GRAS Flavoring Substances 23
The 23rd publication by the FEMA Expert Panel presents safety and usage data on 174 new generally recognized as safe flavoring ingredients.
The Expert Panel of the Flavor and Extract Manufacturers Association continues to perform its primary function of evaluating the safety of flavoring substances under the conditions of intended use.
For more than four decades, the FEMA Expert Panel has maintained a safety evaluation program to respond to the provision in the 1958 Food Additives Amendment to the Federal Food, Drug, and Cosmetic Act—Public Law 85-929, 72 Stat. 1784 (1958), codified at 21 USC Sec. 348 (1988)—that exempted from food additive status those substances “generally recognized, among experts qualified by scientific training and experience to evaluate its safety, as having been adequately shown through scientific procedures … to be safe under the conditions of its intended use.” Substances “generally recognized as safe” (GRAS) by the FEMA Expert Panel under the conditions of their intended use are not considered to be food additives and are excluded from mandatory premarket approval by the U.S. Food and Drug Administration.
In May 2010, the FEMA Expert Panel will have completed 50 years of continuous operation evaluating the safety of flavoring substances. During this time, the Panel has rigorously supported the meaning and intent of the GRAS provision. First, the Panel has been and is now composed of well-recognized experts from academic scientific disciplines that are related to the safety evaluation of flavoring substances. The disciplines of toxicology, pathology, biochemistry, organic chemistry, pharmacology, and medicine are well represented on the Panel. Second, the Panel members are not only well-recognized in their respective disciplines but they are experienced in applying that expertise and scientific judgment to the safety evaluation of flavor ingredients. Currently, the duration of membership on the Panel is in the range from two to more than 25 years, with a mean Panel tenure of 11 years. Third, consistent with the “generally recognized as safe” evaluation process, the Panel regularly publishes its GRAS decisions in the peer-reviewed literature. To this end, the Panel not only publishes articles identifying substances newly determined as GRAS (e.g., Newberne et al., 2000; Smith et al., 2001, 2003, 2005), but also publishes the scientific data supporting the GRAS determination for these substances (Adams et al., 1996, 1997, 1998, 2002, 2004, 2005a, b, c, 2007; Newberne et al., 1999; Smith et al., 2002). In addition, the Panel periodically evaluates and publishes the criteria and scientific procedures it applies in reaching its GRAS decisions (Hall and Oser, 1977; Woods and Doull, 1991; Smith et al., 2005a, b).
In 1996, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) initiated a systematic program to evaluate the safety of flavoring substances. Based on the evaluation of the available safety data, JECFA has reached conclusions consistent with those of the Panel; namely, that more than 1,700 GRAS flavoring substances are considered “no safety concern under current conditions of intake.” In a similar program in the European Union begun in 2001, the European Food Safety Authority has reached similar conclusions on substances structurally related to flavoring substances evaluated by JECFA.
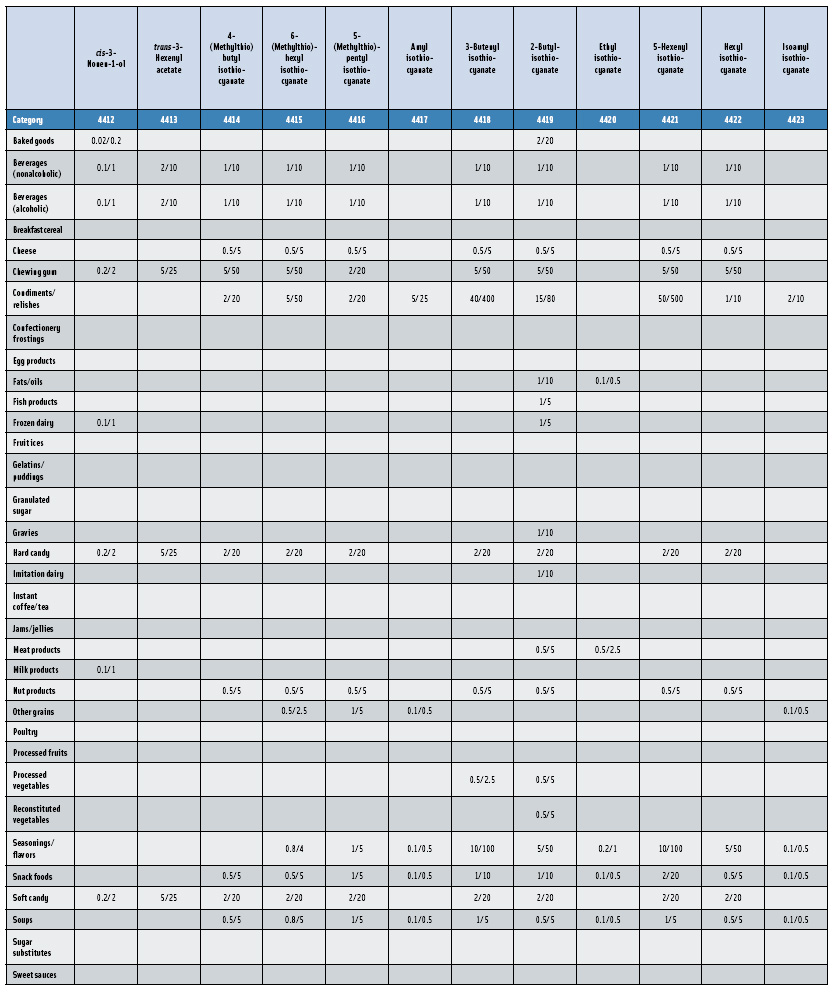
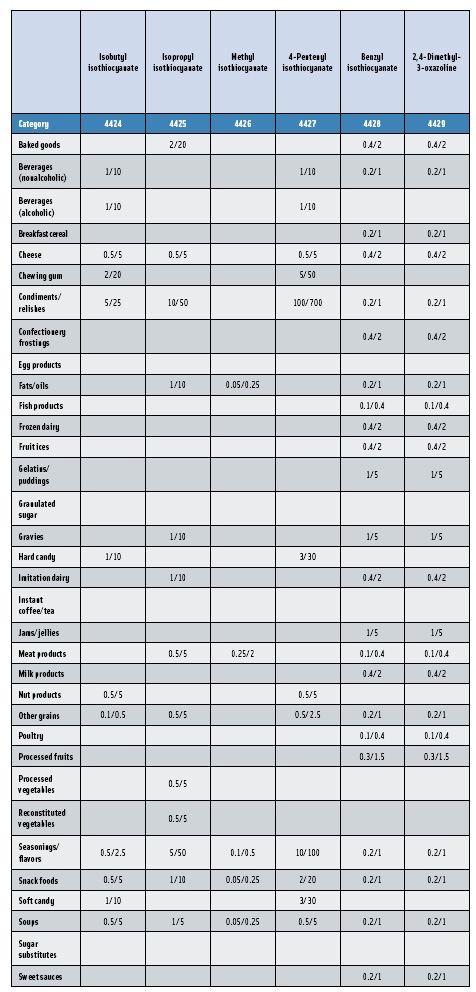
Further confirmation of the key role that the Expert Panel plays in the global safety evaluation of flavoring substances is that non-U.S. flavor companies and trade associations have reached out to the Panel for its scientific evaluation expertise. Beginning in 2005, the Japanese Flavor and Fragrance Materials Association (JFFMA) joined with the International Organization of Flavor Industries (IOFI) and FEMA to sponsor the GRAS evaluations of more than 300 substances previously used in flavorings only in Japan and other Asian countries. The program’s goals are (1) to have Asian-specific flavor ingredients evaluated by the FEMA Expert Panel for GRAS status for use as flavoring substances in the United States; and (2) subsequently submit the data supporting the GRAS substances to JECFA for a second safety evaluation. As expected, some groups of flavor ingredients scheduled for review are specific to an Asian diet. For example, the Panel concluded that a group of substituted isothiocyanates (Nos. 4414–4427) used in “wasabi-type” flavors are GRAS.
