Food and Water Safety Technologies Gone Viral
The enemy of my enemy is my friend: How bacteriophages are being used to reimagine food and water safety.
Article Content
Providing the world population with sufficient quantities of safe food and drinking water is hampered by several factors, including erratic weather patterns from climate change and global overpopulation (Berners-Lee et al. 2018). Within the United States, the annual aggregated economic burden of foodborne disease is estimated to be between $51 billion and $71 billion, depending on the model used (Scharff 2012). The World Health Organization (WHO) estimates that the annual global burden of foodborne illness is approximately 600 million people, with 40% of the associated deaths coming from children under five years of age (WHO 2015). These staggering statistics have led many scientists to look for new technologies to help combat pathogenic bacteria, while others are looking to revive older strategies.
Bacteriophages (phages) have been used as tools to address several of the emerging global issues that now plague humanity. Food safety is one of the areas where the unique characteristics of phages have shown promise as effective biocontrol agents of bacteria. Phages are the natural viral predators of bacteria and have evolved over billions of years to efficiently bind, infect, and kill their host bacteria with a high degree of specificity and speed. What’s more, phages are ubiquitous and naturally exist on food surfaces, are regularly consumed by humans, and do not influence the organoleptic profile of the products they come in contact with (Moye, Woolston, and Sulakvelidze 2018, O’Sullivan et al. 2019). Since the topic was last addressed in Food Technology in 2014, interest in general phage research as well as applications of new food-related phage technologies has grown significantly as emerging methodologies and technological advances in genetic engineering accumulated and became less expensive to employ (Wheatley et al. 2020, Peng and Chen 2020).
This ever-expanding niche in the field of biotechnology boasts a number of new and exciting developments that have caught the eye of many food processors along the farm-to-fork chain. Examples of phage-based technologies currently on the market include Agriphage (Certis USA), which is being used to control bacterial spot and speck on tomatoes and peppers; EcoShield (Intralytix) and PhageGuard E. (PhageGuard), which target E. coli O15:H7; PhageGuard S. (PhageGuard) and SalmoFresh (Intralytix), which target Salmonella spp.; and ListShield and ShigaShield (both from Intralytix), which target Listeria monocytogenes and Shigella spp., respectively (Moye, Woolston, and Sulakvelidze 2018). The continual global impact of food outbreaks means that new and innovative ways for combating these dangerous bacterial food and water pathogens are actively needed. Researchers can take advantage of natural systems (i.e., phage infections) that have been so evolutionarily successful in controlling their targeted bacteria’s population, they’ve gone viral. After all, the enemies of our bacterial enemies are promising food safety friends.
Understanding Phage Biology
For billions of years, the perpetual bilateral arms race that occurs between phages and their bacterial targets has led to each organism’s respective evolution being interdependent on the other: As bacteria are pressured to avoid phage infection, phages are then pressured to overcome host defenses. This coevolution has resulted in the incredible specificity and molecular synergy that phages have with their hosts, along with a unique recognition ability for their host bacteria. It is important to note that similar to all viruses, phages are not living organisms but instead are obligate biological parasites that rely exclusively on their bacterial host for their replicative process. Therefore, phage evolution can be attributed to replication mistakes of their host.
