IFT Expert Report on Biotechnology and Foods
Human Food Safety Evaluation of rDNA Biotechnology-Derived Foods
The Institute of Food Technologists convened three panels of experts, consisting of IFT members and other prominent biotechnology authorities, to prepare a comprehensive scientific review of biotechnology. The report consists of four sections. The first section—Introduction—appeared in the August 2000 issue of Food Technology; the next two sections—Safety and Labeling—appear in this issue; and the fourth—Benefits and Concerns—will appear in the October issue.
This section begins with a discussion of issues relevant to safety evaluation of recombinant DNA biotechnology-derived foods, including the concept of substantial equivalence, safety of introduced genetic material and gene product, unintended effects, allergenicity, and products without conventional counterparts. It is followed by the scientific consensus of international scientific groups regarding safety of rDNA biotechnology-derived foods.
Issues Relevant to Safety Evaluation
Food manufacturers are required by law to ensure the safety and quality of their products regardless of the source or identity of the ingredients. Traditional foods are viewed by the Food and Drug Administration as “safe” based on a long history of use. The consuming public also views traditional foods as safe. However, many traditional foods contain naturally occurring toxins that can present hazards to consumers under some circumstances of exposure. Fortunately, in most circumstances, these naturally occurring toxins are present in concentrations that are not hazardous to consumers ingesting typical quantities of the food prepared under typical conditions. Also, some traditional foods are allergenic to some consumers, even though they are safe for the vast majority of consumers.
New foods produced through conventional breeding or introduced into the marketplace from other parts of the world are not required to undergo any type of safety assessment. They are assumed to be safe because they are comparable to other varieties (if newly introduced through conventional breeding) or because they have been safely consumed in other parts of the world. In fact, these newly introduced foods may contain numerous unique components that are not individually or collectively assessed for safety.
In contrast, products derived through rDNA biotechnology are assessed for safety before their introduction into the food marketplace. Food manufacturers also must ensure the safety and quality of products that contain ingredients derived from rDNA biotechnology. In 1992, FDA provided a general outline for the safety assessment of rDNA biotechnology-derived food products based on risk analysis related to the characteristics of the products (FDA 1992). All of the existing foods produced using rDNA biotechnology have undergone a rigorous science-based safety assessment focusing on the characteristics of the products, especially the unique components. While this practice has been voluntary in the United States, FDA announced in May 2000 that it intends to propose a premarket notification system for rDNA biotechnology-derived foods that would make this unofficial policy into a regulatory requirement (HHS, 2000). Thus, in practice, the safety assessment of foods derived using rDNA biotechnology has been more stringent than for conventionally derived products.
Substantial Equivalence
In the safety assessment of rDNA biotechnology-derived foods, it is helpful to compare the new plant variety to its traditional counterpart because the counterpart has a history of safe use as a food. The concept of substantial equivalence effectively focuses the scientific assessment on potential differences that might present safety or nutritional concerns.
Substantial equivalence is not an absolute determinant of safety per se, since compositional changes in an rDNA biotechnology-derived food may have no impact on the safety of the food. However, substantial equivalence provides a process to establish that the composition of the plant has not been changed in such a way as to introduce any new hazards into the food, increase the concentration of inherent toxic constituents, or decrease the customary content of nutrients. For example, high-oleic-acid soybean oil from rDNA biotechnology-derived soybeans has an oleic acid concentration that falls outside the range typically found in soy oils. From a scientific perspective, this food is nevertheless considered safe, based on scientific knowledge about the safety of oleic acid, a common fatty acid in foods.
--- PAGE BREAK ---
A determination of substantial equivalence considers the intentional and unintentional effects of genetic modification, and includes an evaluation of phenotypic and compositional characteristics. With respect to food safety, substantial equivalence involves the quantitative assessment of the concentration of inherent constituents in the modified food, compared to the often wide range typically found in its traditional counterpart, under similar food production conditions.
Most food sources (e.g., soybeans, corn) are exceedingly complex mixtures that vary widely in composition, so it is necessary to consider all of the factors that determine the normal range of variation (IFBC, 1990). Key constituents measured include nutrients, such as proteins, fats, carbohydrates, vitamins, and minerals, as well as inherent antinutritional factors, toxins, and allergens (Miraglia et al., 1998). The breadth of technology used to measure these constituents is evolving rapidly, with new methods available to assess the integrity of metabolic pathways and to measure secondary metabolites, functional proteins, and gene expression at the molecular level.
A recent report (FAO/WHO, 2000) of the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) considered the concept of substantial equivalence:
A comparative approach focusing on the determination of similarities and differences between the genetically modified food and its conventional counterpart aids in the identification of potential safety and nutritional issues and is considered the most appropriate strategy for the safety and nutritional assessment of genetically modified foods.
The Consultation was of the view that there were presently no alternative strategies that would provide a better assurance of safety for genetically modified foods than the appropriate use of the concept of substantial equivalence. Nevertheless, it was agreed that some aspects of the steps in safety assessment process could be refined to keep abreast of developments in genetic modification technology. The concept of substantial equivalence was developed as a practical approach to the safety assessment of genetically modified foods. It should be seen as a key step in the safety assessment process although it is not a safety assessment in itself; it does not characterize hazard, rather it is used to structure the safety assessment of a genetically modified food relative to a conventional counterpart. The Consultation concluded that the application of the concept of substantial equivalence contributes to a robust safety assessment framework. The Consultation was satisfied with the approach used to assess the safety of the genetically modified foods that have been approved for commercial use.
Similarly, in a May 2000 report, the Organization for Economic Cooperation and Development (OECD) examined the safety of novel foods and feeds. It concluded that:
Safety assessment based on substantial equivalence is the most practical approach to address the safety of food and food components derived through modern biotechnology.