--- PAGE BREAK ---
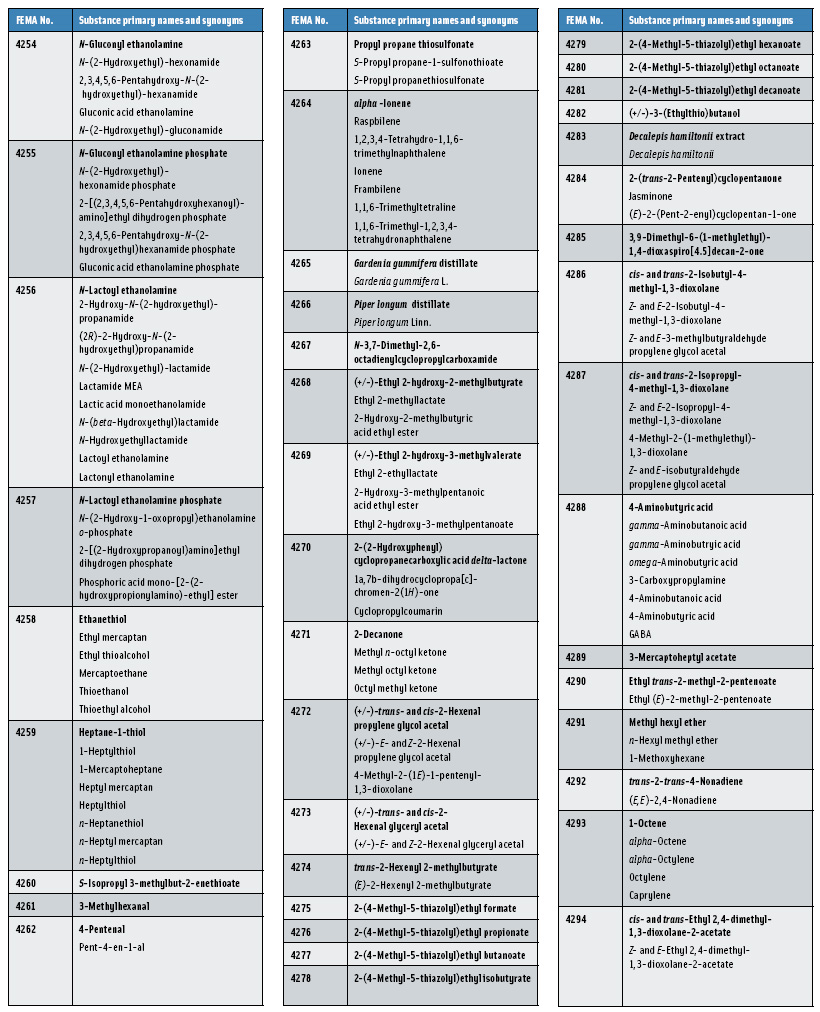
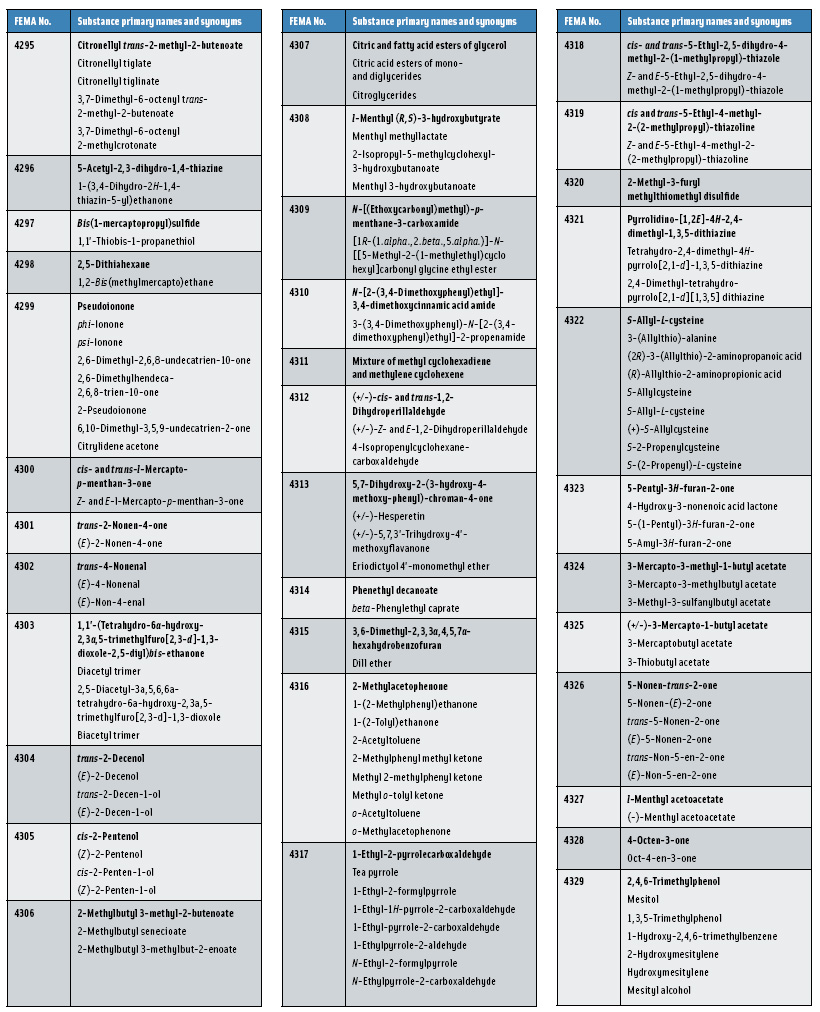
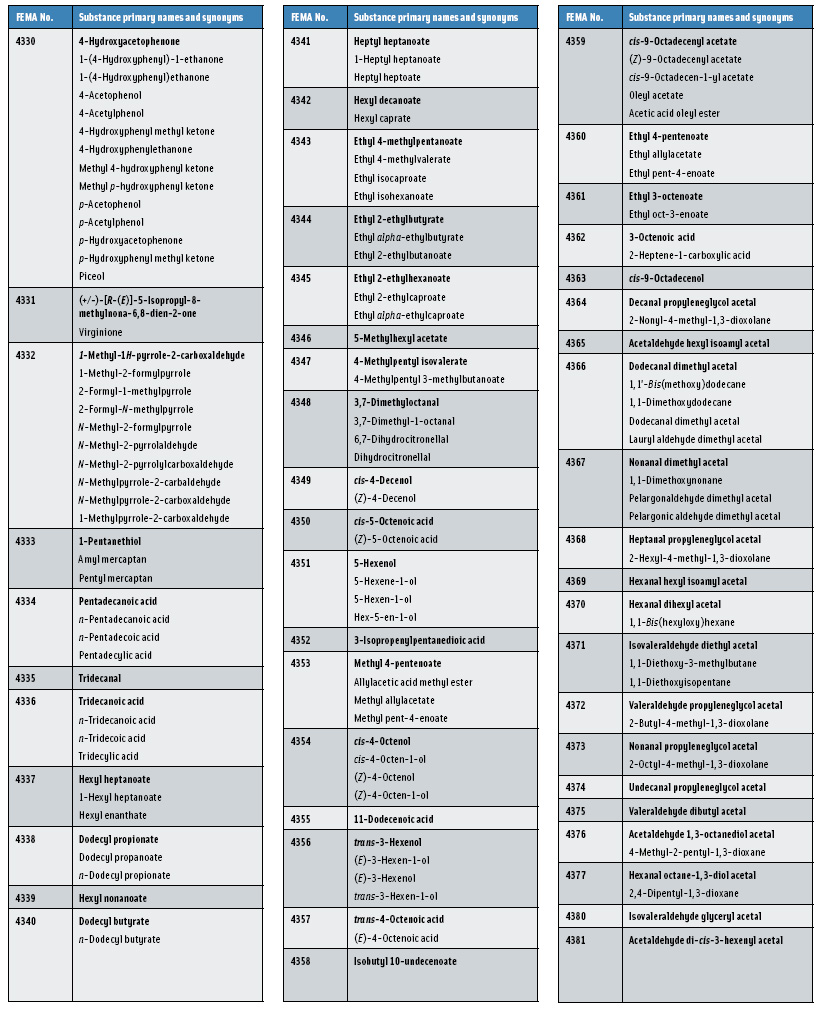
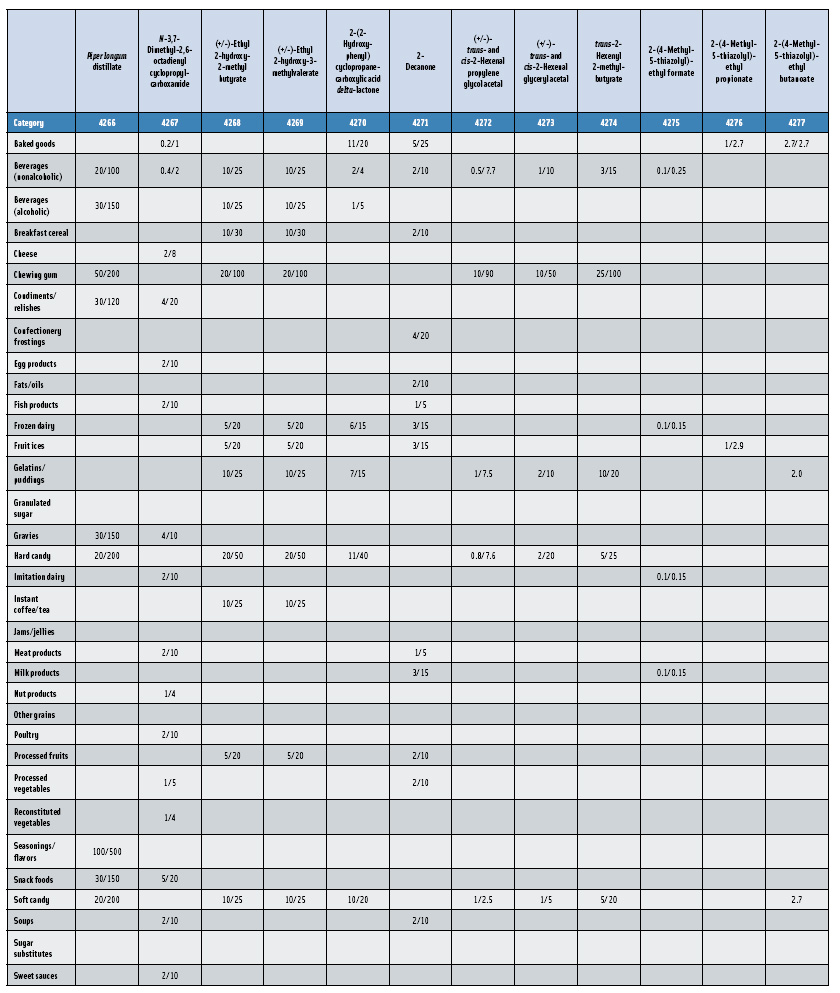
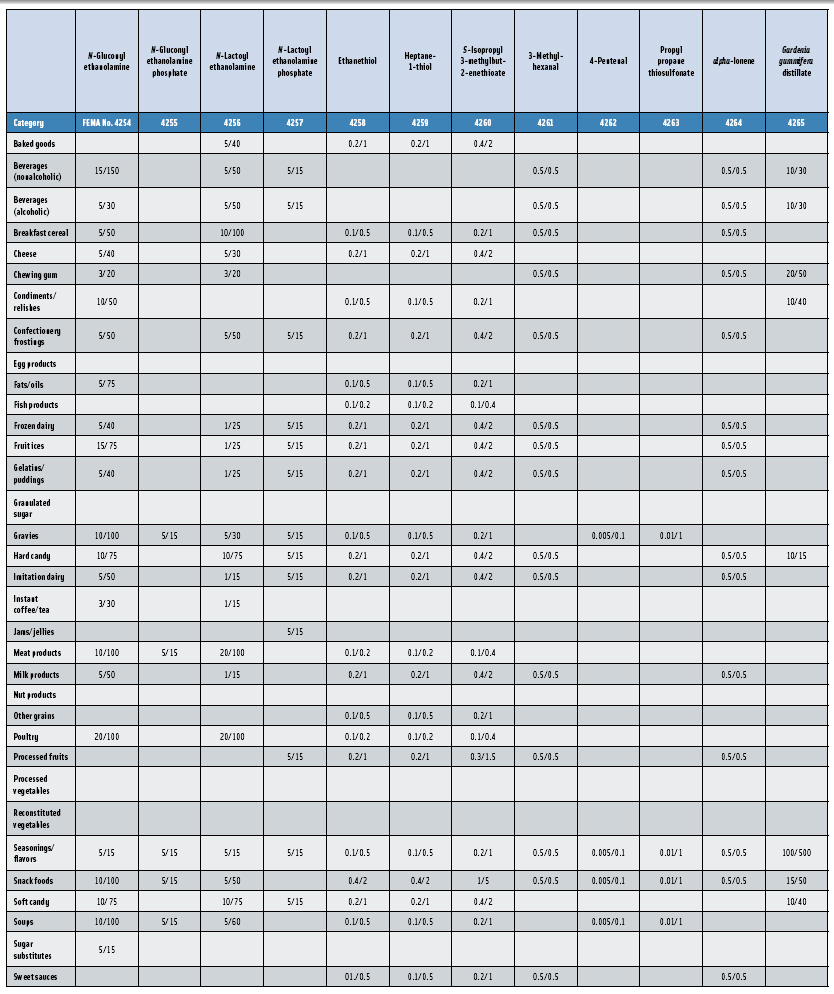
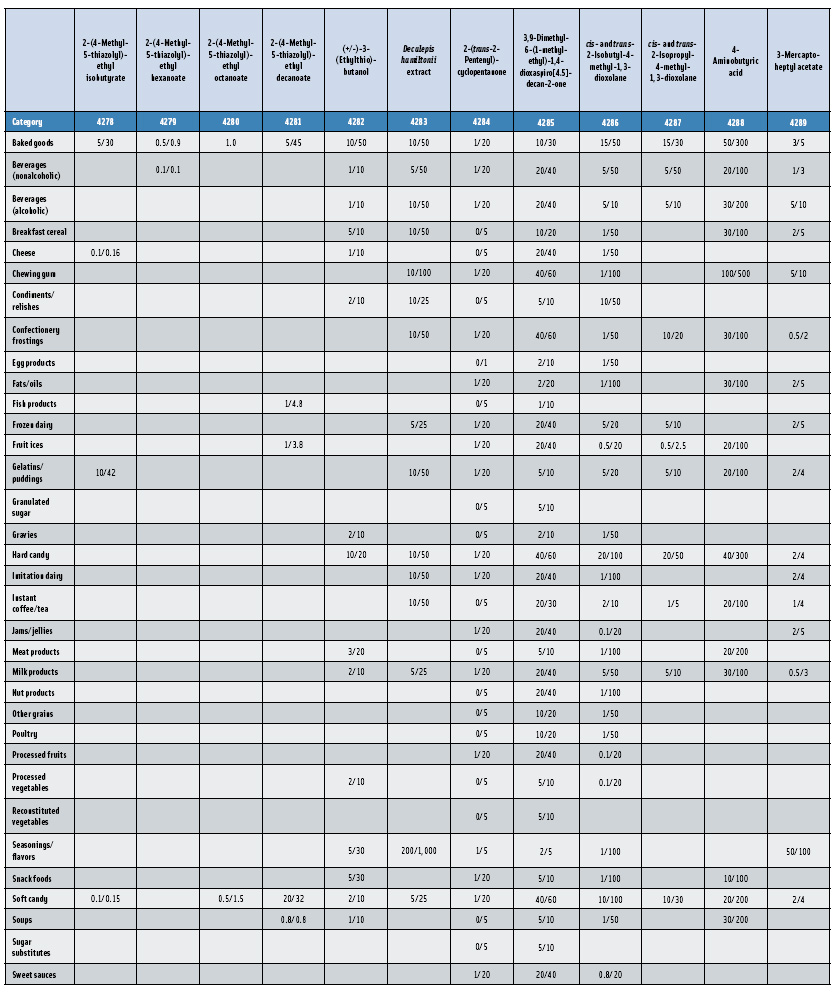
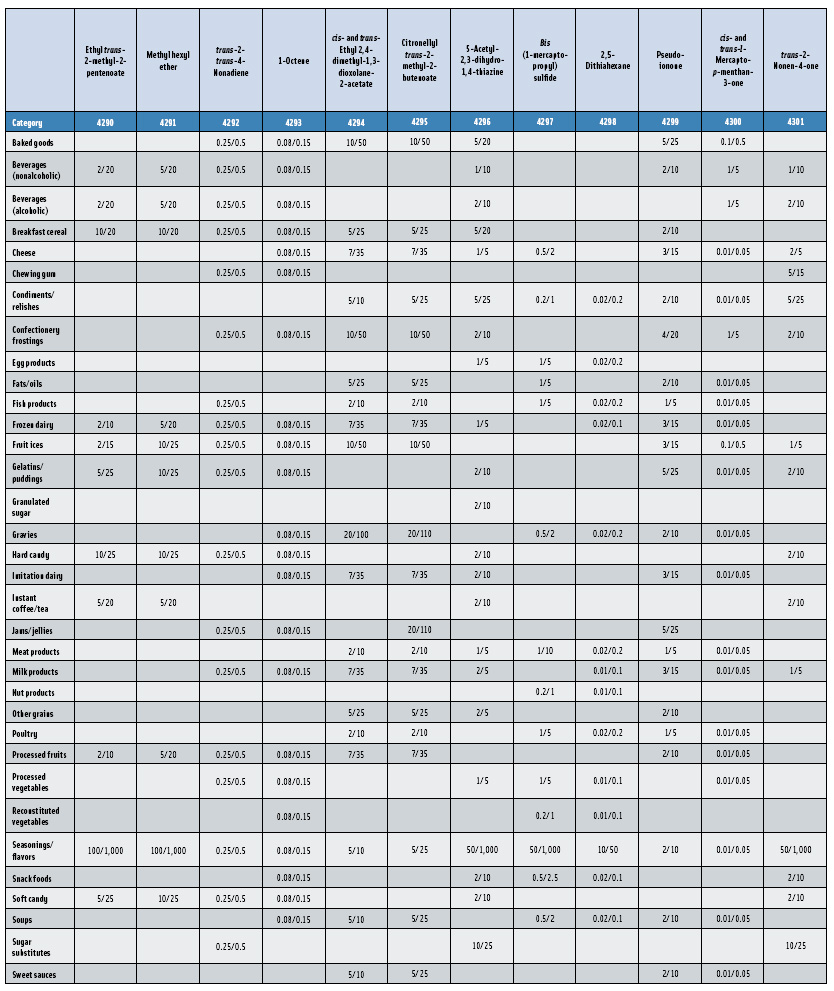
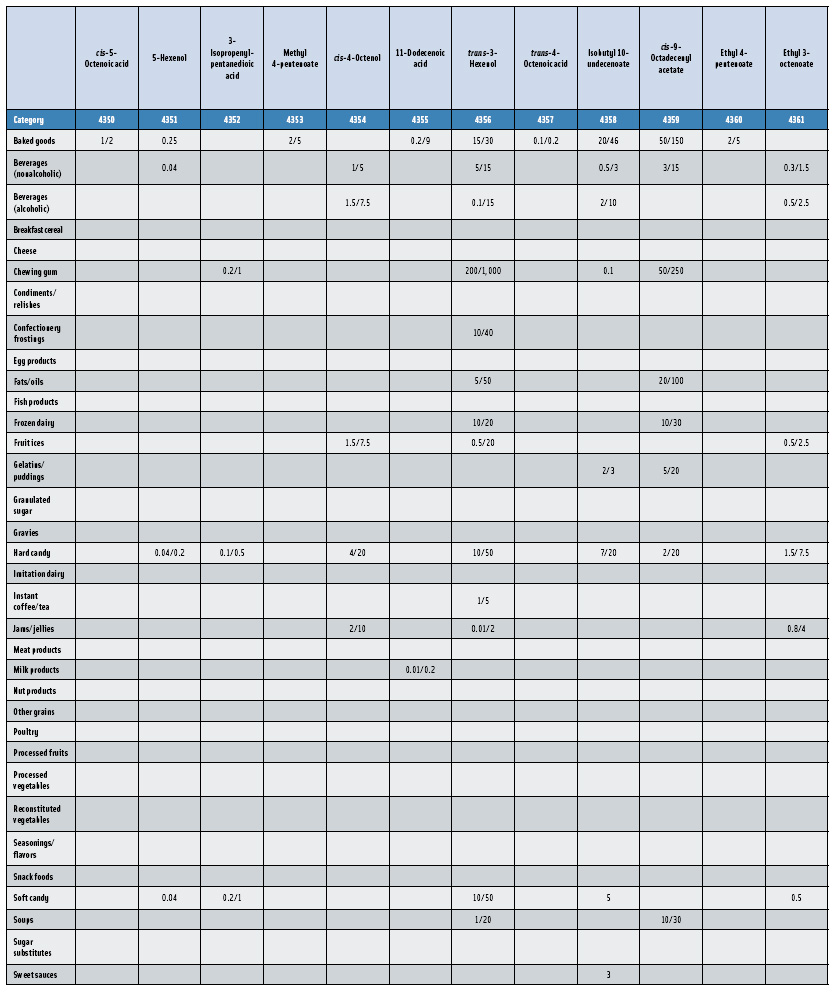
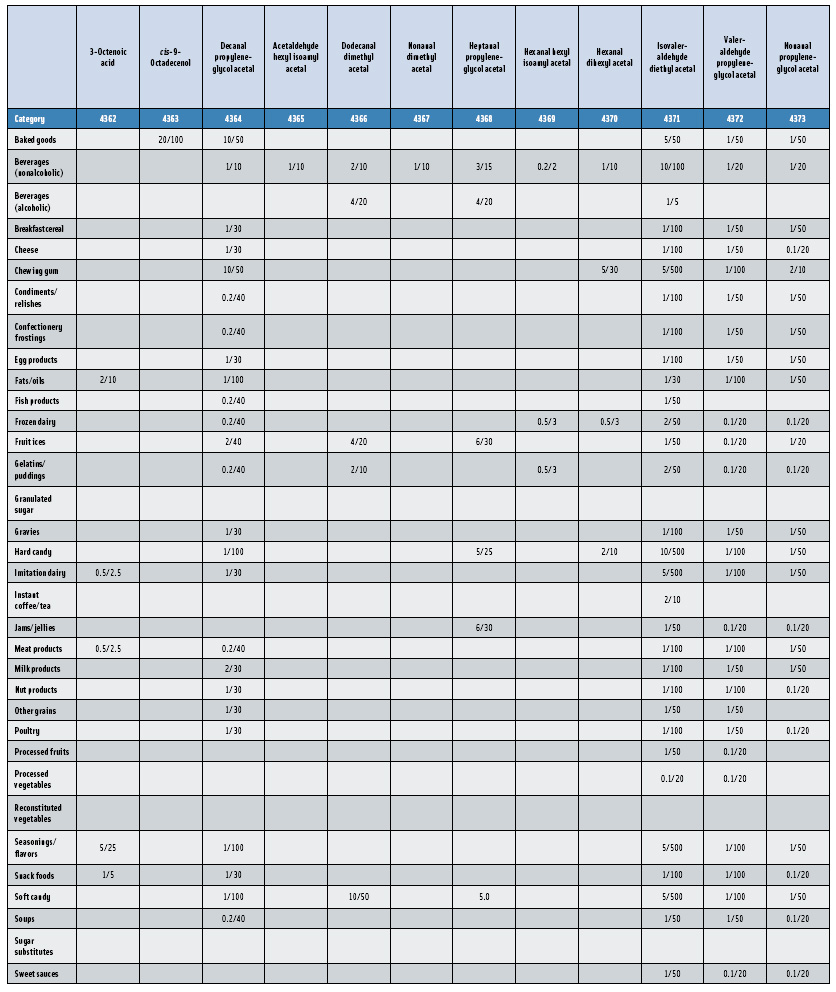
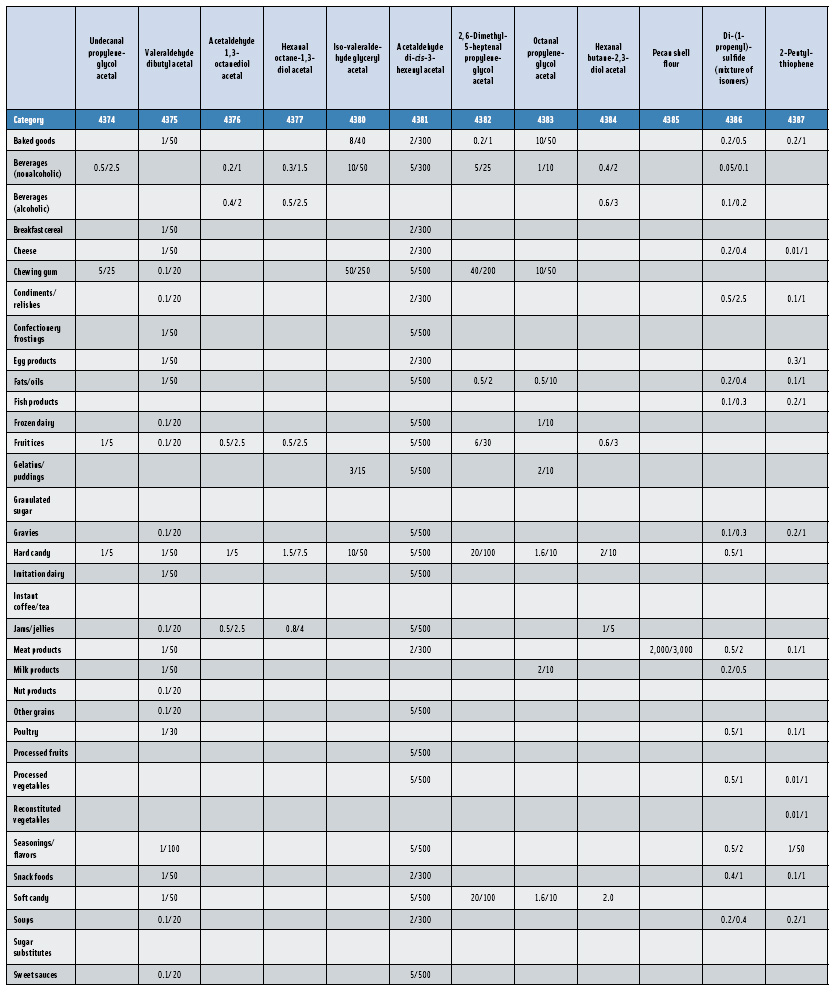
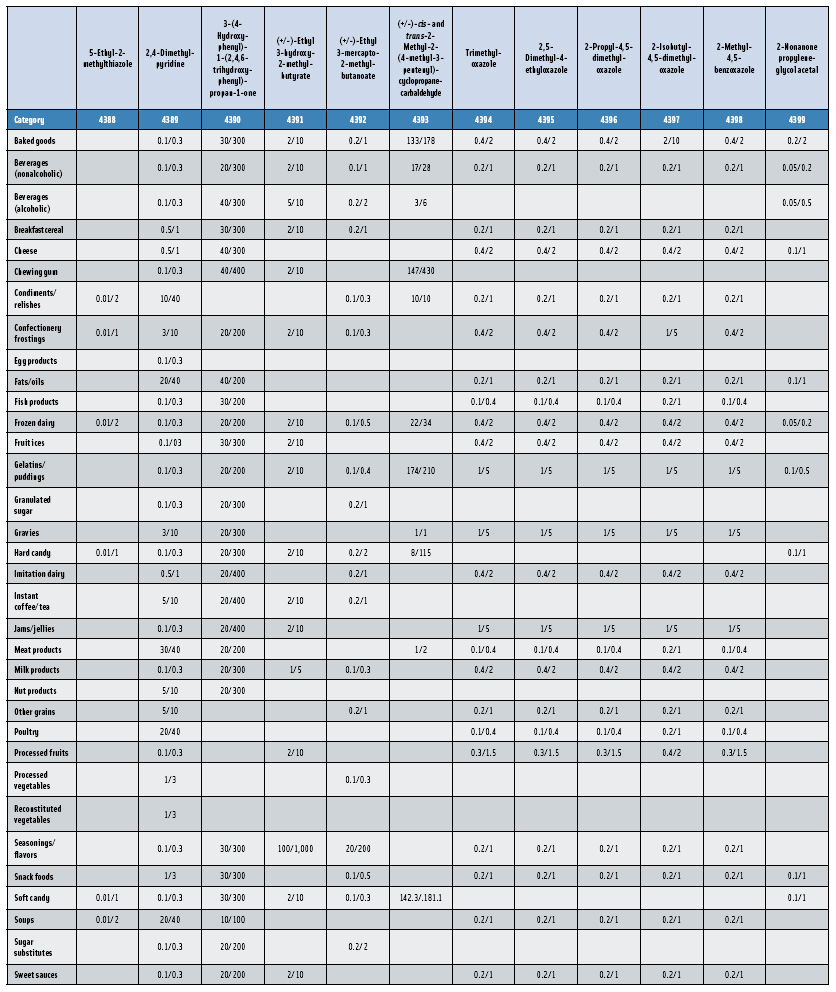
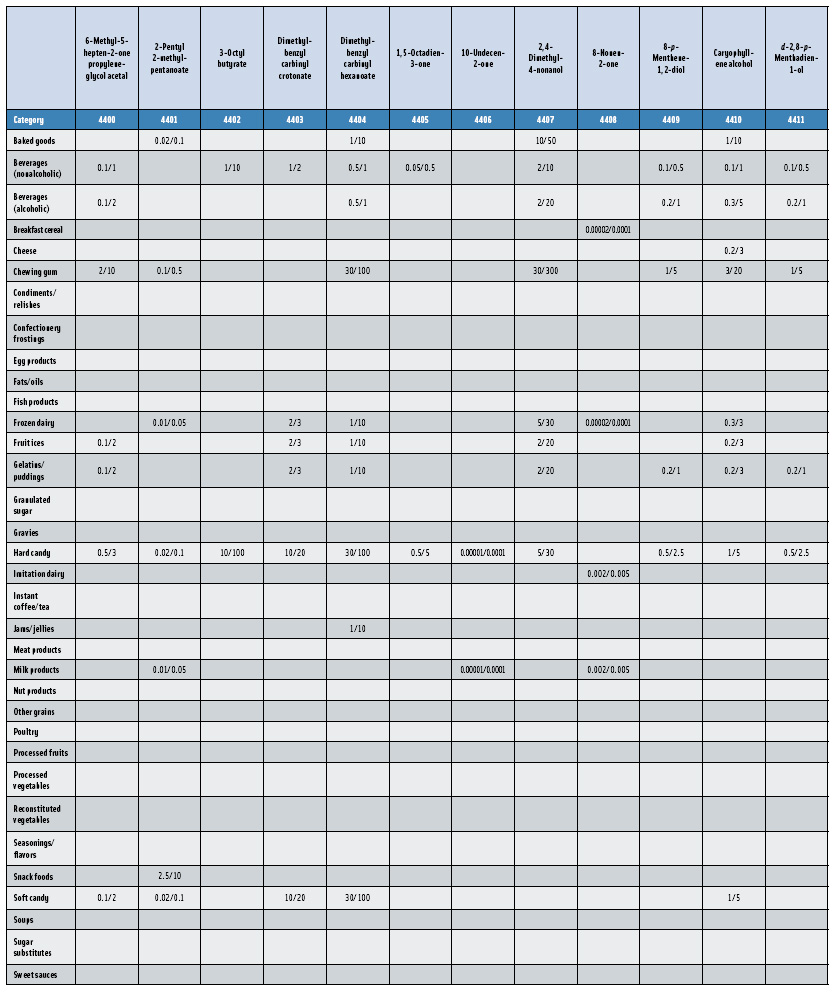
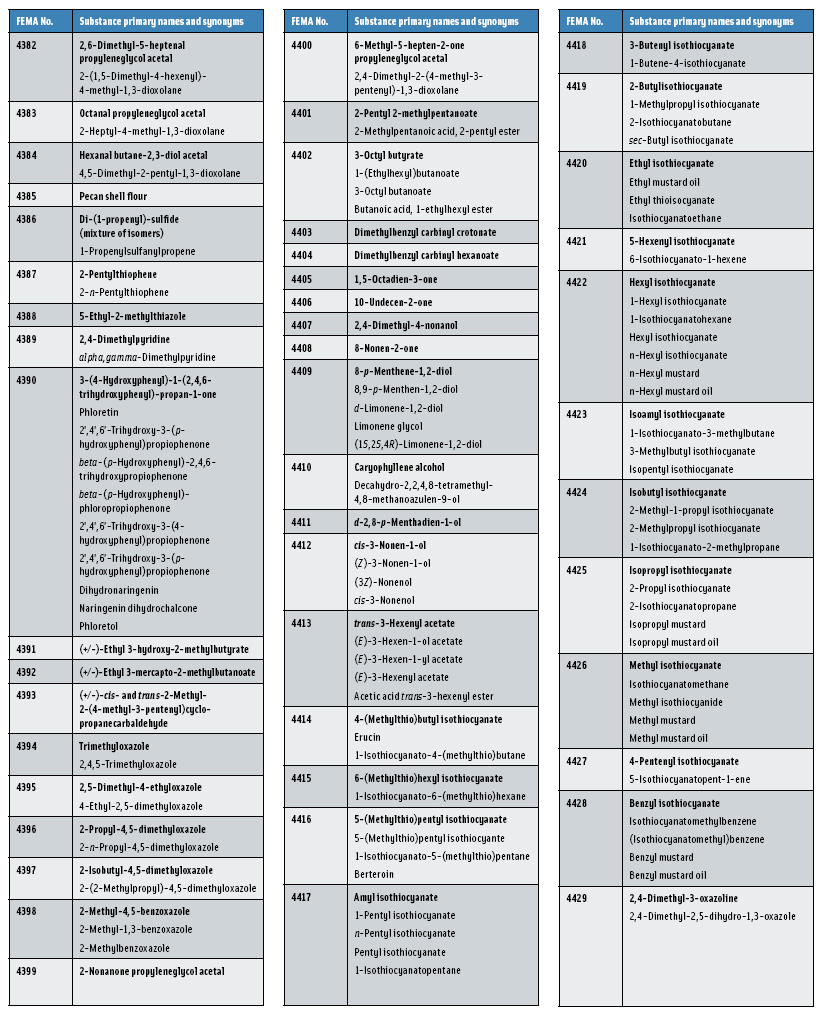 In this, the 23rd GRAS publication, 174 new GRAS flavoring substances—Nos. 4254–4429, except 4378–4379—are identified (Tables 1 and 2). In addition, the Panel determined that new use levels and food categories for five flavoring substances are consistent with their current GRAS status (Table 3). Of these 174 new flavoring substances, four are Natural Flavor