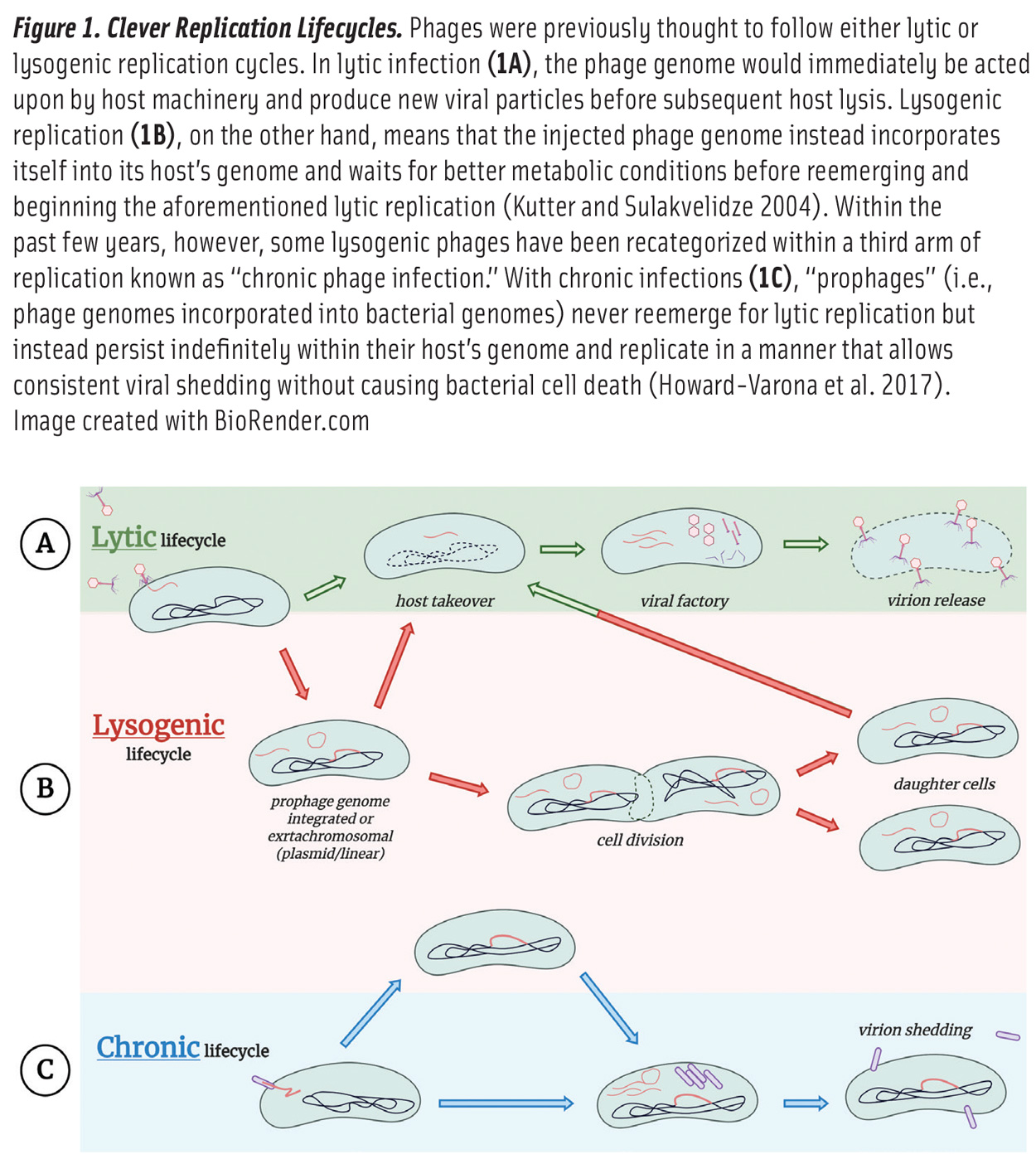
There are an estimated 1032 phage particles on Earth (Kutter and Sulakvelidze 2004), with about 95% of these belonging to the Caudovirales order of tailed bacteriophages that follow either lytic or lysogenic replication cycles, described in Figure 1. Generally speaking, lytic replication begins with receptor-dependent recognition of a host bacterium’s surface, facilitated by receptor-binding proteins located at the end of a phage’s protruding tail fibers (Kutter and Sulakvelidze 2004). Upon injection of the phage genome into the bacterial cytoplasm, a phage must successfully avoid host antiviral defenses while simultaneously hijacking the bacterium’s molecular machinery. During viral replication, phage-encoded endolysins enzymatically degrade the bacterial cell wall from within to release tens to thousands of newly assembled phage particles (virions) back into the surrounding environment. The released virions are then able to infect new hosts, thus perpetuating the phage replication cycle.
