GRAS Flavoring Substances 21
The 21st publication by the Expert Panel of the Flavor and Extract Manufacturers Association on recent progress in the consideration of flavoring ingredients generally recognized as safe under the Food Additives Amendment
During a GRAS assessment of a substance, the Expert Panel evaluates a combination of relevant data, including data specific to the substance as well as data for the chemical group to which the substance belongs. For example, a pyrazine derivative is reviewed individually and in the context of its chemical group (42 other pyrazine derivatives) (Smith et al., 2002a). The analysis of food and flavor exposure data, pharmacokinetics, metabolism, and toxicology for the structurally related group of substances provides the comprehensive basis of the GRAS decision. The breadth of the safety evaluation allows the Expert Panel to arrive at a “weight of evidence” decision concerning the GRAS status of each candidate substance.
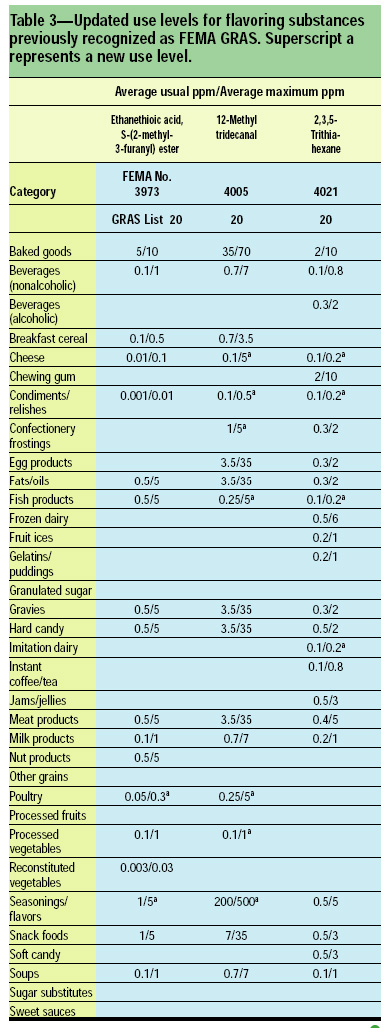
In addition to assessing the GRAS status of new flavoring substances, the Expert Panel operates an ongoing program to comprehensively reassess the GRAS status of existing flavoring substances. In this manner, the Expert Panel revaluates the safety for an individual substance or group of substances based on most recent scientific information. The current reassessment program, known as “GRAS reaffirmation” or “GRASr” began in 1994 and is scheduled for completion in December 2005. As part of the GRASr program, the Expert Panel regularly publishes reviews of key scientific data on structurally related groups of flavoring substances on which GRAS decisions are based (Adams et al., 1996, 1997, 1998; Newberne et al., 1999; Smith et al., 2002a, b).