In its 1992 policy on foods derived from new plant varieties (FDA 1992), FDA employs the concept of substantial equivalence by focusing on the characteristics of the food product. Foremost, this policy on food products from new plant varieties is intended to be applied regardless of the derivation of the plant, i.e., through conventional breeding or rDNA biotechnology methods. FDA has identified certain characteristics of these foods that would dictate the need for further scrutiny to establish safety. These include a substance that is completely new to the food supply, an allergen expressed in an unusual or unexpected circumstance, changes in the concentrations of major dietary nutrients, and increased concentrations of antinutritional factors and toxins inherent to the food. Although the FDA policy does not specifically use the term substantial equivalence, the absence of the characteristics mentioned above would lead to the conclusion that a food from a new plant variety is substantially equivalent to its traditional counterpart.
Safety of Introduced Genetic Material and Gene Product
Under FDA’s current (1992) policy, as a starting point, the characteristics of the product are assessed, including the nucleotide sequence of the DNA of the genetic material that is used for plant transformation. This procedure provides important information on the encoded protein(s), regulatory elements controlling expression, and the presence or absence of additional potential coding sequences within the DNA. Although all extraneous non-coding DNA may not be identified, it can be minimized to very small segments. This level of detail cannot ordinarily be determined for new plant varieties produced in conventional ways such as hybridization.
--- PAGE BREAK ---
Thus, the FDA policy contemplates that the structure and function of proteins encoded by the gene(s) introduced into plants will be understood in considerable detail. This information is used to assess the level of any potential risk, both of the introduced protein and of other products that may be produced or altered by the presence of the introduced protein. An additional factor is the source of the gene. The FDA policy contemplates that the following questions be addressed: Does the source organism have a history of safe use? and Does the source of the gene produce any endogenous toxins or allergens, that would need to be assessed in the genetically modified plant?
Any potential safety concerns associated with the source organism would serve to focus the safety assessment of the rDNA biotechnology-derived plant and the products derived from that plant. For example, if a gene were obtained from a source that produced a known allergen, the proteins encoded by the introduced DNA would have to be assessed to demonstrate that this DNA did not encode an allergen.
• Safety of Introduced Genetic Material. The initial step in a safety assessment is full characterization of the genetic construct being inserted. This step includes identifying the source of the genetic material to establish whether it originates from a pathogenic, toxin-producing, or allergenic source. Parameters measured include the size of the genetic construct that is inserted into the plant genome, the number of constructs inserted, the location of insertion, and the identification of genetic sequences within the construct that allow for its detection (marker sequences) and expression (promoter sequences) in the plant.
The genetic material transferred is composed of DNA. All food, rDNA biotechnology-derived or otherwise, contains DNA. Individuals consume large quantities of DNA when eating conventional foods (Beever and Kemp, 2000). The DNA introduced using rDNA biotechnology represents only a tiny fraction of the total DNA consumed when the food is eaten, and transfer of genes from rDNA biotechnology-derived plants to mammalian cells is extremely unlikely.
Since DNA occurs in all foods, it is not subject to a safety evaluation (IFBC, 1990; Miraglia et al., 1998). It is well-established that DNA is rapidly digested in the gastrointestinal tract, and there is no evidence of DNA transfer from foods to human intestinal cells or gut microorganisms (Donaldson and May, 1999). Any plant DNA that might be found in human tissues is likely to be a small, non-functional fragment resulting from centuries of consumption and does not imply that plant foods are unsafe. Moreover, the likelihood of transfer of rDNA segments from foods produced using rDNA biotechnology is far less than for DNA from conventional foods simply because the novel DNA is less than 1/250,000 of the overall amount consumed (FAO/WHO, 2000).
Earlier rDNA biotechnology-derived foods were based on the use of selectable marker genes that confer resistance to an antibiotic. A workshop convened by the WHO concluded that the presence of marker genes per se in food would not constitute a safety concern (WHO, 1993). FAO/WHO (2000) recently reconsidered the issue of antibiotic resistance marker genes and again found there is no evidence that the markers currently in use pose a health risk to humans or domestic animals. Still, genes that confer resistance to drugs with specific medical use or limited alternative therapies should not be used in widely disseminated rDNA biotechnology-derived foods.
Following extensive examination, FDA decided to permit the use of kanamycin-resistance genes in the development of rDNA biotechnology-derived tomatoes, oilseed rape, and cotton for food and feed use and permitted these crops in food and feed (FDA, 1994). FDA concluded that the DNA for kanamycin resistance was not different from other rDNA in its digestibility and does not pose a food safety concern.
The marker gene used to confer kanamycin resistance was the neomycin phosphotransferase, type II gene (NPTII). The NPTII protein is rapidly degraded, like other dietary proteins, when subjected to conditions which simulate mammalian digestion. This protein has also been tested in acute toxicology studies at levels more than one million times the level that would be consumed by people eating food from rDNA biotechnology-derived plants. Finally, the transformation of intestinal bacteria by kanamycin resistance from plants is negligible, with a calculated theoretical maximum of less than 1 in 100,000 compared to bacterial transfers of resistance (WHO, 1993). Thus, this protein poses no food safety concerns. FDA concluded that there is no inherent danger presented by the presence of the antibiotic resistance markers used in earlier rDNA biotechnology-derived foods. These marker genes, such as the NPTII gene, do not present a food or feed safety concern and are not considered to be either toxic or allergenic.
The risk that the use of antibiotic resistance genes could lead to a transfer of antibiotic resistance and reduced efficacy of antibiotics is extremely small, because it would require a series of events, each of which is highly unlikely. Moreover, if such a move did occur, antibiotic selection would be needed to make the newly resistant strain a common one (Salyers, 2000). These concerns are addressed in additional detail in the Benefits and Concerns section.