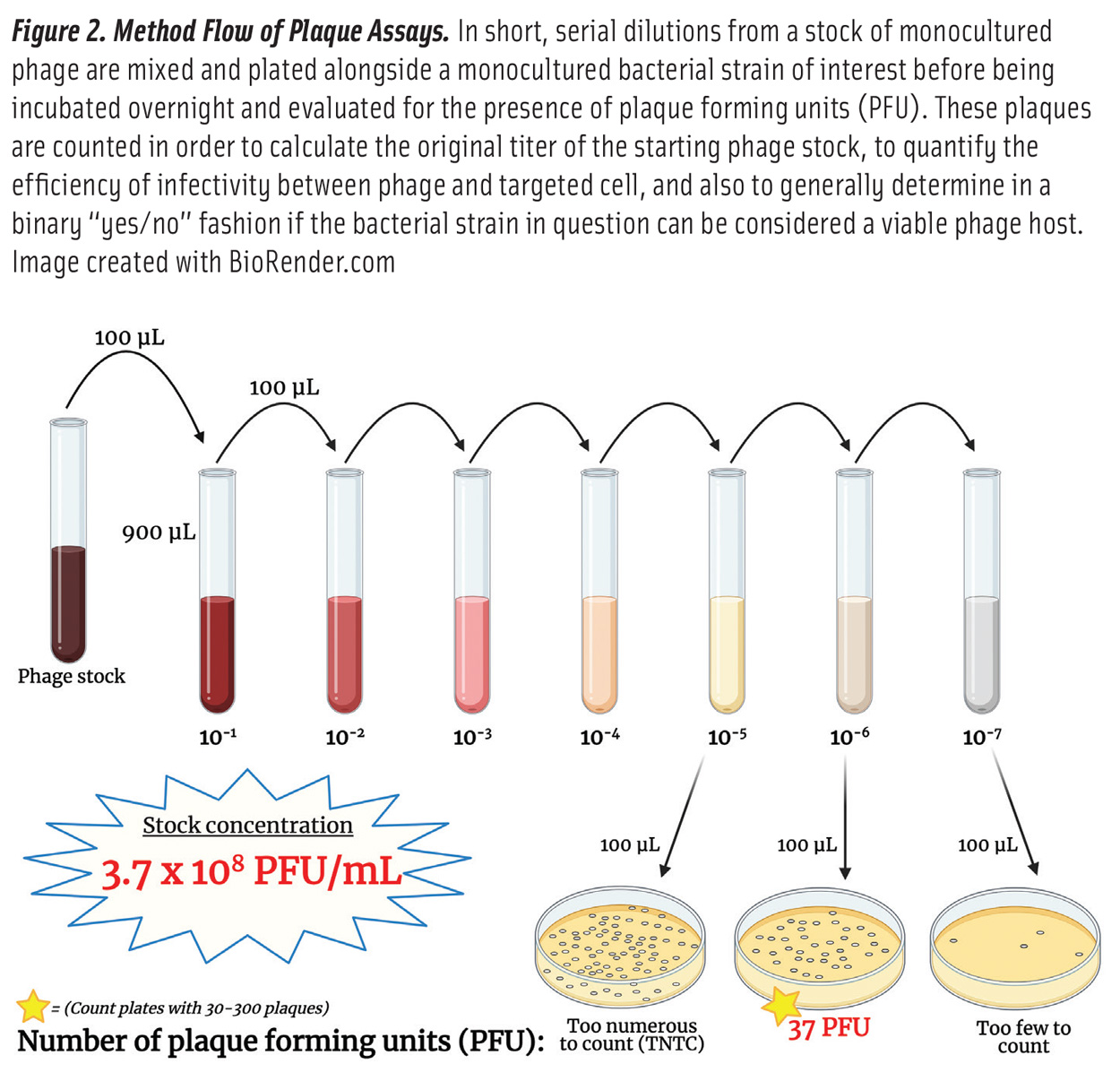
Lytic phages incubated in a petri dish with their host bacteria form clear plaques within a bacterial lawn. The concentration of phages can therefore be quantified as plaque-forming units as seen in Figure 2, which is comparable to bacterial colony-forming units.
