Cornell Researchers Give Microbes the Slip
INSIDE ACADEMIA
 Situated atop a steep hill overlooking Cayuga Lake, the main campus of Cornell University is surrounded by lush trees, green hills, gorgeous gorges, and spectacular waterfalls. Its location in Ithaca, N.Y.—the heart of New York’s Finger Lakes region—provides staff, students, and visitors with acres of stunning topography on a grand scale. Paradoxically, the campus also serves as the setting for a topographical discovery on the nanoscale: the use of anodization to create nanoscale topography that renders metal surfaces resistant to foodborne pathogens.
Situated atop a steep hill overlooking Cayuga Lake, the main campus of Cornell University is surrounded by lush trees, green hills, gorgeous gorges, and spectacular waterfalls. Its location in Ithaca, N.Y.—the heart of New York’s Finger Lakes region—provides staff, students, and visitors with acres of stunning topography on a grand scale. Paradoxically, the campus also serves as the setting for a topographical discovery on the nanoscale: the use of anodization to create nanoscale topography that renders metal surfaces resistant to foodborne pathogens.
 One of the scientists behind this groundbreaking discovery is Carmen Moraru, an associate professor in Cornell University’s food science department. After completing her undergraduate and doctorate degrees in food engineering at the University Dunarea de Jos in Galati, Romania, Moraru came to the United States as a postdoctoral associate at Rutgers University. Since 2003 she has been teaching and conducting research in food safety engineering at Cornell University, where she focuses on intervention strategies that make food safer.
One of the scientists behind this groundbreaking discovery is Carmen Moraru, an associate professor in Cornell University’s food science department. After completing her undergraduate and doctorate degrees in food engineering at the University Dunarea de Jos in Galati, Romania, Moraru came to the United States as a postdoctoral associate at Rutgers University. Since 2003 she has been teaching and conducting research in food safety engineering at Cornell University, where she focuses on intervention strategies that make food safer.
Over the years, Moraru became increasingly interested in how foodborne bacteria attach to different surfaces and how to thwart that attachment. Bacteria can attach or adhere to virtually any surface whether natural or synthetic. This adhesion—a survival mechanism for bacteria—is a powerful phenomenon that can be beneficial in some environments, such as in a human’s oral cavity or intestinal microbiome, and detrimental in others, such as the surfaces of food-processing equipment in a food facility.
While investigating different strategies to decontaminate such surfaces, Moraru made an important discovery: “In my research in the beginning of my position at Cornell, we were looking for different methods of decontaminating surfaces, and it was at that time when we realized that the fact that a surface is relatively smooth does not necessarily guarantee that bacteria will not attach,” Moraru says. Her collaboration with an associate professor at Rensselaer Polytechnic Institute (RPI), Diana Borca-Tasciuc, then led to the theory that altering metal surfaces with controlled nanoscale topography could preclude bacterial adhesion to metal surfaces. “We were looking for a method that would be easily applicable commercially, that would not be too expensive, and [that] could be applied to large surfaces [and] to three-dimensional parts. Based on [Borca-Tasciuc’s] experience, we realized that anodization might in fact work because you can create all sorts of surface details using nano-fabrication methods, clean-room technology, and much more sophisticated methods, but they are prohibitively expensive. They would not be easily applied to food-processing equipment,” Moraru adds.
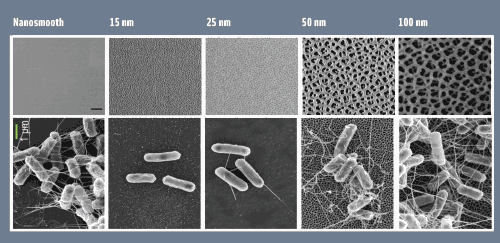
Anodization is an electrochemical process that causes the controlled oxidation of a metal. The process involves submerging the metal in an acid bath containing two electrodes emitting a current. For Moraru and Borca-Tasciuc’s research, anodization was used to change the topography of a metal’s surface, creating nano-sized pores that alter the electrical charge and surface energy of the metal. The anodized nano-pores exert a repulsive force that prevents bacteria from adhering and biofilms from forming. “We have been working specifically with aluminum, which we anodized and converted into aluminum oxide, or alumina, which is a generally recognized as safe [GRAS] material and thus can be used in food applications,” Moraru explains. The alumina structures itself into cylindrical pores with diametric ranges of 10 nanometers up to hundreds of nanometers. “We are able to control the size of the pores that form by controlling the anodization conditions—that is, the current, the temperature, and the chemical composition of the [acid] bath,” Moraru says.