• Safety of Gene Product. FDA’s 1992 policy also contemplates that, once the genetic construct has been fully characterized, an assessment of the safety of the gene product will be conducted. [The gene product is the protein, often an enzyme, that is produced by the newly introduced gene(s) and is present in the rDNA biotechnology-derived food or food ingredient, e.g., the protein expressed in Bt corn, encoded by genes from Bacillus thuringiensis (Bt), that confers pesticidal specificity for lepidopteran insects.] Safety evaluations typically include identification of the composition and structure of the gene product; a quantification of the amount of gene product expressed in the edible portion of the food; a search for similarity to known toxins and antinutritional factors, allergens, and other functional proteins; a determination of the thermal and digestive stability of the gene product; and the results of both in-vivo and in-vitro toxicological assays to demonstrate lack of apparent allergenicity or toxicity (Donaldson and May, 1999).
--- PAGE BREAK ---
Unintended Effects
From a safety perspective, unintended effects of genetic modification have been speculated to manifest as the unintended expression of some unknown or unexpected toxic or antinutrient factor, or the otherwise unintended enhanced production of known toxic constituents (Royal Society, 1998).
However, based on the knowledge gained to date from the multitude of foods derived from rDNA biotechnology, there is no scientific evidence of the occurrence of such unintended effects. Given the more precise and predictable nature of genetic change accomplished through rDNA techniques as compared to the random genetic changes observed in conventional breeding, such unintended effects would be considered less likely in foods derived from rDNA biotechnology. Furthermore, these effects have been observed infrequently in the many thousands of crosses involving conventional crop breeding. In such cases, the source of the toxic constituent can typically be traced back to a related species used in conventional cross-breeding manipulations. For example, high glycoalkaloid concentrations were found in the conventionally bred Lenape potato, and the variety was subsequently withdrawn by the U.S. Department of Agriculture (Zitnak and Johnston, 1970). These toxins are present in all potatoes, and new potato cultivars are routinely screened for glycoalkaloid content. The unusually high glycoalkaloid content in Lenape was attributed to the use of the wild, non-tuber-bearing Solanum chacoense in its parentage. Interestingly, Lenape is a parent of Atlantic, a current potato variety with a glycoalkaloid content typical of the range for edible potatoes.
Allergenicity
Food allergies involve abnormal immunological responses to substances in foods, usually naturally occurring proteins found in commonly allergenic foods such as peanuts, milk, and seafood. Allergic reactions can be manifested by symptoms ranging from mild cutaneous or gastrointestinal symptoms to life-threatening anaphylactic shock reactions. Virtually all food allergens are proteins, although only a small fraction of the proteins found in nature (and in foods) are allergenic. Since genetic modifications involve the introduction of new genes into the recipient plant and since these genes would produce new proteins in the improved variety, the potential allergenicity of the newly introduced protein should be a key component of the safety assessment process.
An assessment of the potential allergenicity of rDNA biotechnology-derived foods typically follows the decision-tree process outlined by the International Food Biotechnology Council (IFBC) and the Allergy and Immunology Institute of the International Life Sciences Institute (ILSI) (Metcalfe et al., 1996). This strategy focuses on specific scientific criteria, including the source of the gene(s), the sequence homology of the newly introduced protein(s) to known allergens, the immunochemical reactivity of the newly introduced protein(s) with immunoglobulin E (IgE) antibodies from the blood serum of individuals with known allergies to the source from which the genetic material was obtained, and the physicochemical properties, e.g., digestive stability, of the introduced protein.
At the recently concluded expert consultation (FAO/WHO, 2000), several other criteria, including the level of expression of the newly introduced protein(s) in the edible portions of the improved variety and the evaluation of the functional category for the introduced protein (some functional categories of proteins, e.g., high-methionine 2S albumins, are known to contain several allergens from different sources), were suggested for addition to the IFBCILSI allergenicity assessment strategy.
The first step of the allergenicity assessment (Fig. 1) involves the classification of the source of the genetic material as either commonly allergenic, less commonly allergenic, or of unknown allergenic potential. Eight foods or food groups, including milk, eggs, fish, crustacean shellfish, peanuts, soybeans, tree nuts, and wheat, are well accepted as commonly allergenic; these eight foods or food groups account for more than 90% of all food allergies in the world (FAO, 1995). More than 160 other foods have been described to cause allergic reactions (Hefle et al., 1996), and would be classified as less commonly allergenic. However, many of the genes that have been and will be used to produce rDNA biotechnology-derived foods are obtained from sources with no history of allergenicity as foods. Certainly, if the source contains well known environmental allergens, e.g., ragweed that contains common ragweed pollen allergens, then the allergenicity of newly introduced protein(s) from such sources must be carefully evaluated.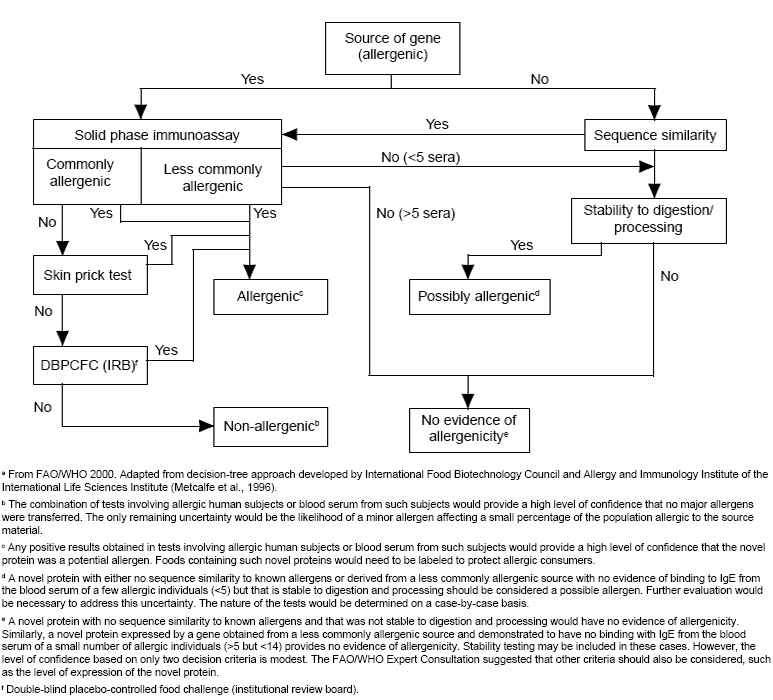
The approaches to allergenicity assessment vary according to the nature of the source of the transferred genetic material. If the genetic material is obtained from a known allergenic source, either commonly or less commonly allergenic, and the encoded protein is expressed in the edible portion of the rDNA biotechnology-derived food, then the protein must be considered to be an allergen unless proven otherwise.