Because phages do not infect all strains within their host range equally, an equal titer of phages may display a different number of plaques on various strains when performing a plaque assay. Still, the method of titering phages by using a plaque assay has barely changed since its creation over a century ago (Kutter 2009).
Phage Technologies for the Food Industry
In the early 1900s, phages were administered to patients infected with bacterial pathogens as a means to control the infection. This practice is called “phage therapy”: where a cocktail of phages that target the same bacterial host(s), often via different surface receptors, are administered together in order to cover a wider number of bacterial strains within this cumulative host range. Although using phages was originally met with enthusiasm, the discovery of penicillin in 1928 combined with controversies within the scientific community over the efficacy of phage treatments (due to inconsistent results and poorly controlled experiments) resulted in phage research being effectively abandoned in the United States at the end of World War II. In many Eastern European countries and the former Soviet Union, however, phage research has continued to thrive and is widely practiced (Pires et al. 2016). Phage cocktails have been successfully used in the past for bacterial-induced diseases like cholera and dysentery (Kutter 2009), and more recently in a highly publicized case of multidrug resistant Acinetobacter baumannii in San Diego (Schooley et al. 2017).
In the United States, phage therapy has only been approved for “compassionate use” by the U.S. Food and Drug Administration (FDA) although many other phage-based technologies have been approved by U.S. regulatory agencies, including the FDA, the U.S. Department of Agriculture, and the Environmental Protection Agency (O’Sullivan et al. 2016). Some of the most popular phage products, such as ListShield, have been reliably used in the United States since the mid-2000s, with many products having also achieved GRAS (Generally Recognized as Safe) status for equally as long (Endersen et al. 2014). Similar phage applications are prevalent in European countries, improving upon both food and water safety as well as highlighting the immense