Analyzing for Histamine in Seafood
FOOD SAFETY & QUALITY
Certain bacteria naturally occurring in the aquatic environment and marine fish produce histidine decarboxylase enzymes during their growth. The enzymes may react with free histidine, a naturally occurring amino acid in fish muscle, to form histamine and other biogenic amines, generally as a result of time and temperature abuse of harvested fish. Dietary consumption of fish with elevated histamine levels can have an adverse effect on humans.

Everyone is susceptible to histamine poisoning. Symptoms range from a slight tingling sensation in the mouth to flushing of the face and upper body, headache, palpitations, dizziness, swelling of the tongue, nausea, vomiting, diarrhea, and stomach pain. The symptoms occur within minutes to a few hours after consumption but are self-limiting in 24–36 hr, and recovery is complete. But, as David Green ([email protected]), Professor and Extension Leader in the Dept. of Food, Bioprocessing and Nutrition Sciences at North Carolina State University and Director of the Center for Marine Sciences and Technology Seafood Laboratory, told me, they certainly don’t make for a pleasant dining experience.
Histamine Poisoning and Its Cause
A significant cause of foodborne disease in the United States, histamine poisoning accounted for 7.5% of all foodborne disease outbreaks and 38% of all seafood-related foodborne illnesses reported to the Centers for Disease Control and Prevention in 1990–2003.
The illness is often referred to as scombrotoxin poisoning or scombroid fish poisoning, since the first case study—reported in the Edinburgh Medical Journal in 1830—involved the consumption of bonito, a member of the Scombridae family. The disease has been implicated in other scombroid fish such as tuna and mackerel, although non-scombroid species such as mahi mahi and bluefish are capable of supporting development of elevated levels of histamine when they are temperature abused. According to the Sea Grant brochure, Ice Your Fish (www.iceyourfish.sea-grant.org), fish most likely to cause scombrotoxin poisoning are amberjack, mahi mahi, mackerel, bluefish, herring, marlin, bonito, wahoo, tunas, jacks, and shad.
The bacteria most often associated with histamine development include Gram-negative bacteria such as Morganella morganii, Raoultella planticola, and Enterobacter aerogenes that naturally exist on the gills and in the gut of saltwater fish. When the fish die, their defense mechanisms no longer inhibit bacterial growth, and the bacteria start to grow and produce the enzymes capable of producing histamine in the flesh of the fish. Temperature control—keeping the fish chilled below 40°F at all times between catching and consumption—is very important not just for quality but also for safety purposes. Heating (cooking or retorting) inactivates the bacteria and their enzymes, but neither heating nor freezing eliminates the histamine once it is formed.
Measurement of histamine levels in seafood is an important part of the seafood industry’s Hazard Analysis Critical Control Point (HACCP) programs. The Food and Drug Administration has established a 50-ppm upper limit (defect action level) for histamine in seafood. Fresh fish muscle contains <1 ppm histamine; 10 ppm means the fish have been mildly temperature abused, and 50 ppm means they may not have been properly iced, allowing for bacteria to grow and produce levels of histamine that may cause human illness. Generally, fish with 200 ppm histamine will cause a reaction in some individuals and fish with >500 ppm histamine are likely to cause the disease even in healthy individuals.
--- PAGE BREAK ---
The analytical method specified by FDA for determination of histamine levels in seafood is AOAC Official Method 977.13, a fluorometric method. It involves excitation with a mercury lamp and the use of a narrow bandpass filter at 350 nm. The samples used for analysis are passed over an ion-exchange resin and the test portion of the mixture is mixed with o-phthalaldehyde, which has a fluorescence maximum near 444 nm, in strong acid to produce the fluorescent product. The histamine concentration is determined from a calibration curve against a known standard. Thermo Scientific Inc. (www.thermo.com) says that its Quantech Digital Filter Fluorometer can detect histamine at the 0.1 ppm level, well below the 10 ppm levels found in mildly temperature-abused seafood samples.
Other quantitative analytical methods for histamine include gas chromatography, high-performance liquid chromatography, high-performance thin-layer chromatography, and capillary electrophoresis. Semi-quantitative methods include immunoenzymatic kits, colorimetry, thin-layer chromatography, and enzymatic methods.