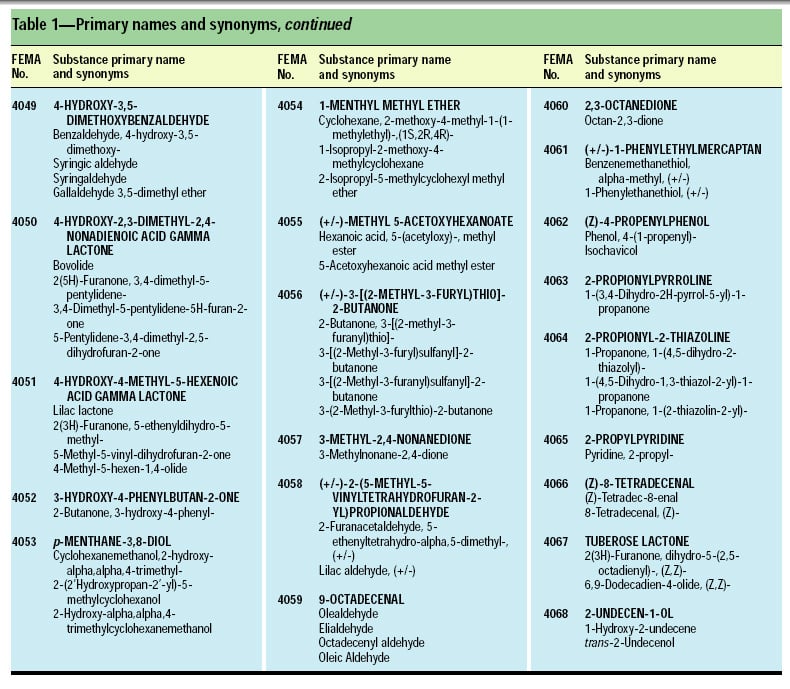
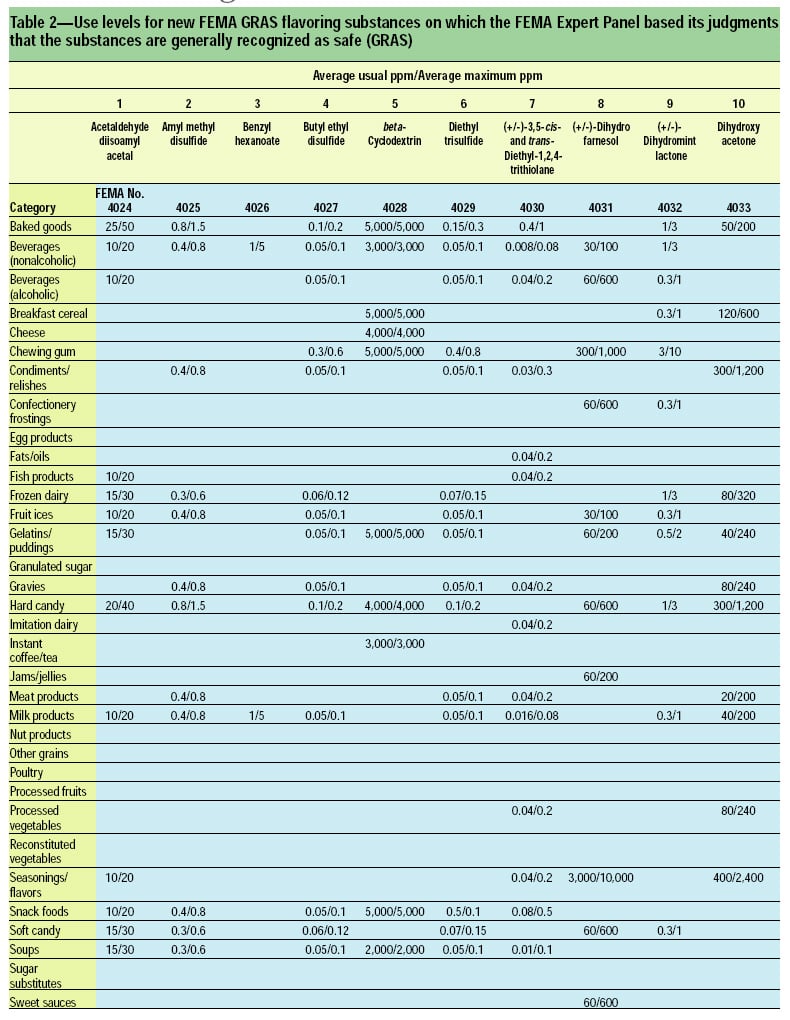
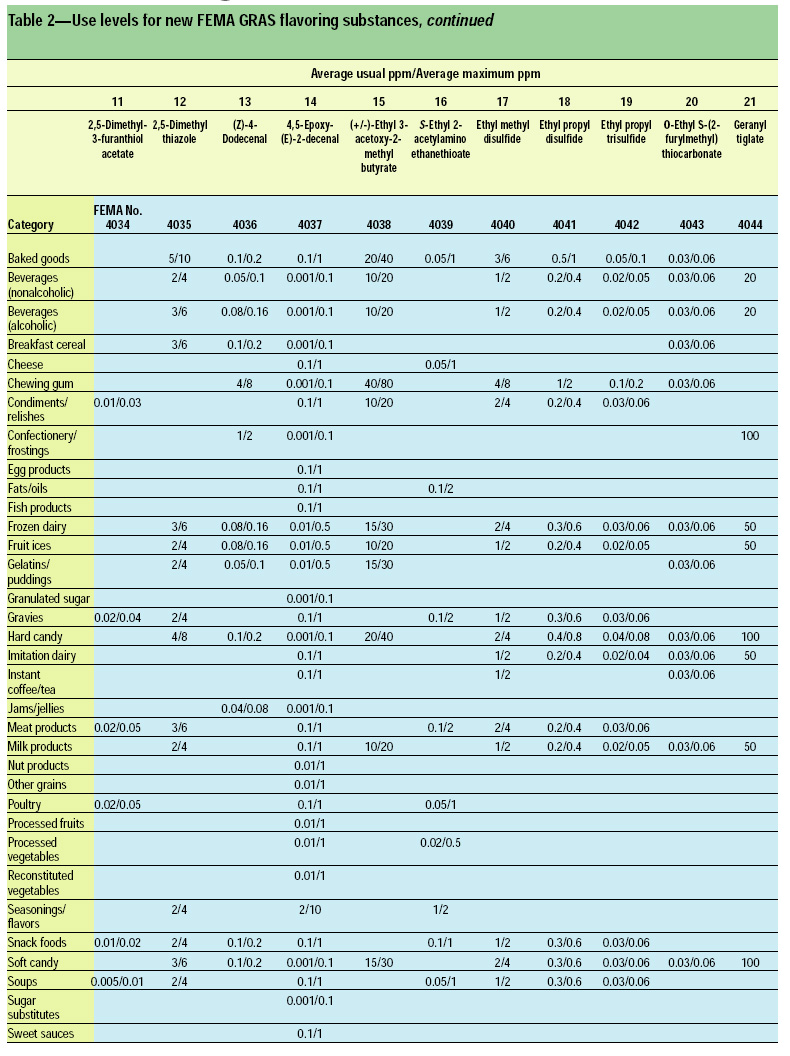
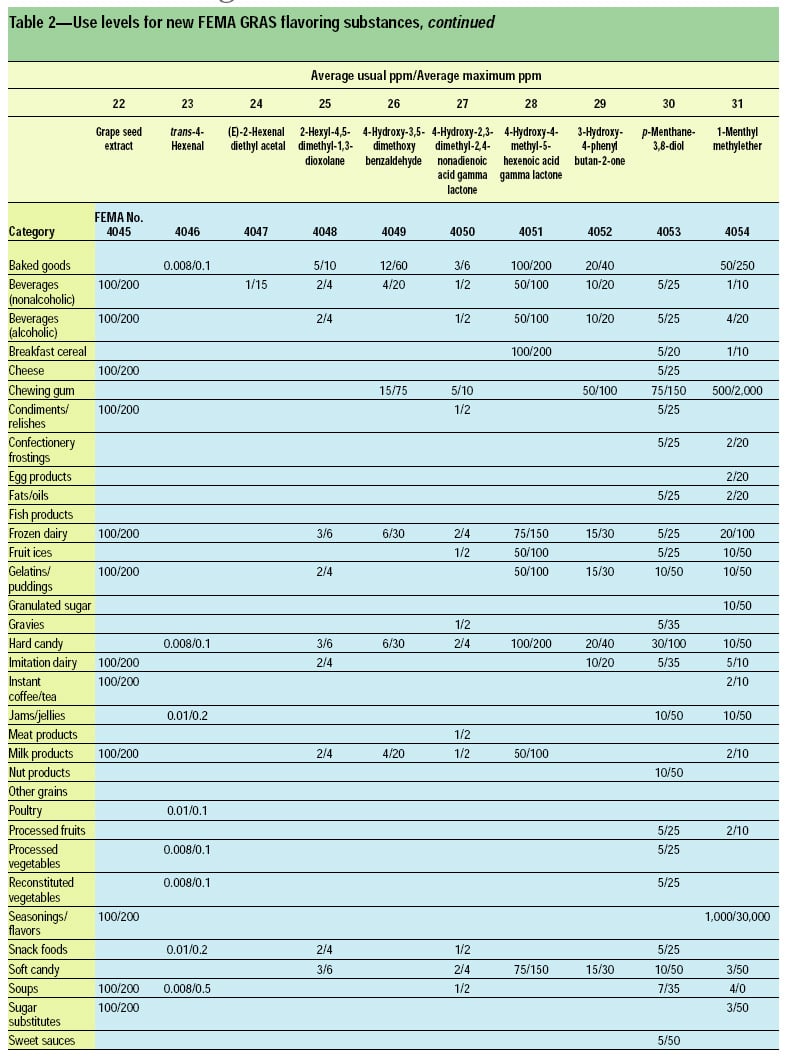
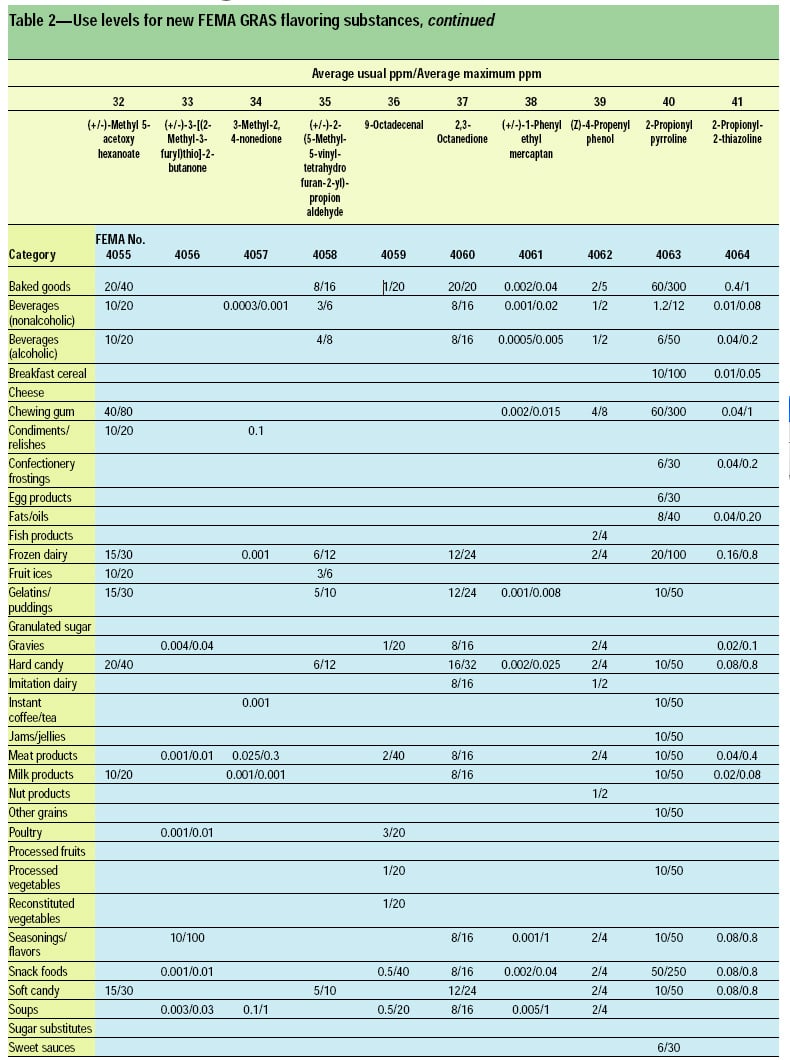
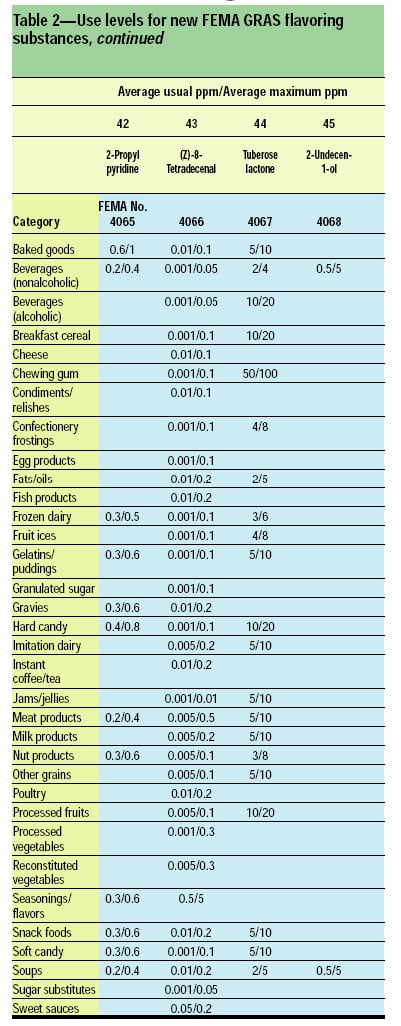
This article, the 21st GRAS publication, includes the results of the Expert Panel’s review of 45 new GRAS flavoring substances (Tables 1 and 2). In addition, the Expert Panel has determined that new use levels and food categories for three flavoring substances are consistent with their current GRAS status (Table 3). In this article, the Expert Panel also critically reviews the results of chronic two-year bioassay studies performed at the National Toxicology Program (NTP) for trans,trans-2,4-hexadienal (FEMA No. 3429) and trans-cinnamaldehyde (FEMA No. 2286).
