Optical Biosensors for Food Pathogen Detection
Optical biosensors have been developed for rapid detection of contaminants in foods, including pathogens, and several have evolved into commercial prototype systems.
The Principle
Biosensors have been described as the offspring of the marriage of biology and electronics (DeYoung, 1983). Modern biosensors evolved from the combination of these two disciplines, with electronics/information technology exemplified by microcircuits and optical fibers, and biology exemplified by molecular biology in the form of enzymes or antibodies (Schultz, 1991; Richter, 1993).
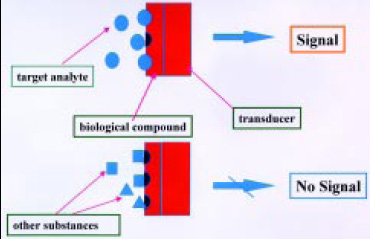 The basic principle utilizes electronic or optical transduction technology to monitor a parameter of the reaction between a biomolecule and an analyte into a quantifiable electrical or optical signal (Byfield and Abuknesha, 1994; Griffiths and Hall, 1993). A visual definition is shown in Fig. 1. This signal can be related to concentration. Analytes that do not fit biological recognition produce no signal. Optical fibers coated with a selective antibody, for example, can be used to detect the presence of a particular pathogen in a food. This type of combination can be used to create an optical biosensor (Shukla et al., 1993).
The basic principle utilizes electronic or optical transduction technology to monitor a parameter of the reaction between a biomolecule and an analyte into a quantifiable electrical or optical signal (Byfield and Abuknesha, 1994; Griffiths and Hall, 1993). A visual definition is shown in Fig. 1. This signal can be related to concentration. Analytes that do not fit biological recognition produce no signal. Optical fibers coated with a selective antibody, for example, can be used to detect the presence of a particular pathogen in a food. This type of combination can be used to create an optical biosensor (Shukla et al., 1993).
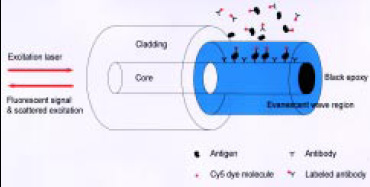 Fiber-optic biosensors are analytical devices in which the attached bioactive compound serves as the chemical recognition element and a fiberoptic probe serves as the transducer (Richter, 1993). When the specific compound to be analyzed in the food interacts with the bioactive molecule, this interaction is detected as a change in an optical signal measured through the fiber-optic assembly. This type of biosensor has commonly used tapered fiber-optic probes coated with antibodies to detect pathogens and toxins in foods (Golden et al., 1992; Zhou et al. 1997). In these systems, the light from a diode laser travels through an all-fiber system to the fiber tip and penetrates as an evanescent wave into the area outside the tip (Fig. 2). A fluorescently labeled complex binds to the antibodies on the tip and interacts with the evanescent wave of the fiber-optic waveguide. The fluorescent signal then radiates in all directions, and some of it travels back up the fiber tip to the detector.
Fiber-optic biosensors are analytical devices in which the attached bioactive compound serves as the chemical recognition element and a fiberoptic probe serves as the transducer (Richter, 1993). When the specific compound to be analyzed in the food interacts with the bioactive molecule, this interaction is detected as a change in an optical signal measured through the fiber-optic assembly. This type of biosensor has commonly used tapered fiber-optic probes coated with antibodies to detect pathogens and toxins in foods (Golden et al., 1992; Zhou et al. 1997). In these systems, the light from a diode laser travels through an all-fiber system to the fiber tip and penetrates as an evanescent wave into the area outside the tip (Fig. 2). A fluorescently labeled complex binds to the antibodies on the tip and interacts with the evanescent wave of the fiber-optic waveguide. The fluorescent signal then radiates in all directions, and some of it travels back up the fiber tip to the detector.
The light system of choice in these biosensors has been fluorescence, since this type of optical measurement can greatly amplify the signal from the analyte. Non-selective adsorptions and interferences due to background fluorescence have been the major drawbacks in developing optical biosensors for toxic chemicals and pathogens. These two problems decrease the ability of the biosensor to be selective and limit the minimum level of detection.
--- PAGE BREAK ---
Optical biosensors can also be based on an effect referred to as surface plasmon resonance (SPR). In this method, antibodies are immobilized by a coupling matrix on the surface of a thin film of a precious metal deposited on the reflecting surface of an optically transparent glass waveguide (Liedberg and Johansen, 1998; Medina, 1997). Visible or near-infrared radiation is passed through the waveguide in such a way as to cause an internal reflection at the common face of the waveguide. At a certain wavelength in the red or near-IR region, the light interacts with a plasma of electrons on the metal surface, and the resonance effect causes a strong absorbance. The exact wavelength of this resonance absorption depends on the angle of incidence, the metal (size, amount, and type), the amount of antibody immobilized in the coupling matrix on the metal surface, and the surrounding material. The presence of antigens interacting with the antibody causes a shift in the resonance to longer wavelengths, and the amount of the shift can be related to the concentration of antigens. If monochromatic (single-wavelength) radiation having a wavelength near the resonance is passed through the waveguide, the presence of antigens can be detected by the change in the incident angle that produces the maximum resonance.