versatility of these predators of bacteria. As an example, a prominent biotech company based in the Netherlands, Micreos, has been producing phage-based biocontrol technologies (where “phage biocontrol” is defined as using high concentrations of phages to specifically target and eradicate relevant foodborne or waterborne bacterial organisms) for food safety applications since 2010 (Kazi and Annapure 2016).
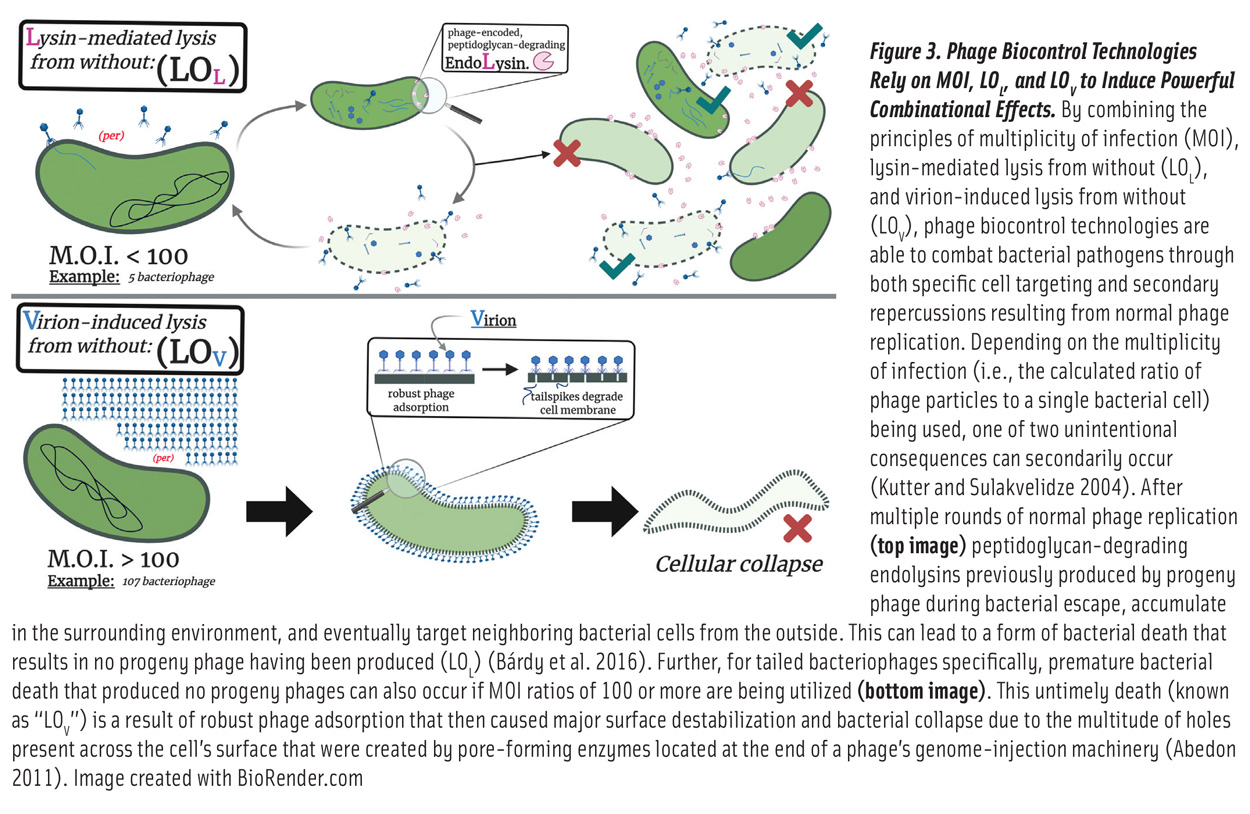
Despite the many available food safety strategies currently in practice, which act as “hurdles” (e.g., pH, water activity, refrigeration) that food pathogens must overcome to grow, outbreaks still occur and remain a substantial burden on global health care and economies. Phages offer an additional pragmatic and natural hurdle for combatting our bacterial enemies. Phage technologies take advantage of many valuable traits that the viruses naturally possess (Figure 3). High concentrations of phages used against their bacterial hosts have both immediate and secondary effects. While phage binding and infection may have a more limited target host range, the endolysins produced as a result of phage infections often target more well-conserved structural components of bacterial peptidoglycan layers, thus acting as an additional layer of biocontrol (Richter et al. 2018, Kazi and Annapure 2016). For phage-based bacterial detection technologies, an additional benefit is that phages can only infect and replicate in growing bacterial cells. Therefore, bacteria that have been killed using an upstream mitigation step, such as heat treatment, would not produce a false positive signal. Additionally, the average lytic replication cycle lasts 15–30 minutes, allowing rapid test results (Nobrega et al. 2018).
The development of phage-based technologies has also demanded that phage production be scalable, utilize preexisting industrial equipment (Jurač, Nabergoj, and Podgornik 2019), and be inexpensive to produce (García et al. 2019). The Sample6 DETECT test system, now marketed by IEH, is an example of a phage-based bacterial detection system that has been used by food processing companies since 2014. The system identifies points of Listeria and Salmonella contamination both on-site and in-shift (Cappillino et al. 2015).