Complexes (Nos. 4265, 4266, 4283, and 4385) while one (No. 4385) is a flavor carrier used in the preparation of finished food flavors. The Panel also comments on the expanding list of GRAS evaluations for substances with non-flavor functions that are added to finished flavorings.
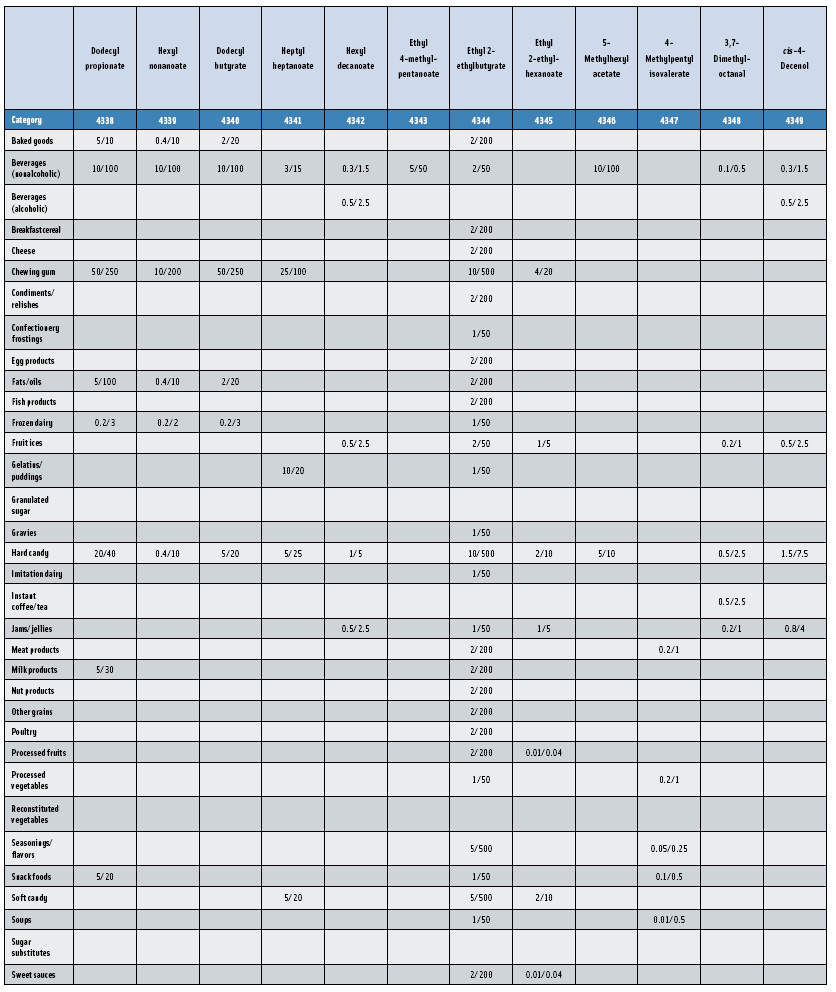
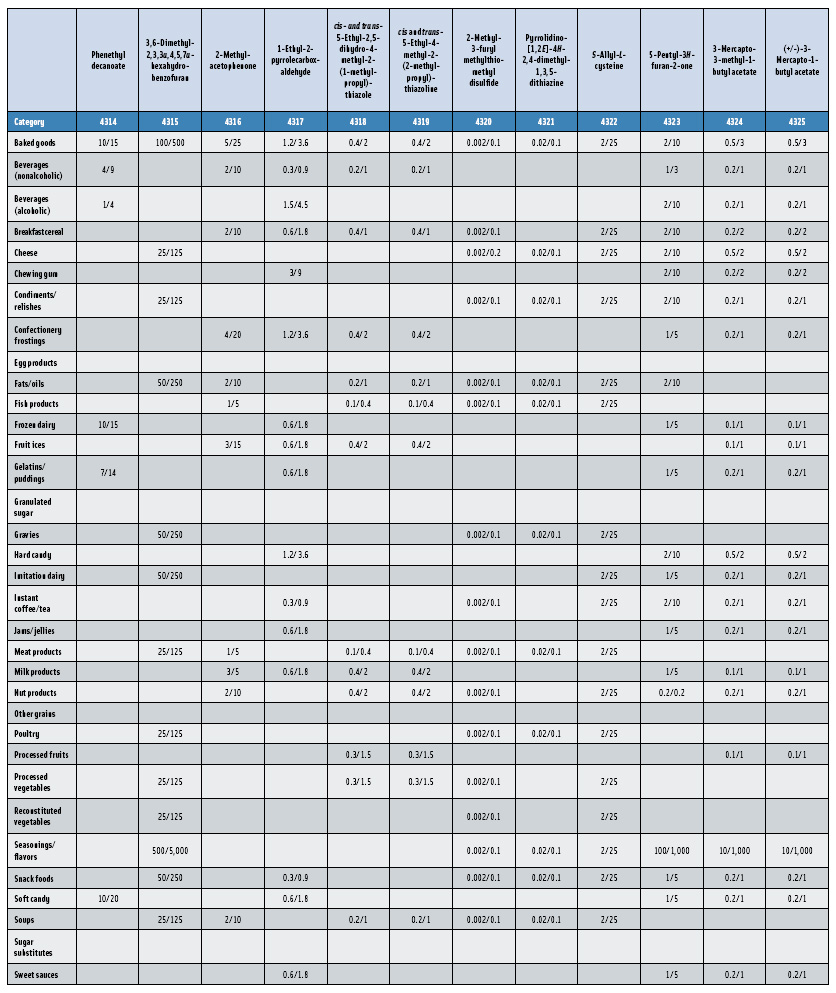
In this, the 23rd GRAS publication, 174 new GRAS flavoring substances—Nos. 4254–4429, except 4378–4379—are identified (Tables 1 and 2). In addition, the Panel determined that new use levels and food categories for five flavoring substances are consistent with their current GRAS status (Table 3). Of these 174 new flavoring substances, four are Natural Flavor Complexes (Nos. 4265, 4266, 4283, and 4385) while one (No. 4385) is a flavor carrier used in the preparation of finished food flavors. The Panel also comments on the expanding list of GRAS evaluations for substances with non-flavor functions that are added to finished flavorings.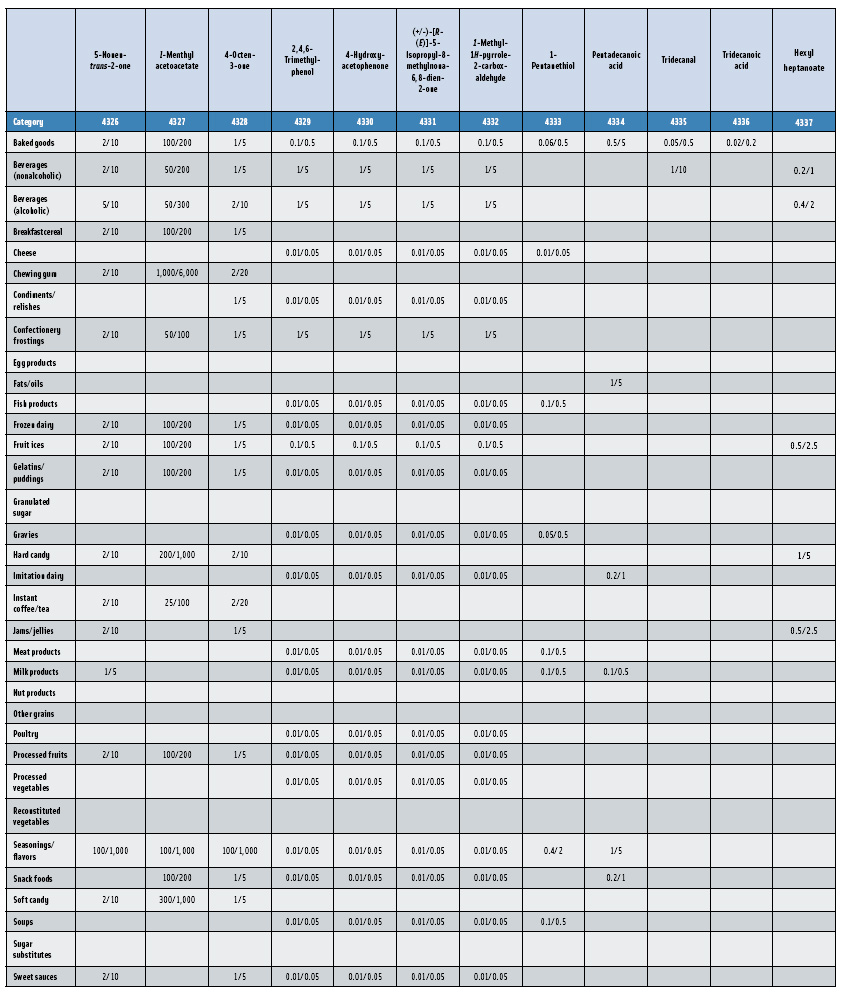