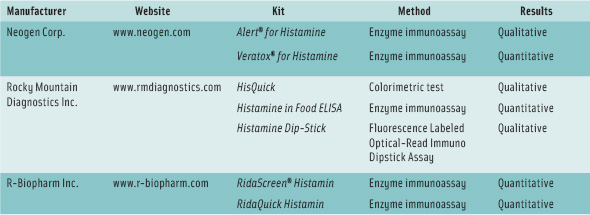
A number of test kits for rapid determination—both quantitative and qualitative (pass/fail)—have also been developed, and some of them are listed in the table on p. 70. Sevim Kose and coworkers at Turkey’s Karadeniz Technical University evaluated eight test kits for their suitability for detecting histamine in several traditional fermented and salted fish products from European countries and Turkey. The researchers, presenting their results in paper 006-02 at the 2009 IFT Annual Meeting, concluded that the Veratox® kit from Neogen Corp. and the RidaScreen® kit from R-Biopharm Inc. can be used for histamine analysis of these types of products.
Histamine Research Studies
Green and his associates have been studying the bacteria involved in histamine production in seafood for a number of years. We hope to improve our understanding, he said, of the physical and chemical factors affecting growth of bacteria responsible for histamine formation in order to design better controls for its production in fish. If we just monitor for the presence of histamine, we haven’t learned how to control its formation.
Histamine—as well as other biogenic amines such as putrescine and cadaverine—is produced by a wide range of bacteria, but the major histamine-producing bacteria in fish are Gram-negative, he said. Some strains produce high levels of histamine (>1,000 ppm) when cultured under optimal conditions, while others produce low levels (<500 ppm). FDA’s Seafood HACCP regulation specifies time and temperature guidelines and a defect action level of 50 ppm histamine.
Green said that there is a need for reliable, rapid, and accurate methods for detection and quantification of histamine and/or histamine-producing bacteria in scombroid fish. Quantification of histamine in fish muscle using HPLC is the “gold standard,” he said, but it is complicated, time-consuming, and expensive. Some food processors use enzyme-based test kits to distinguish products that contain less than 50 ppm histamine from those that contain more than 50 ppm; these methods were designed for use by the fishing industry to verify proper handling of fish at receipt, but he added that they are not quantitative and should only be used to indicate potentially temperature-abused fish.
The most common method used for detection of histamine-producing bacteria is Niven’s agar, a differential growth medium which contains a pH indicator. As histamine accumulates during growth of the bacteria, the pH increases and a color change occurs, allowing histamine-producing colonies to be differentiated. However, this method produces a large number of false positives. More recently, Green said, several nucleic acid amplification/polymerase chain reaction methods have been developed. They can be used for confirmation of bacterial cultures that screen positive on Niven’s agar, he said, but they don’t reduce the number of false positives obtained with initial screening of bacteria using the differential growth medium technique.
--- PAGE BREAK ---
Green and his coworkers studied three methods—use of modified Niven’s agar, conductance change after incubation in histidine decarboxylase broth, and PCR-based assay—for detection of histamine-producing bacteria in fish and recently published their results in the Journal of Food Protection (Vol. 72, No. 9, 2009, pp. 1987–1991). The potentiometric and PCR-based methods were suitable for routine screening for high-histamine-producing bacteria but not for low-histamine-producing bacteria. They require more expensive equipment and a higher degree of training but are faster than the Niven’s agar method. These observations, Green said, support the need for a straightforward method for identifying histamine-producing bacteria.
Consequently, Green and his coworkers have developed a method based on colony lift hybridization that he said provides more-accurate quantitative results because the target organism can be confirmed without the need for sub-culturing. In addition, he said, once a colony is identified by this method, it can be isolated and used in further testing or as part of a strain bank.
The method involves use of a probe specifically targeting the histidine decarboxylase gene of Gram-negative bacteria that produce high levels (>1,000 ppm) of histamine in fish. A cocktail of PCR amplification fragments corresponding to a 709 bp fragment of the histidine decarboxylase gene of four high-producing bacteria successfully reacted specifically (100%) with the high-histamine-producing strains but not with low-histamine strains.
Green and his coworkers have also studied the effect of high hydrostatic pressure on histamine-producing bacteria and the enzymes responsible for histamine formation in yellowfin tuna and mahi-mahi. Treatment at 400 MPa for 5 min reduced M. morganii by 99.9% and resulted in 90% inactivation of its histidine decarboxylase activity. Reporting on their preliminary results in paper 006-05 at the 2009 IFT Annual Meeting, the researchers concluded that the treatment may have potential for use as a secondary control measure for processing of fish to reduce or eliminate both histamine-producing bacteria and the enzymes responsible for histamine formation.
Green added that among the bacteria surviving the high-pressure treatment were 12 isolates that tested positive on Niven’s agar for histamine production and that 10 of the 12 were Gram-positive. He said that the predominance of Gram-positive histamine-producing bacteria contradicts the widely held belief that Gram-negative bacteria are primarily responsible for histamine- formation in fish and that this finding warrants further investigation.