--- PAGE BREAK ---
In such situations, the next step in the allergenicity assessment is a determination of the immunoreactivity of the newly introduced protein with IgE antibodies from the sera of individuals allergic to the donor organism. The blood serum can be tested for reactivity with the purified protein or extracts of the genetically modified food using immunoassays (Yunginger and Adolphson, 1992; Taylor and Lehrer, 1996). If a sufficient number of test sera are used as advocated in the decision tree approach (Metcalfe et al., 1996), the allergenicity of the introduced protein can be determined with a high degree of confidence. However, if negative results are obtained in the immunoassays, the rDNA biotechnology-derived food or extracts of that food should be tested further using in-vivo skin-prick tests (Bock et al., 1977; Taylor and Lehrer, 1996), double-blind, placebo-controlled food challenges (Bock et al., 1988; Taylor and Lehrer, 1996), or digestive stability assessments (Astwood et al., 1996) as advocated by the IFBC-ILSI decision tree. If the immunoassays and these other tests, as appropriate, are negative, then the likelihood that the rDNA biotechnology-derived food contains an allergen would be quite small.
The most difficult assessment occurs when genes are obtained from sources with no history of allergenicity, such as viruses, bacteria, or non-food plants. The likelihood that the proteins derived from such sources of DNA will be allergens is not very high, since most proteins in nature are not allergens (Taylor, 1997). Additionally, many of these proteins will be expressed in the rDNA biotechnology-derived food at very low levels, while allergic sensitization is more likely to occur to the major proteins that exist in foods (Taylor, 1997). The key features of the allergenicity assessment for such foods involve a comparison of the amino acid sequence of the introduced protein with the amino acid sequences of known allergens and the digestive stability of the introduced protein. While the combination of these two criteria provides reasonable assurance that the introduced protein has limited allergenic potential, the ideal approaches to the application of these two criteria have been debated, and the desirability of adding other criteria for the allergenicity assessment of such products has been advocated (Wal, 1998).
The criterion of amino acid sequence homology to known allergens is a logical and increasingly powerful approach. The amino acid sequences of more than 300 known allergens are available for comparative purposes. The IFBC-ILSI strategy defines significant sequence similarity as a match of at least eight contiguous, identical amino acids based on the minimal peptide length needed for T-cell binding, which is a necessary prelude to allergic sensitization; this approach is clearly limited in that it cannot identify discontinuous or conformational epitopes that are dependent on the tertiary structure of the protein (Metcalfe et al., 1996). Others have suggested that the definition of significant sequence homology be modified to a minimal peptide length of less than eight contiguous, identical amino acids (Consumer and Biotechnology Foundation, 1999). While this criterion (amino acid sequence homology to known allergens) is clearly useful, international agreement must be sought on its application.
Known food allergens tend to be quite stable to digestive proteases (Astwood et al., 1996) with the exception of the pollen-related food proteins that cause oral allergy syndrome (Taylor and Lehrer, 1996). Thus, digestive stability can be used as a criterion for the assessment of the allergenic potential of the introduced proteins. Both simulated gastric and intestinal models of mammalian digestion are advocated for such assessments (Astwood et al., 1996; Metcalfe et al., 1996). While the usefulness of this criterion is apparent, consensus is needed on the ideal protocols for assessment of digestive stability. It is recognized that novel proteins may exist that are stable to digestion but will not become allergens. Additional testing is needed to assess the allergenic potential of such proteins (FAO/WHO, 2000).
The development of additional criteria and additional tests to use in the assessment of the allergenicity of rDNA biotechnology-derived foods would be advantageous in cases where the gene is obtained from sources with no history of allergenicity. As mentioned, the level of expression of the introduced protein and the functional category of the introduced protein could be used as additional criteria (FAO/WHO, 2000). In addition, the development of suitable animal models for the prediction of the allergenic potential of the introduced proteins is anticipated in the future. While several animal models appear to be promising (Knippels et al., 1998), none has been sufficiently validated for its routine use in the assessment of the allergenicity of rDNA biotechnology-derived foods.
--- PAGE BREAK ---
The existing decision-tree approach has already been applied in the assessment of the allergenicity of rDNA biotechnology-derived foods. The enzyme introduced into glyphosate-tolerant soybeans has no sequence homology to known allergens and is rapidly digested in simulated mammalian digestion systems (Harrison et al., 1996). Similarly, several of the Bt proteins used in insect-resistant crops and the proteins produced by common marker genes are rapidly digested in simulated mammalian digestion systems (Astwood et al., 1996). A high-methionine protein introduced into soybeans by the transfer of a gene from Brazil nuts to correct the inherent methionine deficiency in soybeans was shown to bind to IgE from the sera of Brazil nut–allergic individuals and to elicit positive skin-prick tests in some of these patients (Nordlee et al., 1996). This protein was thus identified as the major allergen from Brazil nuts that had not previously been characterized. As a result, commercial development of this particular soybean variety was discontinued.