SPR is a sensitive technique capable of direct detection of analytes. However, this type of optical biosensor suffers from the fact that non-selective adsorptions can occur on the immobilized surfaces; e.g., proteins can cling to the surface and cause a change in wavelength or angle, and this would be falsely interpreted as the antigen. In addition, some large analytes may not be able to effectively reach the antibody in the coupling matrix and produce a strong signal, thus limiting the level of detection.
Planar waveguides can also have evanescent fields that are sensitive to changes in index of refraction just above the optical surface (Campbell et al., 1998). Placing a molecular recognition compound film on the waveguide surface provides the basis for another type of optical biosensor (Hartman, 1997). Interaction with the biological layer changes the index of refraction, causing changes in the direction of the light and providing for direct detection of analytes without adding labels to provide for an optical signal. However, these waveguide structures may be too expensive for routine applications.
Optical Biosensors with Fiber-Optic Tips
Fundamental fluorescence-based fiber-optic immunosensors start with the primary antibodies immobilized directly on the fiber, either on the blunt end or along the sides of a fiber tip with its cladding removed (Fig. 2). A sandwich assay can then be accomplished, because of the localization of the primary antibodies (Golden et al., 1992). This approach could be developed with replaceable and reusable fiber-optic tips of different geometries to produce a practical biosensor system (Gao et al., 1995).
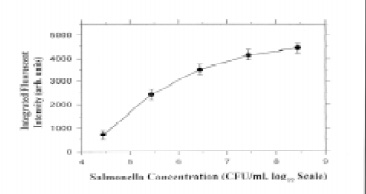 Zhou et al. (1997) developed a compact fiber-optic evanescent-wave biosensor that featured an all-fiber optical design and red semiconductor-laser excitation. Tapered fiber tips with immobilized antibodies for Salmonella attached were studied in different shapes and treatments and optimized. The system response for Salmonella concentration was established and could determine as few as 104 cfu/mL in 1 hr with a sandwich immunoassay format (Fig. 3). The process involved was generally inefficient in terms of time, requiring 30 min for the pathogen to bind to the tip and another 30 min of binding time to attach the fluorescent label. In addition, the size of the microorganism cells could limit the signal recovery from the evanescent wave region, affecting sensitivity. However, more recent results with tapered optical fibers demonstrated improved sensitivity (Demarco et al., 1999). Escherichia coli O157:H7 in seeded ground beef samples was detected at 3–30 cfu/mL, with results obtained within 20 min of sampling.
Zhou et al. (1997) developed a compact fiber-optic evanescent-wave biosensor that featured an all-fiber optical design and red semiconductor-laser excitation. Tapered fiber tips with immobilized antibodies for Salmonella attached were studied in different shapes and treatments and optimized. The system response for Salmonella concentration was established and could determine as few as 104 cfu/mL in 1 hr with a sandwich immunoassay format (Fig. 3). The process involved was generally inefficient in terms of time, requiring 30 min for the pathogen to bind to the tip and another 30 min of binding time to attach the fluorescent label. In addition, the size of the microorganism cells could limit the signal recovery from the evanescent wave region, affecting sensitivity. However, more recent results with tapered optical fibers demonstrated improved sensitivity (Demarco et al., 1999). Escherichia coli O157:H7 in seeded ground beef samples was detected at 3–30 cfu/mL, with results obtained within 20 min of sampling.
--- PAGE BREAK ---
Optical Biosensors with Particles
Fluorescent immunosensors require that the fluorescent dye molecules, which indicate unambiguously the presence of the antigen, find their way to the optically active region of the detecting optical fiber. This can be accomplished by immobilizing the capture antibodies directly on the tip or the tapered core of the fiber, but this means that the capture process is localized and therefore not very efficient. Performing the immunoassay on microparticles that are distributed throughout the sample cell and subsequently concentrated into an optical fiber’s sensing volume promises greatly improved efficiency.
