Replicating the Taste of Sugar
Ongoing research in sweetness modulation of high-potency sweeteners and in enhancement of the sweetness of carbohydrate sweeteners may lead to reduced-calorie foods & beverages with true sugar-like qualities.
Chemists have identified thousands of sweet-tasting organic compounds since the early 1800s. Most compounds are much more potent than the common carbohydrate (CHO) sweeteners and are generally referred to as high-potency (HP) sweeteners. The diversity of chemical structure among sweeteners has fascinated chemists for decades, and now, more than 50 structural classes of sweet-tasting organic compounds are known (Shallenberger 1993, DuBois et al., 2008). Few, however, are commercially viable. 
To be attractive for commercialization, a sweetener must rate acceptably on all of the six metrics: 1) safety, 2) taste quality, 3) stability, 4) solubility, 5) cost, and 6) patentability. A detailed discussion of these metrics is available (DuBois, 2008). A major challenge, however, is accurate replication of sugar taste. HP sweeteners invariably show all or some of low maximal sweetness intensities, “off ” tastes (e.g., bitter, metallic, and licorice-like), non-sugar-like temporal behaviors, and desensitization.
In 2001, Charles Zuker (Univ. of Calif., San Diego), Nicholas Ryba (National Institutes of Health), and their coworkers discovered the rat sweetener receptor (Nelson et al., 2001), and follow-up work by Xiaodong Li and coworkers (Senomyx) led to the discovery of the human sweetener receptor (Li et al., 2002). These breakthroughs enabled, for the first time, sweetener discovery programs independent of human taste testing and which could be rapidly carried out with in vitro assays on large synthetic and natural product libraries.
Following is a discussion of recent advances in modulation of the tastes of known HP sweeteners to render them more sugar-like as well as the discoveries of the first sweetener receptor positive allosteric modulators (PAMs), novel ingredients which enhance the sweetness intensities of CHO sweeteners and have breakthrough potential for caloric reduction with retention of the taste of sugar.
Sweetness Modulation
A problem limiting the utilities of many HP sweeteners is a flavor profile which includes bitter and other negative taste attributes. An even more serious problem, general for all HP sweeteners, is a temporal profile with delayed sweetness onset and lingering sweet aftertaste. Advances in understanding the biochemical mechanisms responsible for these problems are leading to progress.
--- PAGE BREAK ---
• Flavor profile modulation. Saccharin was the first HP sweetener to be commercialized. But unpleasant bitter and metallic off tastes limited its use. This changed dramatically with the discovery (Helgren, 1957) that a blend with cyclamate was much improved in taste with negligible bitterness, a finding that led to the beginning of the diet beverage industry and many other reduced-calorie products. However, the 1969 U.S. Food & Drug Administration (FDA) ban on cyclamate nearly wiped out this new industry and provided impetus for research to find saccharin bitterness inhibitors.
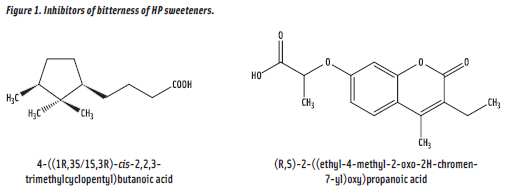 With the discovery that human bitter taste is initiated by a family of 25 receptors known as T2Rs, the specific receptors for saccharin bitterness were recently identified. And knowledge of these receptors enabled Givaudan scientists to conduct a cell-based high-throughput-screening (HTS) program, leading to the discovery of 4-((1R,3S/1S,3R)-cis-2,2,3-trimethylcyclopentyl)butanoic acid (TCB) as an inhibitor for saccharin bitterness (Slack et al., 2010). Disappointingly, however, while highly effective in vitro, TCB had limited effectiveness in sensory testing. Further work by Givaudan scientists led to the reports that TCB (Slack & Evans-Pennimpede, 2009), as well as (R,S)-2-((ethyl-4-methyl-2-oxo-2H-chromen-7-yl) oxy)propanoic acid (EMCC) (Figure 1), are effective as bitterness inhibitors for stevia sweetener rebaudioside A (Ungureanu & Van Omerren, 2010).