Clearly, the assessment of the allergenicity of rDNA biotechnology-derived foods should be a key component of the overall safety assessment process in all cases. A useful strategy has been developed for such assessments, although this strategy should be viewed as dynamic and new approaches and criteria should be added once they are validated and accepted.
Products without Conventional Counterparts
Recombinant DNA-derived biotechnology foods without conventional counterparts need to be evaluated on a case-by-case basis and would be subject to some types of toxicity assessments, depending on the nature of the modification (IFBC, 1990). This situation has not yet arisen with rDNA biotechnology derived foods, although at some point it undoubtedly will. When it does, the situation will raise a variety of issues that will need to be addressed in a scientifically based, flexible manner.
Whole foods are complex mixtures of chemical components characterized by wide variations in composition and nutritional qualities, and are not well suited for traditional toxicological studies designed to assess individual chemical entities. The testing of whole foods—rDNA biotechnology-derived or conventional—in animal feeding studies, for example, is limited by factors such as the animal’s qualitative and quantitative feeding preferences and the levels of nutritional and antinutritional factors and other substances that are present. When one researcher attempted to ascertain the toxic threshold for an rDNA biotechnology-derived tomato by feeding rats freeze-dried tomato extract, the experiments were limited to the human equivalent of 13 tomatoes a day by negative effects of inorganic compounds, such as potassium, that are present in rDNA biotechnology-derived and conventional tomatoes alike. But, as noted by MacKenzie (1999), “Toxicologists still said we hadn’t fed them enough to get a meaningful result.”
Another limitation is that animal toxicity tests are seldom sufficiently sensitive to distinguish differences between the toxicity of a new variety and its conventional counterparts. Indeed, most foods will produce adverse effects in long-term animal feeding studies when fed in high proportions of the diet, regardless of the nature of production. The results of such studies are not easily interpreted, and apparent adverse effects are often the indirect effects of related nutritional dietary imbalance, rather than any specific compound in question. OECD (2000) recognized that there is no scientific justification for requiring long-term feeding studies for rDNA biotechnology-derived foods, and that such studies would be unlikely to provide meaningful information in the great majority of cases. FAO/WHO (2000) concurred, finding that the practical difficulties in the application of conventional toxicology studies to whole foods preclude their use as a routine safety assessment technique.
The key differences between the testing of whole foods and the testing of individual chemical substances in animal feeding studies are indicated in Table 1.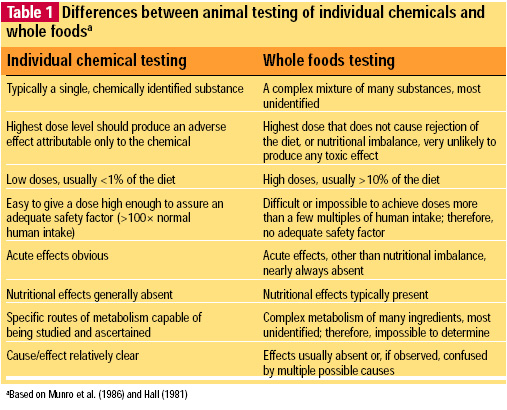
Thus, given a hypothetical rDNA biotechnology-derived food without a conventionally derived counterpart, animal studies would need to be designed to address specific nutritional or toxicological concerns. However, these studies would need to be carefully designed to avoid or minimize the limitations discussed above that are associated with the testing of whole foods or major food constituents (Munro et al., 1996). For example, toxicological studies could be used to examine the potential for acute, chronic, carcinogenic, genotoxic, reproductive, and teratogenic effects of components or fractions of concern in a food derived from a new plant variety. A complete assessment would also include pharmacokinetic data regarding absorption, distribution, metabolism, and excretion of the new product or a novel component thereof. By focusing toxicological examination on carefully selected fractions or components of a food derived from a new plant variety, and excluding major components of no concern, it may be possible to reduce or eliminate the difficulties associated with testing whole foods.
--- PAGE BREAK ---
The assessment of macronutrient substitutes or other major food constituents should follow a tiered approach (Munro et al., 1996), whereby the physical and chemical properties of the constituent are determined, in addition to its potential to disrupt or alter nutrient uptake. Initial predictive effect studies would dictate the physiologically relevant endpoint determinants of subsequent in-vitro and in-vivo studies (Munro et al., 1996). Further, the choice of animal model for any such invivo studies would have to be carefully considered for relevance when applying results to humans (Battershill et al., 1999).
Without precedence, the above discussion outlines a proposal which seems best calculated to provide the data needed for a persuasive showing of safety. Clearly, such novel foods without conventional counterparts, when they do become available, will need careful testing, evaluation, and regulatory scrutiny using a flexible process that contains case-by-case adaptation based on the novel nature of the issues presented.
Scientific Consensus About Safety
The Human Food Safety Panel reviewed available information about the safety of rDNA biotechnology-derived foods and found that there is striking congruence in the conclusions and recommendations of various international scientific groups that have considered the issue.
The National Academy of Sciences published a white paper (NAS, 1987) on the planned introduction of organisms derived using rDNA biotechnology into the environment. This white paper has had wide-ranging impacts in the United States and other countries. Its most significant conclusions and recommendations include (1) there is no evidence of the existence of unique hazards, either in the use of rDNA biotechnology techniques or in the movement of genes between unrelated organisms, and (2) the risks associated with the introduction of rDNA biotechnology-derived organisms are the same in kind as those associated with the introduction of unmodified organisms and organisms modified by other methods.
In a 1989 extension of this white paper, the National Research Council (NRC), the research arm of the NAS, concluded that “no conceptual distinction exists between genetic modification of plants and microorganisms by classical methods or by molecular techniques that modify DNA and transfer genes” (NRC, 1989). The NRC report supported this statement with extensive observations of past experience with plant breeding, introduction of rDNA biotechnology-derived plants, and introduction of rDNA biotechnology-derived microorganisms:
The committees [of experts commissioned by NRC] were guided by the conclusion (NAS, 1987) that the product of genetic modification and selection should be the primary focus for making decisions about the environmental introduction of a plant or microorganism and not the process by which the products were obtained.