The first approach to combining particles with optical biosensors for food pathogen detection was reported by Yu and Bruno (1996). They utilized immunomagnetic separation, employing mobile and suspended magnetic microbeads for capture and concentration of Salmonella typhimurium and E. coli O157 with electrochemiluminescent detection. The microbeads were coated with antibodies, used to trap the bacteria, and washed, and the bacteria were labeled with antibodies complexed with a ruthenium chelate. The microbeads were suctioned into an Origen analyzer (Igen International, Inc., Gaithersburg, Md.) for measurement of the electrochemiluminescence. This approach demonstrated the potential to decrease the sensitivity to 102–103 bacteria/mL and the assay time to less than 1 hr.
A second approach was the application of a particle-based immunosensor to detect staphylococcal enterotoxin B (Strachan et al., 1997). This system employed a fluorometer coupled to a 1.5-mm-diameter glass capillary with an attached lens and an in-line 53-μm filter opposite the lens to catch flowing particles and position them in the light path for excitation and fluorescent emission, as designed by Sapidyne Instruments, Idaho City, Idaho. Polymethlymethacrylate beads were coated with antibodies for the target analyte and pumped into the system, which trapped them on the filter in the flow cell. Samples containing the staphylococcal enterotoxin were pumped over the beads for 4 min, followed by a fluorescently labeled antibody for 4 min, then rinsed for 3 min. The fluorescent signal was then determined. Compared to the common ELISA methods for staphylococcal toxins, this assay was much faster, 10 min vs 1.5–5 hr, but less sensitive.
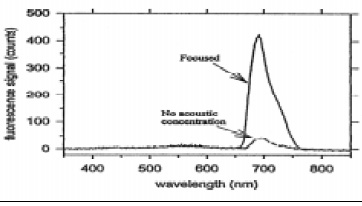
 Acoustic enhancement with ultrasonic manipulation of suspended particles has been shown to be an effective way to increase the sensitivity of fiber-optic biosensors (Zhou et al., 1998). Polystyrene microspheres (6 μm diameter) coated with immobilized antibodies were allowed to capture the antigens (Salmonella), which in turn captured antibodies labeled with fluorescent dye molecules. When the process was complete, an ultrasonic wave with a Gaussian profile focused the complexes along the axis of the cell where an optical fiber was located, with its cladding removed and its core tapered. The fluorescent signal was greatly increased over the signal without acoustic positioning (Fig. 4). Multiple use of the system was also rapid because the filter tip could be reused indefinitely, since no antibodies were attached.
Acoustic enhancement with ultrasonic manipulation of suspended particles has been shown to be an effective way to increase the sensitivity of fiber-optic biosensors (Zhou et al., 1998). Polystyrene microspheres (6 μm diameter) coated with immobilized antibodies were allowed to capture the antigens (Salmonella), which in turn captured antibodies labeled with fluorescent dye molecules. When the process was complete, an ultrasonic wave with a Gaussian profile focused the complexes along the axis of the cell where an optical fiber was located, with its cladding removed and its core tapered. The fluorescent signal was greatly increased over the signal without acoustic positioning (Fig. 4). Multiple use of the system was also rapid because the filter tip could be reused indefinitely, since no antibodies were attached.
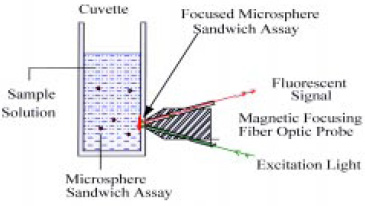 Acoustic focusing of the immunoassay demonstrated that microparticles could be distributed throughout the sample cell for efficient interaction with the bacteria and then concentrated into the fiber’s sensing volume for detection of the fluorescent signal. The next step was to replace the relatively costly and awkward acoustic focusing with simpler magnetic focusing (Pivarnik et al., 1999). This type of immunoassay could be performed on paramagnetic microspheres that are distributed throughout a small transparent cuvette. When the cuvette was placed in front of, and in contact with, a magneto-optic probe, the spheres would be attracted to a spot on the side of the cuvette in the optical sensing region by a small permanent magnet in the probe (Fig. 5).
Acoustic focusing of the immunoassay demonstrated that microparticles could be distributed throughout the sample cell for efficient interaction with the bacteria and then concentrated into the fiber’s sensing volume for detection of the fluorescent signal. The next step was to replace the relatively costly and awkward acoustic focusing with simpler magnetic focusing (Pivarnik et al., 1999). This type of immunoassay could be performed on paramagnetic microspheres that are distributed throughout a small transparent cuvette. When the cuvette was placed in front of, and in contact with, a magneto-optic probe, the spheres would be attracted to a spot on the side of the cuvette in the optical sensing region by a small permanent magnet in the probe (Fig. 5).
