimulate Th-2 type T-cell proliferation, bind serum IgE, elicit a positive prick skin test, and cause histamine and mediator release from mast cells and basophils of sensitive individuals. Characteristic biochemical properties of allergens are that they are proteins or glycoproteins with molecular weights that fall in the 5–100 kDa range, which are usually abundant in the food source and often stable to digestion by gastrointestinal enzymes. Specific structural features and functional similarities, such as protease activity, have also been associated with both food and inhaled allergens.
Effects of Food Processing
Thermal processing, such as frying, roasting, curing, and various types of cooking, can cause multiple, non-enzymatic, biochemical reactions to occur in foods. The browning process in many foods is due to a reaction known as the Maillard reaction, which is one of the major reactions that occurs during cooking or browning of foods (e.g., roasting, frying, and curing). The Maillard reaction is important in the development of flavor and color in foods such as peanuts and tree nuts during roasting, enhances flavors in beverages such as beer and coffee, and involves a process in which amino groups of proteins are modified via non-enzymatic condensation with reducing sugars, and can be described as similar to caramelization.
These thousands of biochemical reactions can affect food proteins, and food allergens are proteins. In turn, the allergenic or immunogenic potential of food can be changed by food processing, which means that the protein encountered by serum IgE, T cells, and other immune cells can be different following processing. A perfect example of this observation is that when milk or egg is baked into a muffin, it elicits a less intense allergic reaction in milk or egg allergic individuals than drinking a glass of milk or eating a fried or boiled egg. This is important because it has been shown that you can more safely (with lower risk of severe reactions) desensitize allergic individuals to milk or egg with baked products than with raw or standard egg or milk consumption (Lambert et al. 2017, Bird et al. 2019). However, it is important to point out that this approach is not safe or effective for all egg or milk allergic individuals as the immune responses among allergic individuals can vary significantly based on different degrees of food processing, the allergenic molecules recognized by the immune system, and the doses tolerated by an individual. Each process can affect the allergenic potency of different foods in a different way. For example, it is known that while roasting or frying peanut enhances the IgE recognition of peanut allergens (Maleki et al. 2000), boiling and high pressure treatment can result in a reduction in allergenic potential of peanuts (Cabanillas et al. 2018). Consuming pistachios soaked in lemon caused significantly reduced reactions in allergic individuals than raw pistachios (Noorbakhsh et al. 2010).
The Effects of Roasting at a Molecular Level
As mentioned previously, one of the major reactions that occurs during cooking or browning of foods (e.g., roasting) is the Maillard reaction, which is important to the food industry and therefore has been extensively studied and is fairly well understood. The amino groups of proteins are modified via non-enzymatic condensation with reducing sugars, ketose, or aldehydes to form glycosylamines that can undergo rearrangement to form reactive dicarbonyl intermediates, which react with or cross-link amino groups to form stable end products called advanced Maillard reaction products, better known as advanced glycation end products (AGEs). The immunological and allergic effects of AGEs have shown that roasting increases peanut allergenicity and that AGEs contribute to this observation. Very specific roasting-induced chemical modifications can be identified for individual allergens. For example, one allergen in peanut, Ara h 1, was purified and subjected to the Maillard reaction. As a result, multiple Ara h 1 molecules were cross-linked or glued together with the reactive sugars, resulting in large inseparable stacks of Ara h 1 molecules, which caused them to be more resistant to digestive enzymes (Maleki et al. 2000).
Resistance to digestion makes it more likely for undigested pieces to be absorbed into the bloodstream and encounter the immune system. Also, specific amino acid modifications on the surface were shown to enhance the recognition and binding of Ara h 1 by serum IgE and a receptor on the surface of immune cells, known as the receptor for AGE (RAGE) (Mueller et al. 2013). These are some examples of how research is carried out to understand the food proteins that are known to be allergenic, how processing may alter them, and how these changes influence the recognition and response by the immune system.
Geographic and Cultural Differences
The most recent insights into the pathogenesis of food allergy involve the increased understanding of environmental and non-oral (i.e., inhaled or via skin) sensitizing agents and routes. Regional lifestyles, environmental pollen and parasite exposure, dietary habits, the varieties of allergenic plant foods and allergen sequences, and methods of food preparation that are culturally defined play a role in the prevalence of specific food allergies in various countries (Beyer et al. 2001, Cochrane et al. 2009, Hoffmann-Sommergruber 2005). An electronic survey in the United States estimated that of the 8% of children who have food allergies, 2.4% have multiple food allergies and about 3% report severe reactions.
A systematic review of both U.S. and European populations found that hazelnut was reported as the most prevalent tree nut allergy in Europe while walnut and cashew were most prevalent in the United States (McWilliam et al. 2015). While severe peanut allergy is mostly a disease of more developed countries, peanut is a staple food in many developing countries like Western Africa, with volumes of consumption exceeding those in North America but minimal reported severe allergy to peanut. In Sweden, 16% of individuals had elevated serum IgE, with 13% to hazelnut, 4.9% to peanut, and 3% to almond. A retrospective analysis of 99 Singaporean children with IgE to cashew reported that 71% had cutaneous reaction (skin), anaphylaxis was seen in 3.8%, and 53% were cross-reactive (also reacted) with peanut, while in the United States, cashew causes more severe reactions than peanut but is less prevalent. There are also differences of IgE binding at the protein level.
Allergy Advances
The specific reasons for the increase in allergic diseases, particularly in developed countries, are unknown. Some theories suggest reasons such as better hygiene, vitamin D deficiency, environmental exposures, or globalization of markets. However, two major breakthroughs have occurred in the field of food allergy. The first finding is that early introduction of peanut to at-risk infants (between the ages of 4 and 6 months), reduces the chances of developing peanut allergy by more than 80% (DuToit et al. 2015). This is likely to be true for other food allergens. The second is the development of peanut as the first food to be characterized as a pharmaceutical and marketed as an oral immunotherapy for the treatment of peanut allergy, which involves ingestion of increasing doses of peanut over time to achieve desensitization (Pepper et al. 2020). This is the first FDA-approved treatment offered for food allergy.