Information about the process used to produce a genetically modified organism is important in understanding the characteristics of the product. However, the nature of the process is not a useful criterion for determining whether the product requires less or more oversight.
The same physical and biological laws govern the response of organisms modified by modern molecular and cellular methods and those produced by classical methods.
Recombinant DNA methodology makes it possible to introduce pieces of DNA, consisting of either single or multiple genes, that can be defined in function and even in nucleotide sequence. With classical techniques of gene transfer, a variable number of genes can be transferred, the number depending on the mechanism of transfer; but predicting the precise number or the traits that have been transferred is difficult, and we cannot always predict the phenotypic expression that will result. With organisms modified by molecular methods, we are in a better, if not perfect, position to predict the phenotypic expression.
Crops modified by molecular and cellular methods should pose risks no different from those modified by classical genetic methods for similar traits. As the molecular methods are more specific, users of these methods will be more certain about the traits they introduce into the plants.
--- PAGE BREAK ---
The types of modifications that have been seen or anticipated with molecular techniques are similar to those that have been produced with classical techniques. No new or inherently different hazards are associated with the molecular techniques.
The same principles were emphasized in a comprehensive report (NIH, 1992) by the U.S. National Biotechnology Policy Board, which was established by Congress and composed of representatives from the public and private sectors:
The risks associated with biotechnology are not unique, and tend to be associated with particular products and their applications, not with the production process or the technology per se. In fact biotechnology processes tend to reduce risks because they are more precise and predictable. The health and environmental risks of not pursuing biotechnology-based solutions to the nation’s problems are likely to be greater than the risks of going forward.
These findings are consistent with the observations and recommendations of the United Kingdom’s House of Lords Select Committee on Science and Technology (UK,1993), which was very critical of that nation’s policy of subjecting rDNA biotechnology-derived products to additional regulatory requirements:
As a matter of principle, GMO-derived products [i.e., those from genetically manipulated organisms, or recombinant organisms] should be regulated according to the same criteria as any other product. . . . U.K. regulation of the new biotechnology of genetic modification is excessively precautionary, obsolescent, and unscientific. The resulting bureaucracy, cost, and delay impose an unnecessary burden to academic researchers and industry alike.
Three joint FAO/WHO consultations, addressing specifically the question of the safety of rDNA biotechnology-derived foods, came to similar conclusions. The first of these expert consultations (FAO/WHO, 1991) concluded:
Biotechnology has a long history of use in food production and processing. It represents a continuum embracing both traditional breeding techniques and the latest techniques based on molecular biology. The newer biotechnological techniques, in particular, open up very great possibilities of rapidly improving the quantity and quality of food available. The use of these techniques does not result in food which is inherently less safe than that produced by conventional ones.
The second consultation (FAO/WHO, 1996) reaffirmed the conclusions and recommendations of the first FAO/WHO consultation:
Food safety considerations regarding organisms produced by techniques that change the heritable traits of an organism, such as rDNA technology, are basically of the same nature as those that might arise from other ways of altering the genome of an organism, such as conventional breeding. . . . While there may be limitations to the application of the substantial equivalence approach to safety assessment, this approach provides equal or increased assurance of the safety of food products derived from genetically modified organisms as compared to foods or food components derived by conventional methods.
The most recent consultation (FAO/WHO 2000) examined the evidence to date and concluded:
A comparative approach focusing on the determination of similarities and differences between the genetically modified food and its conventional counterpart aids in the identification of potential safety and nutritional issues and is considered the most appropriate strategy. . . . The Consultation was of the view that there were presently no alternative strategies that would provide better assurance of safety for genetically modified foods than the appropriate use of the concept of substantial equivalence.
OECD (1993) offered several conclusions and recommendations that are wholly consistent with the NAS, NRC, and FAO/WHO findings:
In principle, food has been presumed to be safe unless a significant hazard was identified.
Modern biotechnology broadens the scope of the genetic changes that can be made in food organisms and broadens the scope of possible sources of foods. This does not inherently lead to foods that are less safe than those developed by conventional techniques.
--- PAGE BREAK ---
Therefore, evaluation of foods and food components obtained from organisms developed by the application of the newer techniques does not necessitate a fundamental change in established principles, nor does it require a different standard of safety.
For foods and food components from organisms developed by the application of modern biotechnology, the most practical approach to the determination of safety is to consider whether they are substantially equivalent to analogous conventional food product(s), if such exist.
OECD (1998) reaffirmed the conclusions and recommendations of previous consultations of both FAO/WHO and OECD. Regarding the specific question of potential allergenicity of novel proteins introduced in rDNA biotechnology-derived foods, the report stated:
While no specific methods can be used for proteins derived from sources with no history of allergy, a combination of genetic and physicochemical comparisons exist which can be used as a screen. The application of such a strategy can provide appropriate assurance that foods derived from genetically modified products can be introduced with confidence comparable to other new plant varieties.
In 2000, OECD acknowledged the public concerns about the safety assessment of rDNA technology (OECD 2000), stating:
Although [the] food safety assessment is based on sound science, there is a clear need for increased transparency and for safety assessors to communicate better with the public. Much progress has already been made in this regard. . . . However, more could be done in this area.