--- PAGE BREAK ---
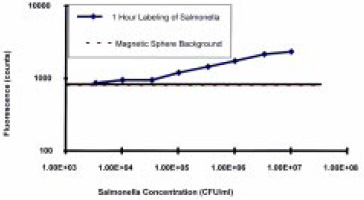
A custom-designed magneto-optic probe was constructed in the Physics Dept. at the University of Rhode Island and tested to prove the principle. Two optical fibers were embedded in a tapered magnetic tip—one for transmitting the exciting laser light and one for detecting the fluorescent signal. The optical fibers were aligned to provide optimum overlap of the laser light emerging from the excitation fiber with the sensing region of the detection fiber. Initial trials showed that 105 cfu/mL concentrations of Salmonella could be detected with a focusing time of 2 min and that the fluorescent signal was proportional to the microbial cell concentration from 105 to 107 cfu/mL (Fig. 6). The sensitivity was comparable to that of competing ELISA and fluorescent immunoassays, but the assay time was significantly improved, with the total assay completed within 1 hr.
As the microspheres focus on the interior wall of the cuvette, they form an opaque solid surface in front of the excitation and detection fibers. This allows for the readings to be completed in the presence of excess secondary antibodies left behind in the cuvette, thus eliminating several rinsing steps, saving time, and increasing ease of use. The spheres are inexpensive, which makes them disposable, which further facilitates cleanup and ease of use. Since there is no contamination of the probe with pathogens, the probe is readily reusable.
Optical Biosensors with SPR
There have been reports of SPR–immunochemical methods to detect E. coli O157:H7 and Salmonella (Medina, 1997). The direct detection of bacteria cells provided low responses, so sandwich immunoassays were utilized. This approach has produced an assay with detection limits of about 106 cfu for E. coli O157:H7. However, it was possible to regenerate this type of biosensor and perform more than 50 repeat assays. The overall assay time was probably about 60 min for sandwich immunoassays and required high technical skills.
Surface-enhanced infrared absorption (SEIRA) spectroscopy was used for the analysis (detection and identification) of pathogens such as Salmonella (Brown et al., 1998; Seelenbinder et al., 1999). This was an extension of the SPR technique in which visible radiation internally reflected inside a substrate interacts (resonates) with a plasmon of surface electrons in a thin gold film on the substrate. Chemicals in contact with the gold surface affect the wavelength and/or the optimum reflection angle of this resonance. Should antibodies be attached to the gold surface, the resonance wavelength (or optimum angle) is characteristic of the presence of antibodies or antibodies/antigens.
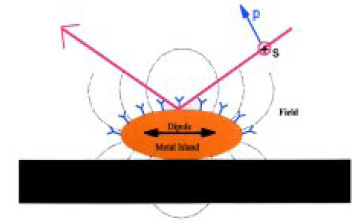 In the SEIRA effect, a very thin film of gold (~10 nm thick) is deposited on a substrate surface in the form of islands on the order of 30 nm in diameter. Infrared radiation generates oscillating dipole moments in these nano particles, and this produces an external electromagnetic field that can interact with molecules on or near the gold surface (Fig. 7). Basically, the nano gold particles act as “antennas” for coupling the radiation to certain vibrations in molecules near the surface. The coupling is such that vibrations perpendicular to the surface are enhanced.
In the SEIRA effect, a very thin film of gold (~10 nm thick) is deposited on a substrate surface in the form of islands on the order of 30 nm in diameter. Infrared radiation generates oscillating dipole moments in these nano particles, and this produces an external electromagnetic field that can interact with molecules on or near the gold surface (Fig. 7). Basically, the nano gold particles act as “antennas” for coupling the radiation to certain vibrations in molecules near the surface. The coupling is such that vibrations perpendicular to the surface are enhanced.
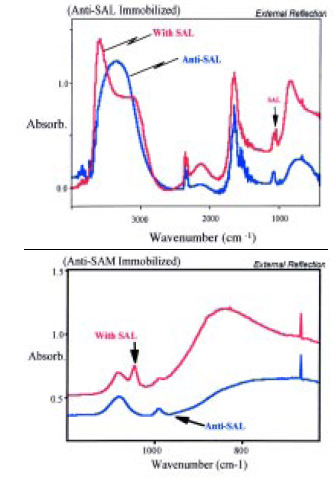 Biosensors were prepared by depositing thin films of gold onto 2-cm2, 1-mm-thick silicon substrates. Antibodies for Salmonella were immobilized onto the gold. The SEIRA spectrum of this sample was measured, and a very simple spectrum was observed. In addition to bands due to water, two very sharp bands due to the monolayer of the antibody were observed. The intensities of these two bands were much greater than had been expected. The sensor was then dipped into a solution containing Salmonella, removed, and blown dry and the spectrum re-measured. A single, very sharp new band was observed with the Salmonella present. This new band was at an entirely different frequency from those observed in the antibody-only spectra (Fig. 8). The concentrations of the samples tested were in the range of 104–106 cfu/mL, and the height of the new band appeared to be proportional to the pathogen concentration. The process for the assay required several steps and a working time of about 30 min.