--- PAGE BREAK --- 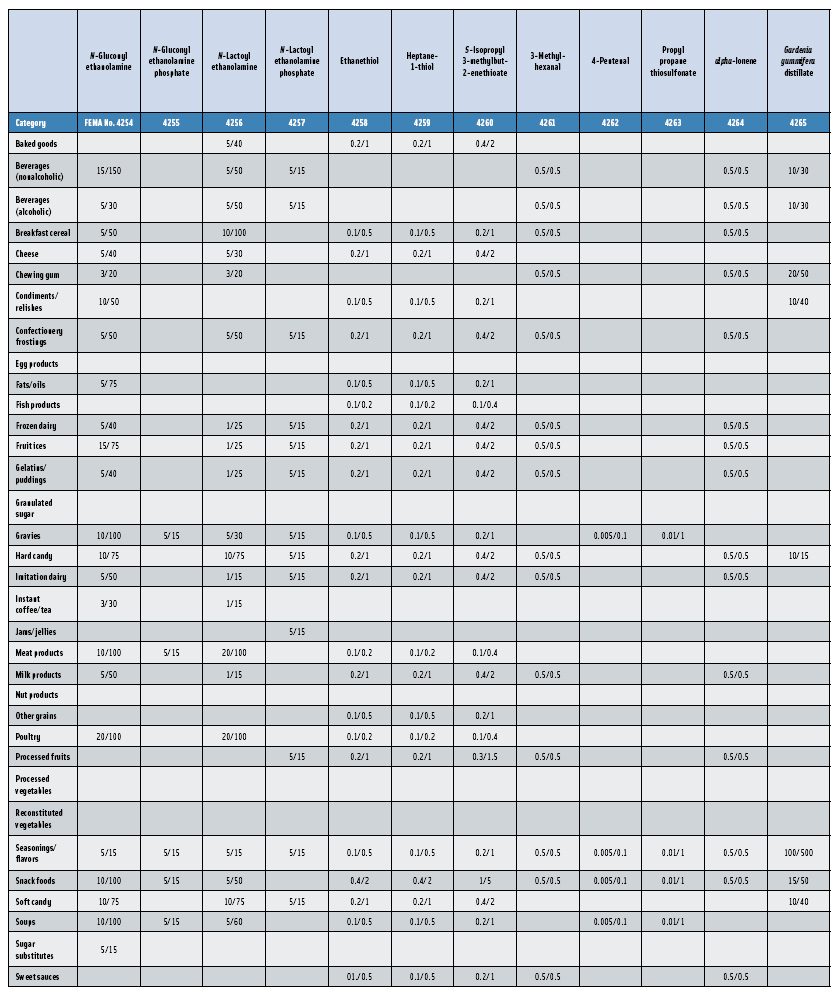

--- PAGE BREAK ---
FEMA GRAS Evaluation of Substances with Non-Flavor Function
Flavorings are typically mixtures of substances, most of which impart flavor (e.g., menthol and cinnamaldehyde) or, on a more limited basis, modify flavor (e.g., neohespiridin dihydrochalcone and (-)-homoeriodictyol, sodium salt). Compounded flavorings also include substances that act as preservatives (butylated hydroxyanisole, BHA), solvents (ethyl alcohol), encapsulating agents (beta-cyclodextrin), and emulsifiers (carrageenan) (Table 4). Often, substances that act as emulsifiers, solvents, and preservatives in the preparation of compounded flavors serve the same function in the food supply. In these instances, the Panel evaluates