The NRC’s Committee on Genetically Modified Pest-Protected Plants published a report (NRC, 2000) that reaffirmed the principles set forth in the 1987 NAS white paper. Specifically, the committee found that “there is no strict dichotomy between, or new categories of, the health and environmental risks that might be posed by transgenic and conventional pest-protected plants” and that the “properties of a genetically modified organism should be the focus of risk assessments, not the process by which it was produced.” The committee concluded that “[w]ith careful planning and appropriate regulatory oversight, commercial cultivation of transgenic pest-protected plants is not generally expected to pose higher risks and may pose less risk than other commonly used chemical and biological pest-management techniques.” (While the report focused on rDNA biotechnology-derived pest-protected plants, the committee stated that many of its conclusions are also applicable to rDNA biotechnology-derived plants generally.)
In summary, the safety of rDNA biotechnology-derived foods has been extensively reviewed by a number of scientific organizations, at the national and international level. The use of rDNA biotechnology in itself has no impact on the safety of such foods. Foods derived using rDNA biotechnology are subject to rigorous and systematic scientific evaluations under existing principles of food safety—far more than are routinely applied to the products of traditional breeding. Thus, the level of field testing and premarket review for food safety provide assurance that foods derived from plants and microorganisms through rDNA biotechnology are at least as safe as existing foods, and are consistent with all existing standards of food safety.
Conclusions
Based on its evaluation of the available scientific evidence, the Human Food Safety Panel reached the following conclusions:
• Biotechnology, broadly defined, has a long history of use in food production and processing. It represents a continuum that encompasses both centuries-old traditional breeding techniques and the latest techniques based on molecular modification of genetic material, which are a major step forward by virtue of their precision and reach. The newer rDNA biotechnology techniques, in particular, offer the potential to rapidly and precisely improve the quantity and quality of food available.
--- PAGE BREAK ---
• Crops modified by modern molecular and cellular methods pose risks no different from those modified by earlier genetic methods for similar traits. Because the molecular methods are more specific, users of these methods will be more certain about the traits they introduce into the plants.
• The evaluation of food, food ingredients, and animal feed obtained from organisms developed with the newer rDNA biotechnology techniques of genetic manipulation does not require a fundamental change in established principles of food safety; nor does it require a different standard of safety, even though, in fact, more information and a higher standard of safety are being required.
• The science that underlies rDNA biotechnology-derived foods does not support more stringent safety standards than those that apply to conventional foods.
• The use of rDNA biotechnology and molecular techniques of genetic manipulation significantly broadens the scope of the genetic changes that can be made in food organisms and broadens the scope of possible sources of foods, but this does not inherently lead to foods that are less safe than those developed by conventional techniques. By virtue of their greater precision, such products can be expected to be better characterized, leading to more predictability and a more reliable safety assessment process.
Human Food Safety Panel
Dallas Hoover, Professor, Dept. of Animal and Food Science, University of Delaware, Newark
Bruce M. Chassy, Associate Director of Campus Biotechnology Center, Assistant Dean for Science Communications and Outreach, University of Illinois, Urbana
Richard L. Hall, Consultant, Franklin, Maine; Towson, Md.
Harry J. Klee, Eminent Scholar, Horticultural Sciences Dept., University of Florida, Gainesville
John B. Luchansky, Research Leader, Eastern Regional Research Center, U.S. Dept. of Agriculture, Agricultural Research Service, Wyndmoor, Pa.
Henry I. Miller, Robert Wesson Fellow, Stanford University, Stanford, Calif.
Ian Munro, President, Cantox Health Sciences International, Mississauga, Ontario, Canada
Ronald Weiss, Research Program Manager, Food Research Institute, University of Wisconsin, Madison
Susan L. Hefle, Assistant Professor, Food Allergy Research and Resource Program, University of Nebraska, Lincoln
Calvin O. Qualset, Director, Genetic Resources Conservation Program, University of California, Davis
References
Astwood, J.D., Leach, J.N., and Fuchs, R.L. 1996. Stability of food allergens to digestion in vitro. Nature Biotechnol. 14: 1269-1273.
Battershill, J., Hattersley, S.J., and Sanderson, M. 1999. Critical issues for the safety assessment of novel foods when no conventional counterpart exists: Discussion meeting, Dept. of Health, London, UK, 12 February 1998. Food Additives and Contaminants 16(1): 37-45.
Beever, D.E. and Kemp, C.F. 2000. Safety issues associated with the DNA in animal feed derived from genetically modified crops. A review of scientific and regulatory procedures. Nutr. Abstr. Reviews, Series B: Livestock Feeds and Feeding 70: 175-182.
Bock, S.A., Buckley, J., Holst, A., and May, C.D. 1977. Proper use of skin tests with food extracts in diagnosis of hypersensitivity to foods in children. Clin. Allergy 7: 375-383.
Bock, S.A., Sampson, H.A., Atkins, F.M., Zeiger, R.S., Lehrer, S., Sachs, M., Bush, R.K., and Metcalfe, D.D. 1988. Doubleblind, placebo-controlled food challenges (DBPCFC) as an office procedure: A manual. J. Allergy Clin. Immunol. 82: 986-997.
Consumer and Biotechnology Foundation. 1999. Genetically modified foods and allergenicity: Safety aspects and consumer information. Report of Workshop, Breukelen, Netherlands, May 28-29.
Donaldson, L. and May, R. 1999. Health implications of genetically modified foods. Dept. of Health. www.doh.gov.uk/pub/docs/doh/gmfood.pdf .
FAO. 1995. Report of the FAO Technical Consultation on Food Allergies, Rome, Nov. 13-14. Food and Agric. Org., Rome.
FAO/WHO. 1991. Strategies for assessing the safety of foods produced by biotechnology. Report of a Joint FAO/WHO Expert Consultation. Food and Agriculture Organization of the United Nations and World Health Organization. WHO, Geneva, Switzerland.
FAO/WHO. 1996. Biotechnology and Food Safety. Report of a Joint FAO/WHO Expert Consultation. Food and Agriculture Organization of the United Nations and World Health Organization. WHO, Geneva, Switzerland.