Biosensors were prepared by depositing thin films of gold onto 2-cm2, 1-mm-thick silicon substrates. Antibodies for Salmonella were immobilized onto the gold. The SEIRA spectrum of this sample was measured, and a very simple spectrum was observed. In addition to bands due to water, two very sharp bands due to the monolayer of the antibody were observed. The intensities of these two bands were much greater than had been expected. The sensor was then dipped into a solution containing Salmonella, removed, and blown dry and the spectrum re-measured. A single, very sharp new band was observed with the Salmonella present. This new band was at an entirely different frequency from those observed in the antibody-only spectra (Fig. 8). The concentrations of the samples tested were in the range of 104–106 cfu/mL, and the height of the new band appeared to be proportional to the pathogen concentration. The process for the assay required several steps and a working time of about 30 min.
--- PAGE BREAK ---
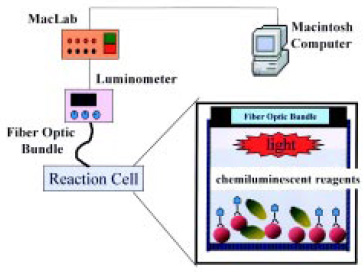 Optical Biosensors with Membranes
Optical Biosensors with Membranes
A simple, sensitive, and rapid chemiluminescent fiber-optic biosensor utilizing monoclonal antibodies to Staphylococcus aureus was developed to detect the pathogen in food (Ye et al., 1999). The S. aureus cells were selectively labeled with a monoclonal horseradish peroxidase (POD) conjugate, collected by membrane filtration, and detected with a luminometer and an enhanced chemiluminescent luminol reagent. The biosensor system utilized a simple but efficient microwell-plate vacuum-filtration unit with an 8-mm membrane sealed at the bottom of the sample well (Fig. 9) The bacteria sample was concentrated on the membrane and positioned directly in front of a fiber-optic light guide to effectively collect and transmit the signal to the luminometer.
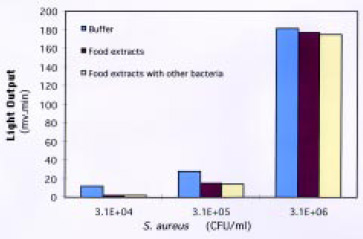 Labeling S. aureus in solution proved to be much more effective than labeling it on the membrane surface. Using the microwell-plate filtration system resulted in less sample handling, better reproducibility, and dramatically reduced assay time. The sensitivity of the biosensor was 3.8 × 104 cfu/mL, with a linear response from 104 to 106 cfu/mL. This was considered adequate to detect the organism at concentrations lower than the level that could result in food poisoning. The performance of the biosensor was not affected by food materials or by the presence of other bacteria (Fig. 10). The biosensor process time was 17 min, providing a simple and specific assay for S. aureus.
Labeling S. aureus in solution proved to be much more effective than labeling it on the membrane surface. Using the microwell-plate filtration system resulted in less sample handling, better reproducibility, and dramatically reduced assay time. The sensitivity of the biosensor was 3.8 × 104 cfu/mL, with a linear response from 104 to 106 cfu/mL. This was considered adequate to detect the organism at concentrations lower than the level that could result in food poisoning. The performance of the biosensor was not affected by food materials or by the presence of other bacteria (Fig. 10). The biosensor process time was 17 min, providing a simple and specific assay for S. aureus.
Comparison to other Methods
The primary objective of optical biosensors for bacteria detection is to drastically shorten the detection time for various pathogens. The speed of detection is critical in preventing and diagnosing food-related illnesses for the food industry and public health agencies. Therefore, to objectively evaluate any new pathogen detection methods, their detection speed has to be compared to that of standard cultural and other existing rapid methods.
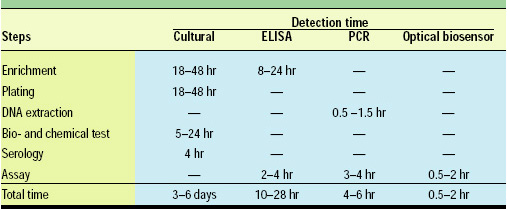 Table 1 shows the average detection time for traditional cultural methods, ELISAs, polymerase chain reactions (PCRs), and the optical biosensors. Cultural methods have long been the golden standard and are still the most trustful method for pathogen detection in foods. However, these assays normally require enrichment, isolation, morphological examination, biochemical testing, and serological testing to positively identify food pathogens. The overall time involved is on average 3–6 days and could range from 3 to 9 days. Thus, cultural methods are not suitable as on-line or real-time monitoring but are off-line solutions that need to be performed in a well-equipped laboratory.