The anodization of aluminum occurs at RPI while the food application, physicochemical modeling of attachment, and theorizing of attachment occur at Cornell. Moraru and her research team at Cornell have tested the repulsion forces of anodized aluminum featuring nanoscale topography against pathogenic bacterial strains such as Listeria monocytogenes, Escherichia coli O157:H7, Staphylococcus aureus, and Staphylococcus epidermidis (a pathogen that is problematic in biomedical settings) as well as a number of non-pathogenic bacterial strains. “The effect has been consistently the same: The surfaces that have the smallest pore sizes, which were 15 to 25 nanometers in diameter, had the least [bacterial] attachment,” Moraru says. Despite not having tested all foodborne pathogens on these surfaces, Moraru is confident that the degree of repulsion will be similar against a large number of microorganisms. “We have screened strains with very different bacterial surface properties, bacterial shapes, and sizes, and the surfaces have been effective against all of those that we tested,” she points out. Nevertheless, she and her team plan to perform further testing on nanoscale topography, including testing how well it repels other foodborne pathogens such as various Salmonella strains.
 Moraru envisions that anodized metals featuring nanoscale topography could be used for metal food-contact surfaces such as equipment parts or work surfaces. However, cost could be a limiting factor, so the applications would need to be strategic: “Even if the technology is relatively affordable, there will be a slight cost increase compared to a nonanodized surface. Maybe we will not see every single metal food-contact surface made out of anodized metal but we [could] see certain critical parts: parts that are hard to reach, hard to sanitize. For instance, blades for different types of cutters or intricate parts of valves that are not that easy to reach,” Moraru says. “One simple application could be drains, which cause a lot of trouble in food processing because they tend to accumulate bacteria and can become a source of contamination.” Moraru predicts that the technology could be used at water processing facilities, for medical equipment in healthcare settings, and perhaps even for home utensils and sinks—all of which are prone to contamination and the formation of biofilms.
Moraru envisions that anodized metals featuring nanoscale topography could be used for metal food-contact surfaces such as equipment parts or work surfaces. However, cost could be a limiting factor, so the applications would need to be strategic: “Even if the technology is relatively affordable, there will be a slight cost increase compared to a nonanodized surface. Maybe we will not see every single metal food-contact surface made out of anodized metal but we [could] see certain critical parts: parts that are hard to reach, hard to sanitize. For instance, blades for different types of cutters or intricate parts of valves that are not that easy to reach,” Moraru says. “One simple application could be drains, which cause a lot of trouble in food processing because they tend to accumulate bacteria and can become a source of contamination.” Moraru predicts that the technology could be used at water processing facilities, for medical equipment in healthcare settings, and perhaps even for home utensils and sinks—all of which are prone to contamination and the formation of biofilms.
Moraru emphasizes that nanoscale anodization of metals is complementary to the use of bactericides and other antimicrobials: “You don’t kill bacteria with it. You just don’t let them attach. So in a food processing environment or even at home, I think we need both because we do see significantly reduced attachment, but there is still some bacteria that attach. Even if you have some attachment still occur, the density of that film is going to be reduced. It’s not going to be such a continuous biofilm, so that will improve access of sanitizers to bacteria.”
The challenges Moraru and her collaborators face are few but include making the diameter of nano-pores as small as possible and applying the technology to metals such as stainless steel, which is the metal most often used for food-processing surfaces and equipment. “Anodizing stainless steel is not trivial,” Moraru says. “We’re up against a tough challenge there, but we’re very optimistic that we can do it.” Borca-Tasciuc and her research team at RPI “are well on their way of controlling the surface topography of stainless steel surfaces using anodization,” she adds. Another challenge is funding this research. “We were very fortunate to have four years of support from the [U.S. Dept. of Agriculture],” Moraru says, but the grant ended at the end of 2014. “We’re hoping to get more funding from the USDA … as well as industry.” For that reason, it may take a few years before the technology is commercialized.
In the meantime, Moraru and her collaborators continue their research with optimism and a willingness to share their discovery: “There are still aspects of these applications that we need to further explore, and we don’t have all the answers yet,” she says. “What we want to do is help improve the safety of our food supply and help all of us fight these dangerous bacteria.”
Toni Tarver is senior writer/editor of Food Technology
magazine ([email protected]).