With the discovery that human bitter taste is initiated by a family of 25 receptors known as T2Rs, the specific receptors for saccharin bitterness were recently identified. And knowledge of these receptors enabled Givaudan scientists to conduct a cell-based high-throughput-screening (HTS) program, leading to the discovery of 4-((1R,3S/1S,3R)-cis-2,2,3-trimethylcyclopentyl)butanoic acid (TCB) as an inhibitor for saccharin bitterness (Slack et al., 2010). Disappointingly, however, while highly effective in vitro, TCB had limited effectiveness in sensory testing. Further work by Givaudan scientists led to the reports that TCB (Slack & Evans-Pennimpede, 2009), as well as (R,S)-2-((ethyl-4-methyl-2-oxo-2H-chromen-7-yl) oxy)propanoic acid (EMCC) (Figure 1), are effective as bitterness inhibitors for stevia sweetener rebaudioside A (Ungureanu & Van Omerren, 2010).
With 25 T2R bitterness receptors, controlling bitterness in foods and beverages with inhibitors may be difficult. Recently, however, evidence has shown that bitter taste may be even more complex than would be expected from 25 receptors. Meyerhof and coworkers (Kuhn et al., 2010) provided evidence that the 25 T2Rs form hetero- as well as homo-dimers, such that the population of functional bitterant receptors may be as high as 325. Such a large number of bitterant receptors may explain the limited sensory panel efficacy of TCB.
The discovery of potent inhibitors against individual T2Rs may now be routine using cell-based assays and HTS. But the challenge will remain to discover inhibitors that are highly effective in human taste.
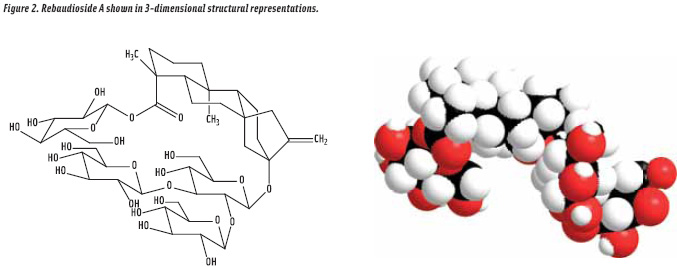 • Temporal profile modulation. The major problem in replication of sugar taste with HP sweeteners is that they invariably exhibit atypical temporal profiles (i.e., delayed sweetness onset accompanied by sweetness linger). In 2003, we (i.e., The Coca-Cola Co.) initiated the development of the stevia sweetener rebaudioside A (Figure 2), also known as rebiana (Prakash et al., 2008). And later, we entered into collaboration with Cargill for commercialization.
• Temporal profile modulation. The major problem in replication of sugar taste with HP sweeteners is that they invariably exhibit atypical temporal profiles (i.e., delayed sweetness onset accompanied by sweetness linger). In 2003, we (i.e., The Coca-Cola Co.) initiated the development of the stevia sweetener rebaudioside A (Figure 2), also known as rebiana (Prakash et al., 2008). And later, we entered into collaboration with Cargill for commercialization.
--- PAGE BREAK ---
In our program, we focused on formulation of rebiana to be more sugar-like in temporal profile. In order to address this challenge, we used a biorational approach (DuBois, 2011). We considered rebiana’s higher binding affinity, relative to sucrose, for the sweetener receptor as a potential explanation. However, on the basis of kinetics considerations, a 200-fold higher binding affinity is insufficient to explain a persistent sweetness.
After considering many potential explanations, in our view, the most probable rationale, which also accounts for slow sweetness onset that always accompanies sweetness linger, is that HP sweeteners engage in extensive non-specific binding throughout the oral cavity. We refer to this rationale for the atypical temporal profiles of HP sweeteners as the Non-Specific Binding (NSB) Hypothesis. If this hypothesis is correct, then the challenge is formulation to reduce non-specific binding. But the question is how? We considered that biological cells are well known to be affected by the tonicity of extra-cellular media, such that at low tonicity cells swell and at high tonicity cells shrink. Thus, since hypertonic sweetener formulations may have the effect of shrinking oral epithelial cells, we speculated that such formulations would reduce non-specific binding, thus accelerating sweetness onset and attenuating sweetness linger.