Table 1 shows the average detection time for traditional cultural methods, ELISAs, polymerase chain reactions (PCRs), and the optical biosensors. Cultural methods have long been the golden standard and are still the most trustful method for pathogen detection in foods. However, these assays normally require enrichment, isolation, morphological examination, biochemical testing, and serological testing to positively identify food pathogens. The overall time involved is on average 3–6 days and could range from 3 to 9 days. Thus, cultural methods are not suitable as on-line or real-time monitoring but are off-line solutions that need to be performed in a well-equipped laboratory.
--- PAGE BREAK ---
ELISAs and PCRs have been increasingly popular for food pathogen detection because of their much-improved speed and reliability. ELISAs normally need 10–28 hr to complete. However, most still require some kind of enrichment of the sample because of the relatively low sensitivity of the detection instruments.
PCR has been a very powerful technique for pathogen identification, being capable of detecting a single cell in the sample. The time involved is also normally shorter than for ELISAs, 4–6 hr in most cases. However, the cost of equipment and reagents, the expertise and skills required, and the sample-handling capacity have limited its use on food samples.
Optical biosensors, on the other hand, have further reduced the assay time to about 0.5–2 hr—less than half the time needed for PCR. Because of the inherent sensitivity of the optic transducers and detectors, the sensitivity of optical biosensors is better than that of ELISAs and comparable to those of most PCRs.
Advantages and Disadvantages
The advantages of optical biosensors include speed of detection, as well as specificity based on the effectiveness of the biological compound used for detection (Singh et al., 2001). Antibodies and enzymes have well-known specificity for their target compounds, and monoclonal antibodies have greatly improved this aspect of immunobiochemistry. Most of the waveguides can be very small, particularly optical fibers, which makes miniaturization and portability possible. The cost of these types of systems can very low, which is important for food testing. The need for reference cells used in many other types of analytical measurements can be minimized or eliminated. The use of optical fibers at least makes real-time measurements directly in foods theoretically possible.
The value of optical signals removes the need for electrical measurements and interferences in the area of measurement (Swatland, 1992; Shukla et al., 1993). This can be a big plus when working around foods, considering the conditions that are possible in the environment of a processing plant. The nature of optical transducers makes them generally immune to hostile environments, again a big plus in the food processing environment. In addition, optical biosensor measurements clearly present the potential for simultaneous detection of several analytes or pathogens at one time.
There can also be some disadvantages. Problems can develop with any biosensor due to stability of the biological component (Richter, 1993). If this material loses structural integrity and breaks down, or is dislodged and/or removed from the transducer, the system will fail. The biological component of the biosensor may not match up in stability to the optical transducer when subjected to hostile environments, or could wear out with repeated measurements. This type of decline could produce lack of response or reduced signal and losses in sensitivity. With many of these systems, particularly those using chemiluminescence or fluorescence, ambient light leaks could produce misleading results.
Optical Biosensors on the Market
While enormous numbers of biosensors have been described in the literature, very few have made the jump from basic research to real products on the diagnostic market (Pfeiffer et al., 1994). There has been a rush over the past couple of years to develop biosensors for detecting the dangerous food pathogens Salmonella and E. coli. These and other pathogenic bacteria continue to contaminate food and poison millions of people each year, causing some 5,000 deaths per year in the United States alone.
--- PAGE BREAK ---
Most biosensors have been adapted from the commercially available rapid ELISA tests for microorganisms, which have been of the sandwich immunoassay format. Many of them were designed to specifically screen Salmonella spp. or other pathogens from clinical or environmental samples such as suspected foods. However, what everyone wants is something like a device they see on the Star Trek TV series, which can make multiple analyses with remote and nondestructive testing. That would be the ultimate goal, but we have only begun the journey toward that objective. At present, only a few commercial optical biosensor prototypes have been built and sold.
Probably the first commercial applications have been with the SPR-type biosensor. One commercial product on the market is the BIAcore, an instrument for various types of food analysis by Biacore International AB, Uppsala, Sweden (www.biacore.com). At this point, this instrument seems better suited for detection of chemical residues and small molecules, rather than food pathogens (Medina, 1997). Another SPR-based biosensor system has been developed by Texas Instruments, Dallas, Tex. (Anonymous, 1999a) and packaged into handheld sensors for small molecular analytes that permit on-line and real time analysis (www.ti.com/spreeta). With both of these SPR-based sensors, there continues to be interest in conducting further research to adapt this technology for pathogen testing in foods.