Over the past few years, more examples of effective phage-based tools intended for both pre- and post-harvest applications have been introduced (Endersen et al. 2014, Doss et al. 2017). As consumer demand for “cleaner” and more “natural” food products intensifies, the use of phages on foods and in food processing environments has gained more attention (Chen et al. 2020, Saleh 2020). Future avenues for phage application include using phages to replace the addition of antibiotics in animal feeds—a once globally accepted but now banned technique used by meat industries to promote rapid animal growth and reduce maturation time before slaughter (Endersen et al. 2014, Pugh 2002, Brüssow 2017). The need for an alternative to antibiotic treatments is of particular interest to the global shrimp market, which has previously relied on the heavy use of antibiotics to stave off bacterial infections responsible for premature death within shrimp colonies (Brüssow 2017). While most available phage technologies utilize whole phage particles, applications using isolated phage components have been investigated recently. Such is the case for utilizing phage-encoded endolysins, which are normally produced by phages at the end of their replication cycles to destroy bacterial peptidoglycan layers in a site-specific manner, and are being investigated by researchers as novel surface disinfectants and agents that help to prevent and break up biofilms (Shannon, Radford, and Balamurugan 2020, Chang 2020).
The variability and complexity of different food matrices means that no single technology, including phage technology, has been able to ensure the safety of all food products. For example, heat treatment is a common technique used to reduce the number of food pathogens within some foods, yet this method may not be suitable for fresh foods such as spinach. To overcome this, food industries will instead rely on chemical sanitizers like chlorine and per acetic acid, a practice that can have its own application issues (Moye, Woolston, and Sulakvelidze 2018). When used in combination with varying hurdle technologies, phage biocontrol represents an innovative and multifaceted approach to targeting and destroying specific spoilage and food safety–related bacteria that are common for many food processing facilities (García-Anaya et al. 2020). It’s important to note that these pairings cannot be used as a one-size-fits-all solution, and are dependent on the properties of the food matrix in question (Shannon, Radford, and Balamurugan 2020, García-Anaya et al. 2020), bacteriophage(s) being used, and the bacterial pathogen being targeted (O’Sullivan et al. 2019).
Final Thoughts
The future of bacteriophage technology is bright and full of potential. There is additional work to be done to assuage the concerns of phage skeptics within the food industry, but the growing interest in this field will help to support and facilitate these critical studies. With such a massive body of primary research having already shown the efficacy and safety of bacteriophages on foods and food contact surfaces, these technologies could begin to be more globally applied. As humanity is tasked to come together and create sustainable solutions for troubling problems such as reliable sources of clean food and water, phages and their respective technologies will likely continue to stand at the forefront of applied biotechnology.
REFERENCES
Abedon, S. T. 2011. “Lysis from without.” Bacteriophage 1(1): 46–49.
Bárdy, P., R. Pantůček, M. Benešík, et al. 2016. “Genetically modified bacteriophages in applied microbiology.” J. Appl. Microbiol. 121(3): 618–633.
Berners-Lee, M., C. Kennelly, R. Watson, et al. 2018. “Current global food production is sufficient to meet human nutritional needs in 2050 provided there is radical societal adaptation.” Elem. Sci. Anth. 6(1).
Brüssow, H. 2017. “Adjuncts and alternatives in the time of antibiotic resistance and in-feed antibiotic bans.” Microb. Biotech. 10(4): 674.
Cappillino, M., R. P. Shivers, D. R. Brownell, et al. 2015. “Sample6 DETECT/L: an in-plant, in-shift, enrichment-free Listeria environmental assay.” J. AOAC Int. 98(2): 436–444.
Chang, Y. 2020. “Bacteriophage-Derived Endolysins Applied as Potent Biocontrol Agents to Enhance Food Safety.” Microorganisms 8(5): 724.
Chen, L., Z. Song, S. Y. Tan, et al. 2020. “Application of Bacteriocins Produced from Lactic Acid Bacteria for Microbiological Food Safety.” Current Topics in Lactic Acid Bacteria and Probiotics 6 (1): 1-8.
Doss, J., K. Culbertson, D. Hahn, et al. 2017. “A review of phage therapy against bacterial pathogens of aquatic and terrestrial organisms.” Viruses 9(3): 50.