the substance for its GRAS status based strictly on its intended use as a component of a food flavor. In order to complete the GRAS evaluation, the applicant must demonstrate that the substance provides the specified function in flavors under conditions and at levels of use that do not serve other non-flavor functions in the finished food. For example, neohesperidin dihydrochalcone (FEMA No. 3811) provides a sweetening effect at levels >300 ppm in a finished food product. However, it was recently evaluated by the Panel and received GRAS status to be added as a flavor modifier at 30 ppm, where it does not display a sweetening effect. Fundamentally, these substances are GRAS for their function in modifying, preserving, emulsifying, etc., the flavoring that is added to food. They are not GRAS for their direct addition to modify, preserve, emulsify, etc., the food. Based on the fact that flavorings are added to food in such minute quantities, normally 1% or less, the function of the substances added to flavorings occurs at a concentration that is far below that required to exert the same function in food.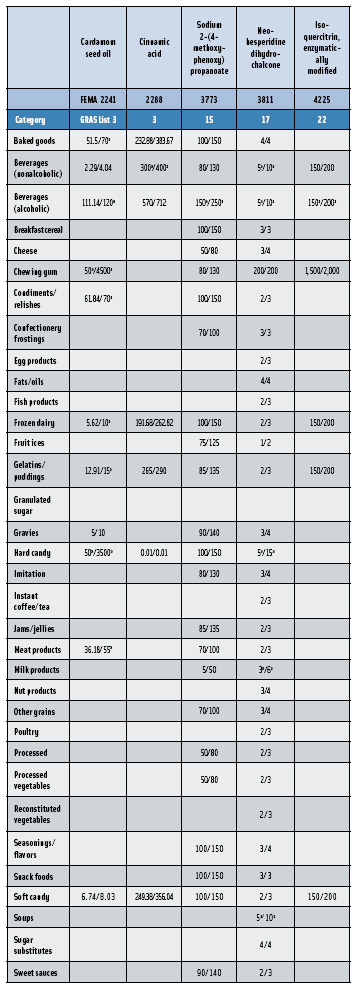
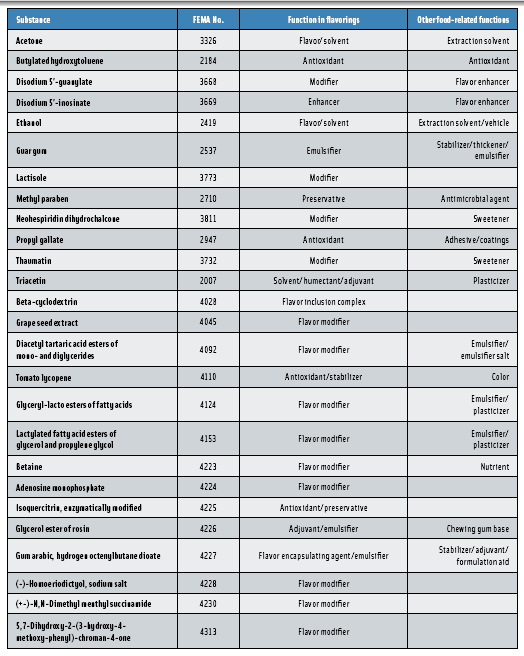 This criterion is entirely consistent with Section 201(s) of the Federal Food, Drug, and Cosmetic Act, which states that in order for a substance to be considered GRAS it must be “safe under conditions of intended use.” It is also consistent with past Panel decisions in which substances possessing non-flavor functions (solvents, modifiers, antioxidants, etc.) have been recognized as GRAS for their intended use in compounded flavors (Table 4). For example, methyl paraben (FEMA No. 2710) is FEMA GRAS for its intended use as a preservative for flavorings. It is not GRAS for its use in food as an antimicrobial agent.