Safety Assessment of trans, trans -2,4-Hexadienal (FEMA 3429)
trans,trans-2,4-Hexadienal (CAS No. 142-83-6) is a linear aliphatic α,β-unsaturated dienal. In concentrated form, it exhibits a powerful irritating odor, but at concentrations used in flavorings (<1 ppm) it provides a sweet-green aroma. Based on a reported annual volume of 1.4 kg (Lucas et al., 1999), the daily percapita intake (PCI) for “eaters only” is estimated to be approximately 0.003 μg/kg body weight /day from its use as a flavoring substance. The intake for the U.S. population in 1995 (260 million) was calculated as follows:

where 0.8 represents the assumption that only 80% of the flavor volume was reported in the survey (Lucas et al., 1999).
trans,trans-2,4-Hexadienal occurs naturally in a wide variety of foods, including kiwifruit, mango, peanuts, clams, and beer (Maarse et al., 1999). Based on quantitative data for its presence in traditional foods, human consumption of this aldehyde as a natural component of food has been estimated to be approximately 1,000 times its consumption from use as a flavoring substance (Stofberg and Grundschober, 1987).
In a two-year bioassay (NTP, 2001a), groups of 50 F344/N rats of both sexes were administered oral doses of 0, 22.5, 45, or 90 mg/kg bw/day of trans,trans-2,4-hexadienal by gavage for 105 weeks. Groups of B6C3F1 mice of both sexes were administered oral doses of 0, 30, 60, or 120 mg/kg bw/day of trans,trans-2,4-hexadienal by gavage for 105 weeks. The NTP subcommittee concluded:
--- PAGE BREAK ---
“Under the conditions of these 2-year gavage studies, there was clear evidence of carcinogenic activity of 2,4-hexadienal in male and female F344/N rats and male and female B6C3F1 mice based on increased incidences of squamous cell neoplasms of the forestomach. The occurrence of squamous cell carcinoma of the oral cavity (tongue) in male B6C3F1 mice may have been related to the administration of 2,4-hexadienal.”
In F344/N rats, the neoplastic response reported in the NTP study included statistically significant increases in the incidence of squamous cell papillomas of the forestomach at 45 (P < 0.001) and 90 mg/kg bw/day (P < 0.001) in males and at 45 (P = 0.031) and 90 mg/kg bw/day (P < 0.001) in females. There were also statistically significant (P < 0.001) increases in the incidence of forestomach epithelial hyperplasia at all dose levels in both sexes. There was no statistically significant increase in the incidence of squamous cell carcinomas of the forestomach in either males or females at any dose level.
Similarly, in B6C3F1 mice, there were statistically significant increases in the incidence of epithelial hyperplasia (P = 0.007) and squamous cell papillomas (P = 0.030) of the forestomach at 120 mg/kg bw/day in males and statistically significant increases in the incidence of epithelial hyperplasia (P = 0.033 at 60 mg/kg bw/day and P < 0.001 at 120 mg/kg bw/day) and squamous cell papillomas (P = 0.004 at 60 mg/kg bw/day and P = 0.001 at 120 mg/kg bw/day) at 60 and 120 mg/kg bw/day in females. At the highest dose level in females, a statistically significant (P < 0.05) increase (7/50) in the incidence of squamous cell carcinomas was reported. There were no other carcinomas observed in the two lower dose or control groups.
The appearance of forestomach hyperplasia and squamous cell papillomas in rodents is a regular occurrence in bioassay gavage studies in which high concentrations of an irritant material in corn oil is delivered daily by dosing tube into the forestomach. These phenomena are consistently associated with administration of high concentrations of aldehydes, e.g., malonaldehyde, furfural, benzaldehyde, and trans,trans-2,4-hexadienal (NTP, 1988, 1990a, 1993a, 2001a) and other irritating substances, e.g., ethyl acrylate, dihydrocoumarin, and coumarin (NTP, 1990b, 1992) in corn oil by gavage. Squamous cell papillomas are benign lesions associated with squamous epithelium surfaces. A majority of papillomas arise as a result of chronic irritation, or from infection from some strains of viruses (Smith and Ford, 1993). Given these results, high irritating concentrations of aldehyde administered by gavage over the lifetime of a rodent may progress to malignant neoplasms, as was observed in the high-dose group of female mice.
Apparently, the combination of daily introduction of a dosing tube into the forestomach and delivery of high concentrations of an irritating test material in corn oil, which itself is a mild irritant and mitogen, was, in all probability, the source of the papillomas in the rodent forestomach. Gavage administration provides a bolus dose that exerts a traumatic effect on the forestomach epithelium. When repeated in chronic studies, it leads to chronic inflammation and regenerative hyperplasia. In contrast, the same total doses administered to rodents in the diet achieve maximum concentrations in the stomach and circulation that are significantly lower than those achieved by a bolus gavage dose. Therefore, the effects resulting from gavage administration would not be expected when 2,4-hexadienal is administered in the diet.
This conclusion is supported by the observation that the occurrence of squamous cell papillomas and forestomach hyperplasia following gavage administration of an irritant in corn oil for two years (NTP, 1986a) do not develop when the same substance is administered at similar intake levels in the diet (NTP, 1993b). In addition, recent two-year bioassays performed with both aliphatic and aromatic aldehydes [trans-cinnamaldehyde and 3,7-dimethyl-2,6-octadienal (citral)] administered microencapsulated in the diet at higher concentrations than those used in the gavage studies mentioned above show no evidence of either forestomach hyperplasia, forestomach papillomas or forestomach carcinomas (NTP, 2001b, 2002).
The relevance of the appearance of forestomach tumors in rodents to potential carcinogenic targets in humans has been the subject of much investigation (Clayson et al., 1990; Grice, 1988; Wester and Kroes, 1988). Although it has been suggested that the mucosa of the rodent forestomach is similar to that of the human esophagus, this is clearly not the case. The rodent forestomach has a storage function and contains mucosa of keratinizing squamous epithelium that is constantly exposed to the strong acid medium of the gastric contents. Conversely, the esophagus has no storage capacity and contains non-keratinizing squamous epithelium that is extremely sensitive to the adverse effects of strong acid medium. The esophagus has no significant contact with food contents, in that it is a muscle that exerts a motive action on food contents propelling them from the pharynx to the stomach.