FAO/WHO. 2000. Safety aspects of genetically modified foods of plant origin. Report of a Joint FAO/WHO Expert Consultation on Foods Derived from Biotechnology. Food and Agriculture Organization of the United Nations and World Health Organization. WHO, Geneva, Switzerland.
FDA. 1992. Statement of policy: Foods derived from new plant varieties. Food and Drug Admin., Fed. Reg. 57: 22984.
FDA. 1994. Secondary direct food additives permitted in food for human consumption; Food additives permitted in feed and drinking water of animals; Aminoglycoside 3'-Phospho-transferase II; Final rule. Food and Drug Admin., Fed. Reg. 59: 26700.
Hall, R.L. 1981. Evaluating the toxicity and health hazards of dietary adjuvants (spices, herbs, etc.). In Proceedings of the Toxicology Forum, July 20–24, 1981, pp. 404. Toxicology Forum, Washington, D.C.
Harrison, L.A., Bailey, M.R., Naylor, M.W., Ream, J.E., Hammond, B.G., Nida, D.L., Burnette, B., Nickson, T.E., Mitsky, T.A., Taylor, M.L., Fuchs, R.L., and Padgette, S.R. 1996. The expressed protein in glyphosate-tolerant soybean, 5-enolpyruvylshikimate-3-phosphate synthase from Agrobacterium sp. Strain CP4, is rapidly digested in vitro and is not toxic to acutely gavaged mice. J. Nutr. 126: 728-740.
Hefle, S.L., Nordlee, J.A., and Taylor, S.L. 1996. Allergenic foods. Crit. Rev. Food Sci. Nutr. 36: S69-S89.
HHS. 2000. FDA to strengthen premarket review of bioengineered foods. Press release. May 3. U.S. Dept. of Health and Human Services, Washington, D.C.
IFBC. 1990. Biotechnologies and Food: Assuring the Safety of Food Produced by Genetic Modification. Intl. Food Biotechnology Council. Regulatory Toxicol. Pharmacol. 12(3): Part 2.
Knippels, L.M.J., Pennicks A.H., Spanhaak S., and Houben G.F. 1998. Oral sensitization to food proteins: A Brown Norway rat model. Clin. Exp. Allergy 28: 368-375.
MacKenzie, D. 1999. Unpalatable truths. New Scientist, April 17, pp. 18-19.
Metcalfe, D.D., Astwood, J.D., Townsend, R., Sampson, H.A., Taylor, S.L., and Fuchs, R.L. 1996. Assessment of the allergenic potential of food derived from genetically engineered crop plants. Crit. Rev. Food Sci. Nutr. 36: S165-S186.
Miraglia, M., Onori, R., Brera, C., and Cava, E. 1998. Safety assessment of genetically modified food products: An evaluation of developed approaches and methodologies. Microchem. J. 59: 154-159.
Munro, I.C., McGirr, L.G., Nestmann, E.R., and Kille, J.W. 1996. Alternative approaches to the safety assessment of macronutrient substitutes. Regulatory Toxicol. Pharmacol. 23: S6-S14.
NAS. 1987. Introduction of recombinant DNA-engineered organisms into the environment: Key issues. Natl. Acad. of Sciences. National Academy Press, Washington, D.C.
NIH. 1992. National Biotechnology Policy Board report. Natl. Insts. of Health, Bethesda, Md.
Nordlee, J.A., Taylor, S.L., Townsend, J.A., Thomas, L.A., and Bush, R.K. 1996. Identification of a Brazil nut allergen in transgenic soybeans. New Eng. J. Med. 334: 688-694.
NRC. 1989. “Field Testing Genetically Modified Organisms: Framework for Decisions.” Natl. Res. Council. National Academy Press, Washington, D.C.
NRC. 2000. “Genetically Modified Pest-Protected Plants: Science and Regulation.” Natl. Res. Council. National Academy Press, Washington, D.C.
OECD. 1993. “Safety Evaluation of Foods Derived by Modern Biotechnology: Concepts and Principles.” Org. for Economic Cooperation and Development, Paris.
OECD. 1998. Report of the OECD Workshop on Toxicological and Nutritional Testing of Novel Foods. Org. for Economic Cooperation and Development, Paris.
OECD. 2000. Report of the Task Force for the Safety of Novel Foods and Feeds. Org. for Economic Cooperation and Development, Paris . 86/ADDI, May 17.
Royal Society. 1998. Genetically modified plants for food use. www.royalsoc.ac.uk/files/statfiles/document-56.pdf.
Salyers, A. 2000. Genetically engineered foods: Safety issues associated with antibiotic resistance genes. Reservoirs of Antibiotic Resistance Network. www.healthsci.tufts.edu/apua/salyersreport.htm.
Taylor, S.L. 1997. Food from genetically modified organisms and potential for food allergy. Environ. Toxicol. Pharmacol. 4: 121-126.
Taylor, S.L. and Lehrer, S.B. 1996. Principles and characteristics of food allergens. Crit. Rev. Food Sci. Nutr. 36:S91-S118.
UK. 1993. Regulation of the United Kingdom biotechnology industry and global competitiveness. October. United Kingdom’s House of Lords Select Committee on Science and Technology.
Wal, J.M. 1998. Strategies for assessment and identification of allergenicity in (novel) foods. Intl. Dairy J. 8: 413-423.
WHO. 1993. Health aspects of marker genes in genetically modified plants. Report of WHO Workshop. WHO/FNU/FOS/93.6. World Health Org., Geneva.
Yunginger, J.W., Adolphson, C.R. 1992. Standardization of allergens. In “Manual of Clinical Immunology,” 4th ed., pp. 678-684. Am. Soc. of Microbiology, Washington D.C.
Zitnak, A., and Johnston, G.R. 1970. Glycoalkaloid content of B5141-6 potatoes. Am. Potato J. 47: 256-260.