This criterion is entirely consistent with Section 201(s) of the Federal Food, Drug, and Cosmetic Act, which states that in order for a substance to be considered GRAS it must be “safe under conditions of intended use.” It is also consistent with past Panel decisions in which substances possessing non-flavor functions (solvents, modifiers, antioxidants, etc.) have been recognized as GRAS for their intended use in compounded flavors (Table 4). For example, methyl paraben (FEMA No. 2710) is FEMA GRAS for its intended use as a preservative for flavorings. It is not GRAS for its use in food as an antimicrobial agent.
 Anticipated Annual Volumes of Use for New Flavoring Substances
Anticipated Annual Volumes of Use for New Flavoring Substances
In its early years of operation, the Expert Panel evaluated the safety of approximately 1,000 flavoring substances that had been in use prior to the 1958 GRAS regulation. This created an initial list of flavorings that was published in the peer-reviewed literature in 1965 (Hall and Oser, 1965). Under contract to the U.S. FDA, the National Academy of Sciences-National Research Council (NAS-NRC)(NAS, 1970, 1975, 1982 and 1987) and later FEMA (Lucas et al., 1999) conducted a series of surveys on the use of FEMA GRAS flavoring substances and food additives used in the preparation of compounded flavors. These surveys collected data related to exposure, including the volume of flavoring substances that disappeared into the U.S. food supply on an annual basis. Together with a more comprehensive analysis of the intake of a restricted number of flavoring substances (Hall and Oser, 1977), these data were the basis for estimating the exposure of U.S. consumers to these flavoring substances.
--- PAGE BREAK ---
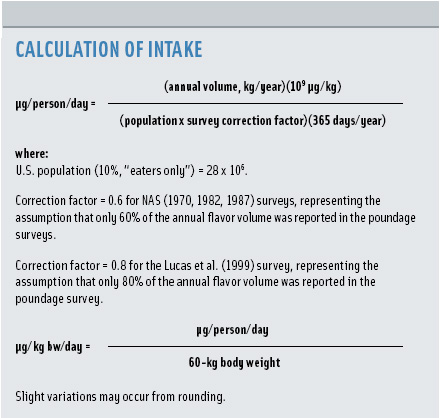
When a new flavoring substance is developed and evaluated for GRAS under conditions of intended use, there is no survey data available upon which to initially estimate exposure. In the absence of such data and until a survey is performed, the Expert Panel requires the company applying for GRAS status for a flavoring substance to provide anticipated poundage for the substance that the company expects to sell into the food and beverage market during the first year. The applicant company is also asked to provide data on potential patterns of use of the flavoring substance in different food categories (e.g., baked goods, soft candy, seasonings, and flavors) and concentrations at which the substance is detected and tolerated by a taste test panel. These data, in conjunction with data on biological and chemical properties of the substance and metabolism and toxicity for the GRAS candidate and structurally related substances, serve the Expert Panel in its safety assessment (Smith et al., 2005b).
In order to assess the relationship of anticipated annual poundage information to actual survey reported poundage data, anticipated poundage reported by GRAS applicants for flavoring agents from FEMA GRAS Lists 6–19 was compared with the results of NAS and FEMA industry-wide poundage surveys performed during the 25-year period from 1970 to 1995. From this analysis, it is apparent that in the vast majority of cases, anticipated poundage (i.e., the amount of a flavoring substance that a flavor company expects to sell in the first year of use) is not realized. Approximately 84% (296/351) of the materials surveyed had reported poundage that averaged less than the anticipated poundage reported in the GRAS application, while roughly 15% (51/351) of the materials averaged more. Therefore, for the vast majority (84%) of flavoring agents the anticipated poundage is an overestimation. Since these anticipated poundages are used to calculate the per capita intake times 10 (PCI x 10) values until surveyed poundage is reported, these PCI x 10 values are, in most cases, overestimates of intake. Of the overestimates, more than one-third of the materials have annual poundage of less than 1 kg reported in recent surveys. For another third of the flavorings substances, anticipated volumes overestimate actual poundage by greater than 90%.
Less than 10% (32/351) of the substances surveyed showed anticipated poundages that underestimated by two-fold or greater the reported annual volumes from later surveys. However, in all of these cases, the anticipated and survey-reported poundages were within the same order of magnitude, providing confidence that intakes calculated using either data would not be significantly different and still provide enormous margins of safety (10,000–1,000,000) when compared to the no-observable-adverse effect level in relevant animal studies. Additionally, the majority of these substances are naturally occurring in traditional food, and intake from food sources far exceeds that from added flavor use.
These data clearly demonstrate that for the vast majority (84%) of FEMA GRAS flavoring substances, the use of anticipated poundage to calculate PCI x 10 values is indeed a highly conservative approach. With the additional assumption that the company applying for GRAS status for a material will represent, at best, 60% of the volume of any material sold into the marketplace, the estimated daily per capita intakes calculated from these numbers are highly inflated and present an additional safety factor. As poundage surveys update the information available for flavoring substances, a more accurate estimate of intake is achieved, but in the interim the anticipated poundage results in a considerable margin of safety.
--- PAGE BREAK ---
FEMA GRAS Lists published in Food Technology, in chronological order
Hall, R.L. 1960. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. Food Technol. 14: 488-495.
Hall, L. and Oser, B.L. 1961. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. II. Food Technol. 15(12): 20, 22-26.
Hall, R.L. and Oser, B.L. 1965. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 3. GRAS substances. Food Technol. 19(2, Part 2): 151-197.
Hall, R.L. and Oser, B.L. 1970. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 4. GRAS substances. Food Technol. 24(5): 25-34.
Oser, B.L. and Hall, R.L. 1972. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 5. GRAS substances. Food Technol. 26(5): 35-42.
Oser, B.L. and Ford, R.A. 1973a. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 6. GRAS substances. Food Technol. 27(1): 64-67.
Oser, B.L. and Ford, R.A. 1973b. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 7. GRAS substances. Food Technol. 27(11): 56-57.
Oser, B.L. and Ford, R.A. 1974. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 8. GRAS substances. Food Technol. 28(9): 76-80.
Oser, B.L. and Ford, R.A. 1975. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 9. GRAS substances. Food Technol. 29(8): 70-72.
Oser, B.L. and Ford, R.A. 1977. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 10. GRAS substances. Food Technol. 31(1): 65-74.
Oser, B.L. and Ford, R.A. 1978. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 11. GRAS substances. Food Technol. 32(2): 60-70.
Oser, B.L. and Ford, R.A. 1979. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 12. GRAS substances. Food Technol. 33(7): 65-73.
Oser, B.L., Ford, R.A., and Bernard, B.K. 1984. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 13. GRAS substances. Food Technol. 38(10): 66-89.
Oser, B.L., Weil, C.L., Woods, L.A., and Bernard, B.K. 1985. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 14. GRAS substances. Food Technol. 39(11): 108-117.
Burdock, G.A., Wagner, B.M., Smith, R.L., Munro, I.C., and Newberne, P.M. 1990. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 15. GRAS substances. Food Technol. 44(2): 78,80, 82, 84, 86.
Smith, R.L. and Ford, R.A. 1993. Recent progress in the consideration of flavoring ingredients under the Food Additives Amendment. 16. GRAS substances. Food Technol. 47(6): 104-117.
Smith, R.L., Newberne, P., Adams, T.B., Ford, R.A., Hallagan, J.B., and the FEMA Expert Panel. 1996a. GRAS flavoring substances 17. Food Technol. 50(10): 72-78, 80-81.
Smith, R.L., Newberne, P., Adams, T.B., Ford, R.A., Hallagan, J.B., and the FEMA Expert Panel. 1996b. Correction to GRAS flavoring substances 17. Food Technol. 51(2): 32.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Hallagan, J.B., and Ford, R.A. 1998. GRAS flavoring substances 18. Food Technol. 52(9): 65-66, 68, 70, 72, 74, 76, 79- 92.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Hallagan, J.B., and Ford, R.A. 1999. Correction to GRAS flavoring substances 18. Food Technol. 53(3): 104.