--- PAGE BREAK ---
Therefore, the appearance of these lesions in the two-year rodent bioassay in which the test material was administered at high concentration by gavage has doubtful relevance to humans consuming trans,trans-2,4-hexadienal as a flavoring substance. The FEMA Expert Panel concludes that trans,trans-2,4-hexadienal is GRAS under conditions of intended use as a flavoring substance and does not present a carcinogenic hazard to humans.
NTP reached a similar conclusion in a recent reevaluation of the results of a two-year NTP gavage study for ethyl acrylate. The study (NTP, 1986b) concluded that ethyl acrylate was carcinogenic due to dose-related increase in the incidence of benign and malignant forestomach neoplasms in rats and mice. Based primarily on the results of the study, ethyl acrylate was listed as “reasonably anticipated to be a human carcinogen.”
However, in 2000, ethyl acrylate was delisted as a human carcinogen, based on the facts that “1) the forestomach tumours induced in animal studies were seen only when the chemical was administered by gavage at high concentrations that induced marked local irritation and cellular proliferation, 2) animal studies by other routes of administration including inhalation were negative, and 3) because significant chronic human oral exposure to high concentrations of ethyl acrylate monomer is unlikely” (NTP, 2000).
An independent analysis of the NTP data for the incidence of forestomach papillomas and carcinomas in male and female rats and mice treated with 2,4-hexadienal revealed that no tumors would be produced by 1020.2 molecules/kg/day (Waddell, 2002). Estimates of daily per capita intake (“eaters only”) (Lucas et al., 1999) of 0.003 μg/kg bw/day of trans, trans-2,4-hexadienal corresponds to intake of 1010.27 molecules/kg bw/day. Therefore, no tumors would have been produced in the rodents even if rats or mice had been fed a daily dose of trans, trans-2,4-hexadienal that was more than ten billion times the daily intake by an “eaters only” population (i.e., 10% of the population consumes 100% of the annual volume of trans, trans-2,4-hexadienal used as a flavoring ingredient).
Safety Assessment of trans-Cinnamaldehyde (FEMA No. 2286)
trans-Cinnamaldehyde is an aromatic aldehyde chemically recognized as (E)-3-phenylpropenal (CAS No. 104-55-2). It occurs naturally as the major constituent of cinnamon shrubs and trees (family Lauraceae) and is therefore the major constituent of cassia oil (ca. 90%) (Cinnamonium cassia) (Lawrence, 1994a) and cinnamon bark oil (ca. 75%) (Cinnamonium zeylanicum) (Lawrence, 1994b). It is used as a flavor ingredient in foods up to an average level of 700 ppm in candy and up to 4,900 ppm in chewing gum (Hall and Oser, 1965). Based on a reported annual volume of 450,400 kg (Lucas et al., 1999), the estimated per-capita intake is 0.099 mg/kg bw/day or “eaters only” intake of 0.99 mg/kg bw/day.
In an NTP (2002) bioassay, groups of 50 male and female F344/N rats and B6C3F1 mice each were administered dietary concentrations of 1,000, 2,100, or 4,100 ppm of microencapsulated trans-cinnamaldehyde for two years. These dietary concentrations correspond to average daily doses of approximately 50, 100 or 200 mg/kg bw/day in male and female rats, respectively, and 125, 270, or 550 mg/kg bw/day in male and female mice, respectively. Additional groups of 50 male and 50 female animals of each species were administered untreated feed or feed containing placebo microencapsules. The NTP Board of Scientific Counselors Technical Report Review Subcommittee met for a peer review of the recently issued draft NTP Technical Report on trans-cinnamaldehyde (NTP, 2002). The Subcommittee concluded, “Under the conditions of these 2-year feed studies there was no evidence of carcinogenic activity of trans-cinnamaldehyde in male or female F344/N rats or B6C3F1 mice.”
The lack of any evidence of carcinogenicity in either rats or mice at levels exceeding 1% of the diet is consistent with the results of other bioassays in which other aldehydes (e.g., citral) (NTP, 2001b) or reactive substances (e.g., benzyl acetate) (NTP, 1993b) were provided in microencapsulated form administered in the diet. A comparison of the two-year bioassay results for dietary administration of microencapsulated cinnamaldehyde to the gavage administration of a structurally related aldehyde, benzaldehyde (NTP, 1993a), provides a basis for evaluating the effect of mode of administration on selected carcinogenic endpoints, specifically the increased incidence of forestomach papillomas and squamous cell carcinomas in rodent species. The increased incidence of forestomach hyperplasia, papillomas, and eventually the appearance of squamous cell carcinomas in the gavage study using high concentrations of an irritating aldehyde confirm the impact of the mode of administration on the toxicological sequelae in the rodent forestomach (see “Safety Assessment of trans,trans-2,4-Hexadienal” above). Future design of two-year bioassay studies with low-molecular-weight irritant substances should avoid the use of gavage as a mode of administration.
--- PAGE BREAK ---
The negative results in the two-year bioassay for trans-cinnamaldehyde provide insight into the mechanism by which hepatic neoplasms are induced in B6C3F1 mice exposed to high dose levels of a related ester, cinnamyl anthranilate, for two years (NTP, 1980). At low dose levels, cinnamyl anthranilate is adequately hydrolyzed to cinnamyl alcohol and anthranilic acid; cinnamyl alcohol is then readily oxidized primarily in the liver to cinnamaldehyde and then cinnamic acid (Keyhanfar and Caldwell, 1996). However, at elevated dietary levels, those exceeding 15,000 ppm in mice, the hydrolysis of cinnamyl anthranilate approaches saturation leading to accumulation of unhydrolyzed ester in the liver compartment. This phenomenon is accompanied by a pattern of hepatic enzyme induction that is characteristic of peroxisome proliferation (Caldwell, 1992; Caldwell and Viswalingam, 1989; Keyhanfar and Caldwell, 1996; Viswalingam et al., 1988).