Another effort to get a planar waveguide optical biosensor on the market has grown out of research at Georgia Tech Research Institute (Hartman, 1977; Campbell et al., 1998). This system is based on an integrated optic interferometer, and was initially used to detect small molecules for pollution control. This optical biosensor has been tested for detection of S. typhimurium at 105–107 cfu/mL and has produced a detection limit of 105 cfu/mL in 10 min when conducted as a sandwich immunoassay (Seo et al., 1999). Increasing the reaction time to 1 hr for the pathogen cells to bind to antibodies on the sensor surface decreased the detection to 104 cfu/mL. This system has been licensed to Photonics Sensors Systems, Atlanta, Ga., for commercial development (Anonymous, 1999b).
The Raptor is a portable, automated fiber-optic biosensor developed by Research International, Woodinville, Wash., based on fiber-optic biosensor research by the Naval Research Laboratory (Golden et al., 1992; Demarco et al., 1999). It is a rapid, automatic, and portable fluorometer assay system (www.resrchintl.com) that can perform 10-min assays for four target analytes. It has been tested for monitoring water quality and testing both toxins and bacteria (Anderson and Rowe-Taitt, 2001). This biosensor has been utilized for the detection of S. auerus enterotoxin for food applications, but so far has not been evaluated for actual food testing with pathogens.
The research by the Fiber Optic and Biosensor Research Group at the University of Rhode Island on optical biosensors with magnetic particles and with membranes has evolved into construction of commercial prototypes (www.newswise.com/articles/2000/7/ECOLI.URI.html). The immunomagnetic biosensor system under a URI patent (Pivarnik et al., 2001) has been built as several portable systems for field testing by Pierson Scientific Associates, Andover, Mass. (Pivarnik et al., 1999; Arthur, 2000). Pierson Scientific recently converted the membrane biosensor laboratory system into a portable prototype device. Units of both types of portable optical biosensors have been built and sold to the U.S. Army SBCCOM—Natick Soldier Center for evaluation as rapid field tests for a variety of food pathogens.
A. Garth Rand, Jianming Ye, Chris W. Brown, and Stephen V. Letcher
Author Rand, a Professional Member of IFT, is Emeritus Professor of Food Science, University of Rhode Island, P.O. Box 181, Grantham, NH 03753. Author Ye is Biosensor Postdoctoral Fellow in Physics, University of Rhode Island, Food Science Research Center, 530 Liberty Ln., West Kingston, RI 02892. Authors Brown and Letcher are, respectively, Professor of Chemistry and Professor of Physics, University of Rhode Island, Kingston, RI 02881. The authors are members of the URI Fiber Optic and Biosensor Research Group and the Sensor and Surface Technology Partnership. Send reprint requests to author Letcher.
This work was supported in part by the Cooperative State Research, Education and Extension Service, U.S. Dept. of Agriculture National Research Initiative Competitive Grant Program on Food Safety (Grant No. 97-35201-4480); the U.S. Army SBCCOM–Natick Soldier Center (Contract No. DAAK6095C2035); and the URI Partnership for Sensors and Surface Technology.
Edited by Neil H. Mermelstein,
Editor
References
Anderson, G.P. and Rowe-Taitt, C.A. 2001. Water quality monitoring using an automated portable fiber optic biosensor: RAPTOR. In “Photonic Detection and Intervention Technologies for Safe Food,” ed. Y-R. Chen and S-I. Tu. Proc. of SPIE 4206: 58-63.
Anonymous. 1999a. SPR comes a step closer to the plant floor. Food Qual., Jan/Feb, pp. 47-49.
Anonymous. 1999b. Biosensor reduces time needed to detect meat contaminants. OE Repts., May 1999. SPIE (Intl. Soc. for Optical Engineering), Bellingham, Wash.
Arthur, M.H. 2000. What will food technologists think up next? Food Technol. 54(9): 112.
Brown, C.W., Li, Y., Seelenbinder, J.A., Pivarnik, P., Rand, A.G., Letcher, S.V., Platek M..J., and Gregory, O.J. 1998. Immuno-assays based on surface enhanced infrared absorption (SEIRA) spectroscopy. Anal. Chem. 70: 2991-2996.
Byfield, M.P. and Abuknesha, R.A. 1994. Biochemical aspects of biosensors. Biosensors & Bioelectronics 9: 373-400.
Campbell, D.P., Moore, J.L., Cobb, J.M., Hartman, N.F., Schneider, B.H., and Venugopal, M.G. 1998. Optical system-on-a-chip for chemical and biochemical sensing: The chemistry. Proc. of SPIE 3540: 153-161.
Demarco, D.R., Saaski, E.W., McCrae, D.A., and Lim, D.V. 1999. Rapid detection of Escherichia coli O157:H7 in ground beef using a fiber optic biosensor. J. Food Protect. 62: 711-716.