Histamine research is an important area, Green said, because it crosses all commodities, not just seafood. He pointed out that histamine is found not only in certain fish species but also in fermented foods like cheese, sausage, sauerkraut, and wines. And the bacteria which produce enzymes capable of producing histamine and other biogenic amines differ by the environment in which the food is found and the enzymatic pathways used for biogenic amine formation.
--- PAGE BREAK ---
 FDA Research Activities
FDA Research Activities
The FDA Gulf Coast Seafood Laboratory (GCSL) in Dauphin Island, Ala., is FDA’s primary seafood research laboratory. Scientists there are working to develop new and improved tests for histamine, histamine-producing bacteria, biogenic amines, and other seafood decomposition metabolites.
Microbiologists Ronald A. Benner Jr., Kristin Butler, Susan McCarthy, and Jessica L. Jones are employing conventional microbiological and molecular methods with the goal of determining which bacteria are significant producers of histamine and other biogenic amines. For example, they are developing a rapid real-time PCR method for enumerating Gramnegative high-histamine-producing bacteria. This method is designed to give quantitative results in just over 1 hr. An enhanced understanding of histamine-producing bacteria will allow FDA regulators to better control scombrotoxin (histamine) poisoning in seafood consumers.
In concert, chemists F. Aladar Bencsath and Ann Abraham are modifying the standard fluorometric method for analyzing histamine in seafood (AOAC Official Method 977.13) to make it more rapid, efficient, and cost effective. For example, instead of purchasing ion-exchange resin, activating with base, rinsing, and preparing a column, they are exploring use of commercially available solid-phase extraction cartridges. This would allow 12 or more cartridges to be run simultaneously, in place of up to 3 columns at a time, as is common practice with the standard method. FDA also intends to reduce the quantity of chemicals used per analysis and convert the fluorometric reading step to a 96-well plate format. If equivalent performance to the official method can be achieved, the modifications should result in significant reductions in cost and time per analysis.
Another analytical technique under development at GCSL is a multi-analyte liquid chromatography-mass spectrometry (LC-MS-MS) method for the simultaneous determination of up to 10 biogenic amines (including histamine). This method will generate more information from a single analysis than testing for histamine alone. Detection and quantification of several biogenic amines simultaneously may provide more information as to the time–temperature history of a fish or fish product, perhaps identifying a new pattern or relationship that is indicative of decomposition and toxic potential.
A Good Source of Information
A good source of information on seafood is the California Sea Grant Seafood Network Information Center (http://seafood.ucdavis.edu) at the University of California, Davis, under the direction of Pamela Tom ([email protected]). To obtain up-to-date information on rapid test methods for seafood hazards, including histamine, visit http://seafood.ucdavis.edu/organize/rapid.html.
Forthcoming Seafood Meetings
February 21-24, 2010
Pacific Fisheries Technologists Conference, Seattle, Wash. This international conference provides a forum to broaden professional networks and exchange information and current research in seafood technology among industry, government, and university researchers and interested participants. Details at http://pftfish.net.
March 1-5, 2010
Aquaculture 2010, San Diego, Calif. Sponsored by the World Aquaculture Society, Fish Culture Section of the American Fisheries Society, and the National Shellfisheries Association. Details at www.was.org.
March 9-10, 2010
2nd European Fish and Seafood Conference, Stavanger, Norway. Sponsored by Nofima, the Norwegian Institute of Food, Fisheries and Aquaculture Research in collaboration with Campden BRI (www.campden.co.uk). The conference will address health and the consumer, sustainability, seafood processing technology, and nondestructive quality measurement. Details at www.nofima.no.
March 14-16, 2010
International Boston Seafood Show and Seafood Processing America Conference, Boston, Mass. Details at www.bostonseafood.com.
May 10-13, 2010
2nd International Conference on Seafood Technology, Anchorage, Alaska. Cosponsored by the Food and Agriculture Organization of the United Nations and the University of Alaska. The congress will address state-of-the-art information regarding the handling, processing, preservation, storage, and transportation of seafood. Details at www.icst2010.com.
May 23-26, 2010
Australasian Aquaculture 2010 International Conference and Trade Show, Hobart, Tasmania, Australia. Cosponsored by the National Aquaculture Council, the Asian Pacific Chapter of the World Aquaculture Society, and the Australian Government Fisheries Research and Development Corp. Details at www.australian-aquacultureportal.com.
by Neil H. Mermelstein,
a Fellow of IFT, is Editor Emeritus of Food Technology
[email protected]