In an earlier GRAS article (Newberne et al., 2000), the Panel concluded that the hepatic neoplasms in the B6C3F1 mouse in the NTP bioassay are secondary responses to peroxisome proliferation, a rodentspecific and dose-dependent phenomenon induced by the intact ester cinnamyl anthranilate (Caldwell, 1992; Caldwell and Viswalingam, 1989; Keyhanfar and Caldwell, 1996; Viswalingam et al., 1988). If the intact ester is responsible for induction of peroxisome proliferation and subsequent appearance of liver neoplasms, then the hydrolysis products (anthranilic acid and cinnamyl alcohol) or their liver metabolites (cinnamaldehyde or cinnamic acid) should show no evidence of hepatocarcinogenicity in bioassay studies in the same species and strain at similar or higher exposure levels.
Two bioassays, one on anthranilic acid and the other on the intermediary metabolite of cinnamyl alcohol, cinnamaldehyde, indicate that this is the case. An intake of 15,000 ppm (i.e., the LOAEL for peroxisome proliferation in the cinnamyl anthranilate study) corresponds to a potential production of 7,945 ppm of cinnamyl alcohol and 8,240 ppm of anthranilic acid, calculated as [(molecular weight of alcohol or acid)/(molecular weight of ester)] × (dietary level in ppm). There was no evidence of carcinogenicity reported when B6C3F1 mice were maintained on diets of (1) 25,000 or 50,000 ppm anthranilic acid 5 days/week for 78 weeks and then observed for an additional 26–27 weeks (NCI, 1980) or (2) 1,000, 2,100, or 4,100 ppm of microencapsulated trans-cinnamaldehyde for 2 years (NTP, 2002). Given the similar levels of exposure, the lack of liver carcinogenicity for the hydrolysis products supports a mechanism of action in which high concentrations of the intact ester are associated with the onset of peroxisome proliferation and the eventual appearance of liver tumors.
The Expert Panel concurs with NTP’s conclusion that trans-cinnamaldehyde exhibits no carcinogenic potential in either species of rodent maintained on diets containing up to and including 4,100 ppm of trans-cinnamaldehyde for two years. Therefore, trans-cinnamaldehyde is considered GRASr as a flavoring substance by the Expert Panel, given its historically low level of use by the flavor industry (Lucas et al., 1999; NAS, 1970, 1982, 1987).
Correction
In the “Safety Assessment of Citral” section of “GRAS Flavoring Substances 20” (Smith et al., 2001), the FEMA number for citral was incorrectly listed as 2045. The correct FEMA number for citral is 2303.
Expert Panel Member Changes
In January 2002, Samuel M. Cohen, Professor of Pathology and Microbiology at the University of Nebraska Medical Center joined the ExpertPanel.
--- PAGE BREAK ---
Previous FEMA GRAS Lists published in Food Technology, in chronological order
Hall, R.L. 1960. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. Food Technol. 14: 488.
Hall, L. and Oser, B.L. 1961. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. II. Food Technol. 15(12):20.
Hall, R.L. and Oser, B.L. 1965. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 3. GRAS substances. Food Technol. 19(2, Part 2): 151-197.
Hall, R.L. and Oser, B.L. 1970. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 4. GRAS substances. Food Technol. 24(5): 25-34.
Oser, B.L. and Hall, R.L. 1972. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 5. GRAS substances. Food Technol. 26(5): 35-42.
Oser, B.L. and Ford, R.A. 1973a. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 6. GRAS substances. Food Technol. 27(1): 64-67.
Oser, B.L. and Ford, R.A. 1973b. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 7. GRAS substances. Food Technol. 27(11): 56-57.
Oser, B.L. and Ford, R.A. 1974. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 8. GRAS substances. Food Technol. 28(9): 76-80.
Oser, B.L. and Ford, R.A. 1975. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 9. GRAS substances. Food Technol. 29(8): 70-72.
Oser, B.L. and Ford, R.A. 1977. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 10. GRAS substances. Food Technol. 31(1): 65-74.
Oser, B.L. and Ford, R.A. 1978. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 11. GRAS substances. Food Technol. 32(2): 60-70.
Oser, B.L. and Ford, R.A. 1979. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 12. GRAS substances. Food Technol. 33(7): 65-73.
Oser, B.L., Ford, R.A., and Bernard, B.K. 1984. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 13. GRAS substances. Food Technol. 38(10): 66-89.
Oser, B.L., Weil, C.L., Woods, L.A., and Bernard, B.K. 1985. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 14. GRAS substances. Food Technol. 39(11): 108-117.
Burdock, G.A., Wagner, B.M., Smith, R.L., Munro, I.C., and Newberne, P.M. 1990. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 15. GRAS substances. Food Technol. 44(2): 78,80, 82, 84, 86.
Smith, R.L. and Ford, R.A. 1993. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 16. GRAS substances. Food Technol. 47(6): 104-117.
Smith, R.L., Newberne, P., Adams, T.B., Ford, R.A., Hallagan, J.B., and the FEMA Expert Panel. 1996a. GRAS flavoring substances 17. Food Technol. 50(10): 72-78, 80-81.
Smith, R.L., Newberne, P., Adams, T.B., Ford, R.A., Hallagan, J.B., and the FEMA Expert Panel. 1996b. Correction to GRAS flavoring substances 17. Food Technol. 51(2): 32.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Hallagan, J.B., and Ford, R.A. 1998. GRAS flavoring substances 18. Food Technol. 52(9): 65-66, 68, 70, 72, 74, 76, 79-92.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Hallagan, J.B., and Ford, R.A. 1999. Correction to GRAS flavoring substances 18. Food Technol. 53(3): 104.
Newberne, P., Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Waddell, W.J., Wagner, B.M., Weil, C.S., Adams, T.B., and Hallagan, J.B. 2000. GRAS flavoring substances 19. Food Technol. 54(6): 66, 68-69, 70, 72-74, 76-84.
Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Munro, I.C., Newberne, P.M., Portoghese, P.S., Waddell, W.J., Wagner, B.M., Adams, T.B., and McGowen, M.M. 2001. GRAS flavoring substances 20. Food Technol. 55(12): 34-36, 38, 40, 42, 44-55.
by R.L. Smith, S.M. Cohen, J. Doull, V.J. Feron, J.I. Goodman, L.J. Marnett, P.S. Portoghese, W.J. Waddell, B.M. Wagner, and T.B. Adams
Robert L. Smith, Chairman of the FEMA Expert Panel, is Professor, Molecular Toxicology, Imperial College School of Medicine, University of London, South Kensington, London SW7 2AZ, United Kingdom. Other members of the FEMA Expert Panel are Samuel M. Cohen, Professor and Chair, Dept. of Pathology and Microbiology, University of Nebraska Medical Center, Omaha; John Doull, Professor Emeritus, University of Kansas Medical School, Kansas City; Victor J. Feron, TNO Nutrition and Food Research, Professor Emeritus, Utrecht University, The Netherlands; Jay I. Goodman, Professor, Michigan State University, East Lansing; Lawrence J. Marnett, Dept. of Biochemistry, Center in Molecular Toxicology, School of Medicine, Vanderbilt University, Nashville, Tenn.; Philip S. Portoghese, Professor, College of Pharmacy, University of Minnesota, Minneapolis; William J. Waddell, Professor and Chair, Emeritus, Dept. of Pharmacology and Toxicology, University of Louisville School of Medicine, Louisville, Ky.; and Bernard M. Wagner, Emeritus Research Professor of Pathology, New York University Medical Center, New York, N.Y. Timothy B. Adams is Scientific Secretary for the FEMA Expert Panel and Scientific Director of the Flavor and Extract Manufacturers Association, 1620 I St., N.W., Suite 925, Washington, DC 20006. Send reprint requests to author Smith.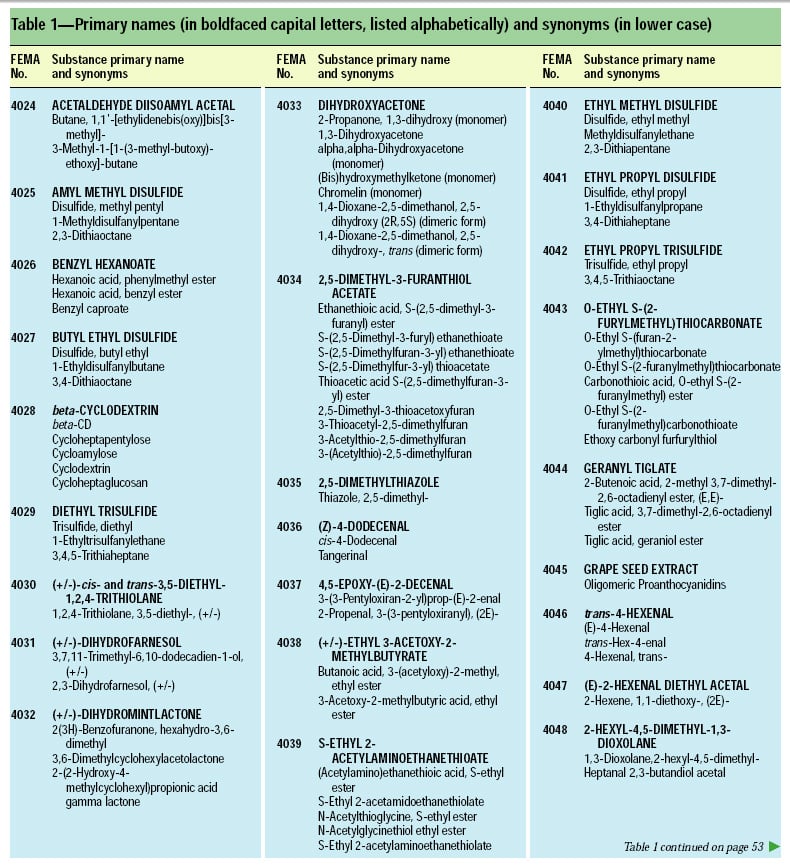
References
Adams, T.B., Hallagan, J.B., Putman, J.M., Gierke, T.L., Doull, J., Munro, I.C., Newberne, P.M., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1996. The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. Food Chem. Toxicol. 34: 763-828.
Adams, T.B., Doull, J., Goodman, J.I., Munro, I.C., Newberne, P.M., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1997. The FEMA GRAS assessment of furfural used as a flavor ingredient. Food Chem. Toxicol. 35: 739-751.
Adams, T.B., Greer, D.B., Doull, J., Munro, I.C., Newberne, P.M., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1998. The FEMA GRAS assessment of lactones used as flavor ingredients. Food Chem. Toxicol. 36: 249-278.
Caldwell, J. 1992. Problems and opportunities in toxicity testing arising from species differences in xenobiotic metabolism. In “Toxicology from Discovery to Experimentation to the Human Perspective.” ed. C.M.C.P.L. Chambers, H.M. Bolt, and P. Preziosi, pp. 651-659. Elsevier, New York.
Caldwell, J. and Viswalingam, A. 1989. A comparative study of the hepatic effects of cinnamyl esters in mice. In “The Fifth International Congress of Toxicology.” Elsevier, New York.
Clayson, D.B., Iverson, F., Nera, E.A., and Lok, E. 1990. The significance of induced forestomach tumors. Ann. Rev. Pharmacol. Toxicol. 30: 441-463.
Grice, H.C. 1988. Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food Chem. Toxicol. 26: 717-723.
Hall, R.L. and Oser, B.L. 1965. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. Food Technol. 19(2): 151-197.
Keyhanfar, F. and Caldwell, J. 1996. Factors affecting the metabolism of cinnamyl anthranilate in the rat and mouse. Food Chem. Toxicol. 34: 241-249.