Endersen, L., J. O’Mahony, C. Hill, et al. 2014. “Phage therapy in the food industry.” Annu. Rev. Food Sci. Tech. 5: 327–349.
García-Anaya, M. C., D. R. Sepúlveda, C. Rios-Velasco, et al. 2020. “The role of food compounds and emerging technologies on phage stability.” Innovative Food Science & Emerging Technologies: 102436.
García, R., S. Latz, J. Romero, et al. 2019. “Bacteriophage production models: An overview.” Front. Microbiol. 10: 1187.
Howard-Varona, C., K. R. Hargreaves, S. T. Abedon, et al. 2017. “Lysogeny in nature: mechanisms, impact and ecology of temperate phages.” The ISME journal 11(7):
Jurač, K., D. Nabergoj, and A. Podgornik. 2019. “Bacteriophage production processes.” Appl. Microbiol. Biotechnol. 103(2): 685-694.
Kazi, M. and U. S. Annapure. 2016. “Bacteriophage biocontrol of foodborne pathogens.” J. Food Sci. Technol. 53(3): 1355–1362.
Kutter, E. 2009. “Phage host range and efficiency of plating.” In Bacteriophages, 141–149. Springer.
Kutter, E. and A. Sulakvelidze. 2004. Bacteriophages: Biology and Applications. CRC Press.
Moye, Z. D., J. Woolston, and A. Sulakvelidze. 2018. “Bacteriophage applications for food production and processing.” Viruses 10(4): 205.
Nobrega, F. L., M. Vlot, P. A. de Jonge, et al. 2018. “Targeting mechanisms of tailed bacteriophages.” Nat. Rev. Microbiol. 16(12): 760–773.
O’Sullivan, L., D. Bolton, O. McAuliffe, et al. 2019. “Bacteriophages in food applications: From foe to friend.” Annu. Rev. Food Sci. Technol. 10: 151–172.
O’Sullivan, L., C. Buttimer, O. McAuliffe, et al. 2016. “Bacteriophage-based tools: recent advances and novel applications.” F1000Research 5: 2782.2
Peng, H., and I. A. Chen. 2020. “Phage engineering and the evolutionary arms race.” Current Opin. Biotechnol. 68: 23–29.
Pires, D. P., S. Cleto, S. Sillankorva, et al. 2016. “Genetically engineered phages: a review of advances over the last decade.” Microbiol. Mol. Biol. Rev. 80(3): 523–543.
Pugh, D. M. 2002. “The EU precautionary bans of animal feed additive antibiotics.” Toxicol. Lett. 128(1–3): 35–44.
Richter, Ł., M. Janczuk-Richter, J. Niedziółka-Jönsson, et al. 2018. “Recent advances in bacteriophage-based methods for bacteria detection.” Drug Discovery Today 23(2): 448–455.
Saleh, F. R. 2020. “Bacteriophage as Bio-Preserving to Enhance Shelf Life of Fruit and Vegetables.” Plant Archives 20(1): 359–362.
Scharff, R. L. 2012. “Economic burden from health losses due to foodborne illness in the United States.” J. Food Prot. 75(1): 123–31.
Schooley, R. T., B. Biswas, J. J. Gill, et al. 2017. “Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumanni Infection.” Antimicrob. Agents Chemother. 61(10): e00954-17.
Shannon, R., D. R. Radford, and S. Balamurugan. 2020. “Impacts of food matrix on bacteriophage and endolysin antimicrobial efficacy and performance.” Crit. Rev. Food Sci. Nutr. 60(10): 1631–1640.
Wheatley, J. P., S. B. W. Liyanagedera, R. Amaee, et al. 2020. “Synthetic Biology for the Rapid, Precise and Compliant Detection of Microbes.” In Advances in Synthetic Biology, 289–306. Springer.
WHO. 2015. WHO estimates of the global burden of foodborne diseases: foodborne diseases burden epidemiology reference group 2007–2015. Edited by the World Health Organization.