Newberne, P., Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Waddell, W.J., Wagner, B.M., Weil, C.S., Adams, T.B., and Hallagan, J.B. 2000. GRAS flavoring substances 19. Food Technol. 54(6): 66, 68-69, 70, 72-74, 76-84.
Smith, R.L., Doull, J., Feron, V.J., Goodman, J.I., Munro, I.C., Newberne, P.M., Portoghese, P.S., Waddell, W.J., Wagner, B.M., Adams, T.B., and McGowen, M.M. 2001. GRAS flavoring substances 20. Food Technol. 55(12): 34-36, 38, 40, 42, 44-55.
Smith, R.L., Cohen, S.M., Doull, J., Feron, V.J., Goodman, J.I., Marnett, I.J., Portoghese, P.S., Waddell, W.J., Wagner, B.M., and Adams, T.B. 2003. GRAS flavoring substances 21. Food Technol. 57(5): 46-48, 50, 52-54, 56-59.
Smith, R.L., Cohen, S.M., Doull, J., Feron, V.J., Goodman, J.I., Marnett, I.J., Portoghese, P.S., Waddell, W.J., Wagner, B.M., and Adams, T.B. 2005. GRAS flavoring substances 22. Food Technol. 59(8): 24-28, 31-32, 34, 36-62.
CORRECTION & CHANGES
In Table 3 of “GRAS Flavoring Substances 19” (Newberne et al., 2000), the synonym for FEMA No. 3933 was incorrectly listed as trans-2-hexenyl propionate. The correct synonym is cis-2-hexenyl propionate.
Jay I. Goodman, Professor of Pharmacology and Toxicology at Michigan State University, retired from the FEMA Expert Panel in October 2005 after 10 years of continuous dedicated service.
Ivonne M.C.M. Rietjens, Professor of Biochemistry and Food Toxicology at Wageningen University, joined the Panel in January 2006.
Victor J. Feron, Professor Emeritus in the Dept. of Biological Toxicology at Utrecht University, retired from the Panel in July 2006 after more than seven years of continuous dedicated service.
William J. Waddell, Chairman of the FEMA Expert Panel, is Professor and Chair, Emeritus, Dept. of Pharmacology and Toxicology, University of Louisville School of Medicine, Louisville, Ky. Other members of the FEMA Expert Panel are Samuel M. Cohen, Professor, Dept. of Pathology and Microbiology, University of Nebraska Medical Center, Omaha; Victor J. Feron, TNO Quality of Life, Professor Emeritus, Biological Toxicology, Utrecht University, Zeist, The Netherlands; Jay I. Goodman, Professor, Dept. of Pharmacology and Toxicology, Michigan State University, East Lansing; Lawrence J. Marnett, Dept. of Biochemistry, Vanderbilt Institute Center in Molecular Toxicology, School of Medicine, Vanderbilt University, Nashville, Tenn.; Philip S. Portoghese, Professor, College of Pharmacy, University of Minnesota, Minneapolis; Ivonne M.C.M. Rietjens, Professor and Chair, Dept. of Toxicology, Wageningen University, Wageningen, The Netherlands; and Robert L. Smith, Professor, Molecular Toxicology, Imperial College School of Medicine, University of London, United Kingdom.
Timothy B. Adams is the Scientific Secretary for the FEMA Expert Panel and Scientific Director of the Flavor and Extract Manufacturers Association, 1620 I St., N.W., Suite 925, Washington DC 20006. Christie Lucas Gavin is Director of Health and Safety at FEMA, and Margaret M. McGowen and Michelle C. Williams are Staff Scientists at FEMA. Send reprint requests to author Adams ([email protected]).
References
Adams, T.B., Hallagan, J.B., Putman, J.M., Gierke, T.L., Doull, J., Munro, I.C., Newberne, P.M., Portoghese, P.S., Smith, R.L., Wagner B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1996. The FEMA GRAS assessment of alicyclic substances used as flavor ingredients. Food Chem. Toxicol. 34: 763-828.
Adams, T.B., Doull, J., Goodman, J.I., Munro, I.C., Newberne, P.M., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford R.A. 1997. The FEMA GRAS assessment of furfural used as a flavor ingredient. Food Chem. Toxicol. 35: 739-751.
Adams, T.B., Greer, D.B., Doull, J., Munro, I.C., Newberne, P.M., Portoghese, P.S., Smith, R.L., Wagner, B.M., Weil, C.S., Woods, L.A., and Ford, R.A. 1998. The FEMA GRAS assessment of lactones used as flavor ingredients. Food Chem. Toxicol. 36: 249-278.
Adams, T.B., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Munro, I.C., Newberne, P.M., Portoghese, P.S., Smith, R.L., Waddell, W.J., and Wagner, B.M. 2002. The FEMA GRAS assessment of pyrazine derivatives used as flavor ingredients. Food Chem. Toxicol. 40: 429-451.
Adams, T.B., Cohen, S., Doul,l J., Feron, V.J., Goodman, J.I., Marnett, L.J., Munro, I.C., Portoghese, P.S., Smith, R.L., Waddell, W. J. and Wagner, B.M. 2004. The FEMA GRAS assessment of cinnamyl derivatives used as flavor ingredients. Food Chem. Toxicol. 42: 157-185.
Adams, T.B., Cohen, S.M., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Munro, I.C., Portoghese, P.S., Smith, R.L., Waddell, W.J., and Wagner, B.M. 2005a. The FEMA GRAS assessment of phenethyl alcohol, aldehyde, acid, and related acetals and esters used as flavor ingredients. Food Chem. Toxicol. 43: 1179-1206.
Adams, T.B., Cohen, S.M. Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Munro, I.C., Portoghese, P.S., Smith, R.L., Waddell, W.J., and Wagner, B.M. 2005b. The FEMA GRAS assessment of benzyl derivatives used as flavor ingredients. Food Chem. Toxicol. 43: 1207-1240.
Adams, T.B., Cohen, S.M., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Munro, I.C., Portoghese, P.S., Smith, R.L., Waddell, W.J., and Wagner, B.M. 2005c. The FEMA GRAS assessment of hydroxyl- and alkoxy-substituted benzyl derivatives used flavor ingredients. Food Chem. Toxicol. 43: 1241-1271.
Adams, T.B., McGowen, M.M., Williams, M.C., Cohen, S.M., Feron, V.J., Goodman, J.I., Marnett, L.J., Munro, I.C., Portoghese, P.S., Smith, R.L., and Waddell, W.J. 2007. The FEMA GRAS assessment of aromatic substituted secondary alcohols, ketones, and related esters used as flavor ingredients. Food Chem. Toxicol. 45: 171-201.
Hall, R.L. and Oser, B.L. 1977. Criteria employed by the Expert Panel of FEMA for the GRAS evaluation of flavoring substances. Food Chem. Toxicol. 15(5): 457-466.
Lucas C.D., Putnam J.M., and Hallagan J.B. 1999. Flavor and Extract Manufacturers Association (FEMA) of the United States 1995 poundage and technical effects update survey. FEMA, Washington D.C.
NAS. 1970. Poundage and technical effects update of substances added to food. Committee on Food Additives Survey Data, Food and Nutrition Board, Institute of Medicine, National Academy of Sciences, Washington, D.C.
NAS. 1975. Poundage and technical effects update of substances added to food. Committee on Food Additives Survey Data, Food and Nutrition Board, Institute of Medicine, National Academy of Sciences, Washington, D.C.
NAS. 1982, Poundage and technical effects update of substances added to food. Committee on Food Additives Survey Data, Food and Nutrition Board, Institute of Medicine, National Academy of Sciences, Washington, D.C.
NAS. 1987. Poundage and technical effects update of substances added to food. Committee on Food Additives Survey Data, Food and Nutrition Board, Institute of Medicine, National Academy of Sciences, Washington, D.C.
Newberne, P., Smith, R.L., Doull, J., Goodman, J.I., Munro, I.C., Portoghese, P.S., Wagner, B.M., Weil, C.S., Woods, L.A., Adams, T.B., Lucas, C.D., and Ford, R.A. 1999. The FEMA GRAS assessment of trans-anethole used as a flavoring substance. Food Chem. Toxicol. 37: 789-811.
Smith, R.L., Adams, T.B., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Portoghese, P. S., Waddell, W.J., Wagner, B.M., Rogers, A.E., Caldwell, J., and Sipes, I.G. 2002. Safety assessment of allylalkoxybenzene derivatives used as flavoring substances – methyl eugenol and estragole. Food Chem. Toxicol. 40: 851-870.
Smith, R.L., Cohen, S.M., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Portoghese, P.S., Waddell, W.J., Wagner, B.M., Hall, R.L., Higley, N.A., Lucas-Gavin, C., and Adams, T.B. 2005a. A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: essential oils. Food Chem. Toxicol. 43: 345-363.
Smith, R.L., Cohen, S.M., Doull, J., Feron, V.J., Goodman, J.I., Marnett, L.J., Munro, I.C., Portoghese, P.S., Waddell, W.J., Wagner, B.M., and Adams, T.B. 2005b. Criteria for the safety evaluation of flavoring substances by the Expert Panel of the Flavor and Extract Manufacturers Association. Food Chem. Toxicol. 43: 1141-1177.
Woods. L.A. and Doull, J. 1991. GRAS evaluation of flavoring substances by the Expert Panel of FEMA. Regulat. Toxicol. Pharmacol. 14: 48-58.