DeYoung, H.G. 1983. Biosensors, the mating of biology and electronics. High Technol. 11: 41-49.
Gao, H.H., Chen, Z., Kumar, J., Tripathy, S.K., and Kaplan, D.L. 1995. Tapered fiber tips for fiber optic biosensors. Optical Eng. 34: 3465-3470.
Golden, J.P., Schriver-Lake, L.C., Anderson, G.P., Thompson, R.B., and Ligler, F.S. 1992. Fluorometer and tapered fiber optic probes for sensing in the evanescent wave. Optical Eng. 31: 1458-1462.
Griffiths, D. and Hall, G. 1993. Biosensors—What real progress is being made? Trends in Biotechnol. 11(4): 122-130.
Hartman, N.F. 1997. Optical biosensors for microbiological analysis. In “Food Microbiological Analysis: New Technologies,” ed. M.L. Tortorello and S.M. Gendel, pp/ 69-76. Marcel Dekker, New York.
Kisaalita, W.S. 1992. Biosensor standards requirements. Biosensors Bioelectronics 7: 613-620.
Liedberg, B. and Johansen, K. 1998. Affinity biosensing based on surface plasmon resonance detection. In “Affinity Biosensors,” ed. K.R. Rogers and A. Mulchandani, pp. 32-53. Humana Press, Totowa, N.J.
Medina, M.B. 1997. SPR biosensor: Food science applications. Food Testing & Anal. 3(5): 14-16.
Pfeiffer, D., Makower, A., Bier, F., and Scheller, F. W. 1994. Commercial development and application of biosensors. Presented at the Third World Congress on Biosensors, June 1-3, New Orleans, La.
Pivarnik, P., Cao, H., Letcher, S., Pierson, A., and Rand, A.G. 1999. Magnetic focusing immunosensor for the detection of Salmonella typhimurium in foods. In “Pathogen Detection and Remediation for Safe Eating,” ed. Y-R. Chen. Proc. of SPIE 3544: 41-49.
Pivarnik, P., Cao, H., Letcher, S., and Rand, A.G. 2001. Magnetic focusing immunosensor for the detection of pathogens. U.S. patent 6,254,830.
Richter, E.R. 1993. Biosensors: Applications for dairy food industry. J. Dairy Sci. 76: 3114-3117.
Schultz, J.S. 1991. Biosensors. Sci. Am. 265(8): 64-69.
Seelenbinder, J.A., Brown, C.W., Pivarnik, P., and Rand, A.G. 1999. Colloidal gold filtrates as novel metal substrates for surface enhanced infrared absorption spectroscopy. Anal. Chem. 71: 1963-1966.
Seo, K.H., Brackett, R.E., Hartmen, N.F., and Campbell, D.P. 1999. Development of a rapid response biosensor for detection of Salmonella typhimurium. J. Food Protect. 62: 431-437.
Shukla, A., Letcher, S.V., Brown, C., and Rand, G. 1993. On the application of fiber optic sensors in physical, chemical and biological problems. In “Experiments in Smart Materials and Structures,” ed K. Kim, AMD 181: 11-21.
Singh, A., Singh, R.K., and Bhunia, A.K. 2001. Light emission based biosensor for detection of food pathogens: A review. In “Photonic Detection and Intervention Technologies for Safe Food,” ed. Y.R. Chen and S.T. Tu. Proc. of SPIE 4206: 7-12.
Strachan, N.J.C., John, P.G., and Millar, I.G. 1997. Application of a rapid automated immunosensor for the detection of Staphylococcus aureus enterotoxin B in cream. Intl. J. Food Microbiol. 35: 293-297.
Swatland, H.J.. 1992. Fiber-optics in the food industry. Food Res. Intl. 25: 227-235.
Ye, J., Pivarnik, P.E., Senecal, A.G., and Rand, A.G. 1999. Rapid detection of Staphylococcus aureus using a membrane fiber optic biosensor. In “Pathogen Detection and Remediation for Safe Eating,” ed. YR. Chen, Proc. of SPIE 3544: 2-9.
Yu, H. and Bruno, J.G. 1996. Immunomagnetic-electrochemiluminescent detection of Escherichia coli O157 and Salmonella typhimurium in foods and environmental water samples. Appl. Environ. Microbiol. 62: 587-592.
Zhou, C., Pivarnik, P., Auger, S., Rand, A., and Letcher, S. 1997. A compact fiber-optic immunosensor based on evanescent wave excitation using a semiconductor laser. Sensors & Actuators B: Chemical 42: 169-175.
Zhou, C., Pivarnik, P., Rand, A.G., and Letcher, S.V. 1998. Fiber-optic biosensor based on ultrasonic concentration of particles and cells. Biosensors & Bioelectronics 13: 495-500.