Lawrence, B.M. 1994a. Progress in essential oils (cassia oil). Perfumer Flavorist 19(4): 33.
Lawrence, B.M. 1994b. Progress in essential oils (cinnamon oil). Perfumer Flavorist 19(3): 59.
Lucas, C.D., Putnam, J.M., and Hallagan, J.B. 1999. “Flavor and Extract Manufacturers Association of the United States 1995 Poundage and Technical Effects Update Survey.” Flavor and Extract Manufacturers Assn., Washington D.C.
Maarse, H., Visscher, C.A., Willemsens, L.C., and Boelens, M.H. 1999. “Volatile Components in Food-Qualitative and Quantitative Data.” Centraal Instituut Voor Voedingsonderzioek TNO, Zeist, The Netherlands.
NAS. 1970. “Evaluating the Safety of Food Chemicals.” Natl. Academy of Sciences, Washington, D.C.
NAS. 1982. “Evaluating the Safety of Food Chemicals.” Natl. Academy of Sciences, Washington, D.C.
NAS. 1987. “Evaluating the Safety of Food Chemicals.” Natl. Academy of Sciences, Washington, D.C.
NCI. 1980. Bioassay of cinnamyl anthranilate for possible carcinogenicity. NCI-CG-TR-196. Natl. Cancer Inst., Bethesda, Md., and Natl. Toxicology Program, Research Triangle, N.C.
Newberne, P.M., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Lucas, C.D. and Ford, R.A. 1999. The FEMA GRAS assessment of trans-anethole used as a flavoring substance. Food Chem. Toxicol. 37: 789-811.
Newberne, P.M., Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Waddell, W.J., Wagner, B.M., Weil, C.S., Adams, T.B., and Hallagan, J.B. 2000. 19. GRAS flavoring substances. Food Technol. 54(6): 66-84.
NTP. 1980. Bioassay of cinnamyl anthranilate for possible carcinogenicity (CAS No. 87-29-6). NTP TR-196. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 1986a. Toxicology and carcinogenesis studies of benzyl acetate (CAS No. 140-11-4) in F344/N rats and B6C3F1 Mice (gavage studies). NTP TR-250. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 1986b. Carcinogenesis studies of ethyl acrylate (CAS 140-88-5) in F344/N and B6C3F1 mice (gavage studies). NTP TR 259. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 1988. Toxicology and carcinogenesis studies of malonaldehyde, sodium salt (3-hydroxy-2-propenal, sodium salt) (CAS No. 24382-04-5) in F344/N rats and B6C3F1 mice (gavage studies). NTP TR-331. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 1990a. Toxicology and Carcinogenesis studies of furfural (CAS No. 98-01-1) in F344/N rats and B6C3F1 mice (gavage studies). NTP TR-382. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 1990b. Toxicology and carcinogenesis studies of 3,4-dihydrocoumarin (CAS No. 119-84-6) in F344/N rats and B6C3F1 mice (gavage studies). NTP TR-423. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 1992. Toxicology and carcinogenesis studies of coumarin (CAS No. 91-64-5) in F344/N rats and B6C3F1 mice (gavage studies). NTP TR-422. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 1993a. Toxicology and carcinogenesis studies of benzaldehyde (CAS No. 100-52-7) in F344/N rats and B6C3F1 mice (gavage studies). NTP TR-378. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 1993b. Toxicology and carcinogenesis studies of benzyl acetate (CAS No. 140-11-4) in F344/N rats and B6C3F1 mice feed studies). NTP TR-431. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 2000. “Report on Carcinogens,” 9th ed. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 2001a. Draft report: Toxicology and carcinogenesis studies of 2,4-hexadienal (89% trans,trans isomer, Cas No. 142-83-6; 11% cis,trans Isomer) in F344/N Rats and B6C3F1 Mice (gavage studies). NTP TR 509. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 2001b. Draft report: Toxicology and carcinogenesis studies of citral (microencapsulated) (CAS No. 5392-40-5) in F344/N rats and B6C3F1 mice (feed studies). NTP TR-505. Natl. Toxicology Program, Research Triangle, N.C.
NTP. 2002. Draft report: Toxicology and carcinogenesis studies of trans-cinnamaldehyde (microencapsulated) (CAS No.14371-10-9) in F344/N rats and B6C3F1 mice (feed studies). NTP TR 514. Natl. Toxicology Program, Research Triangle, N.C.
Smith, R.L. and Ford, R.A. 1993. Recent progress in consideration of flavoring ingredients under the Food Additives Amendment. 16. GRAS Substances. Food Technol. 47: 104-117.
Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Munro, I.C., Newberne, P.M., Portoghese, P.S., Waddell, W.J., Wagner, B.M., and Adams, T.B. 2002a. The FEMA GRAS assessment of pyrazine derivatives used as flavor ingredients. Food Chem. Toxicol. 40: 429-451.
Smith, R.L., Adams, T.B., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Portoghese P.S., Waddell, W.J., Wagner, B.M., Rogers, A.E., Caldwell, J., and Sipes, I.G. 2002b. Safety assessment of allylalkoxybenzene derivatives used as flavoring substances—Methyleugenol and estragole. Food Chem. Toxicol. 40: 851-870.
Stofberg, J. and Grundschober, F. 1987. Consumption ratio and food predominance of flavoring materials. Perfumer Flavorist 12: 27.
Viswalingam, A., Caldwell, J., Fournel, S., Schladt, L., and Oesch, F. 1988. Hepatic effects of cinnamyl anthranilate resemble those of a peroxisome proliferator in mouse, but not in rat. Brit. J. Pharmacol. 93: 32.
Waddell, W.J. 2002. Thresholds of carcinogenicity of flavors. Toxicol. Sci. 68: 275-279.
Wester, P.W. and Kroes, R. 1988. Forestomach carcinogens: Pathology and relevance to man. Toxicol. Pathol. 16: 165-171.