We found support for the NSB Hypothesis in observations of Maruzen Pharmaceutical Co. scientists (Crammer & Ikan, 1987), where hypertonic NaCl was found to increase the sweetness potency of a stevia sweetener composition by approximately 3-fold. In our view, the reported increase in potency is only an apparent increase and actually is caused by acceleration of sweetness onset and elimination of sweetness linger, such that the entire sweetness signal is experienced over a short time interval as is the case for sucrose sweetness. In subsequent work (Prakash et al., 2007), we demonstrated that NaCl is not unique in temporal profile modulation of HP sweeteners and that osmolytes, in general, exhibit this effect. Multiple osmolytes at 500 milliosmolar (e.g., NaCl, KCl, and erythritol) were found to be equally effective in modulating the temporal profile of 500 mg/L of rebiana, thus rendering it sugar-like. Our formulation results support the NSB Hypothesis as the correct rationale for the atypical temporal profiles of HP sweeteners. Further support derives from a recent study on the interactions of sweeteners with phospholipid model membranes. Abe and coworkers (Tokyo Univ.) employed surface plasmon resonance to study interactions of CHO and HP sweeteners and found the levels of interaction to correlate with observed levels of sweetness linger (Miyano et al., 2010).
Sweetness Enhancement 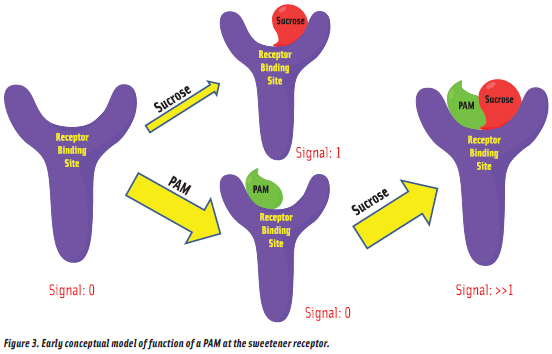 The primary challenge in sweetener research is accurate replication of sugar taste. And while sweetness modulators can be effective in improving the taste of HP sweeteners, we looked for a simpler approach. Thus, we investigated the concept that molecules may exist which bind to the sweetener receptor without activating it and do so in a way that alters the receptor such that CHO sweeteners bind more tightly, as illustrated in Figure 3. Such compounds would be enhancers, or more properly, positive allosteric modulators (PAMs). And, we felt that since a PAM would have no sweetness, a sucrose/PAM formulation should accurately replicate sugar taste. Of course, since a CHO sweetener would still be present in the formulation, some calories would remain. However, if PAMs with ≥ 20-fold enhancement effects could be identified, then beverages could be sweetened with a CHO sweetener, providing great taste, but with ≤ = 5% of the calories, and could qualify for zero-calorie labeling. This logic provided the initial support for a PAM discovery project at The Coca-Cola Co. that was begun in the 1990s (DuBois, 2011).
The primary challenge in sweetener research is accurate replication of sugar taste. And while sweetness modulators can be effective in improving the taste of HP sweeteners, we looked for a simpler approach. Thus, we investigated the concept that molecules may exist which bind to the sweetener receptor without activating it and do so in a way that alters the receptor such that CHO sweeteners bind more tightly, as illustrated in Figure 3. Such compounds would be enhancers, or more properly, positive allosteric modulators (PAMs). And, we felt that since a PAM would have no sweetness, a sucrose/PAM formulation should accurately replicate sugar taste. Of course, since a CHO sweetener would still be present in the formulation, some calories would remain. However, if PAMs with ≥ 20-fold enhancement effects could be identified, then beverages could be sweetened with a CHO sweetener, providing great taste, but with ≤ = 5% of the calories, and could qualify for zero-calorie labeling. This logic provided the initial support for a PAM discovery project at The Coca-Cola Co. that was begun in the 1990s (DuBois, 2011).
--- PAGE BREAK ---
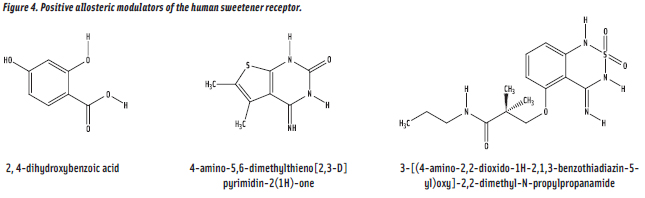 In search of precedent for sweetness enhancement, we found many claims, but were unable to replicate them. Interestingly, however, while not reporting CHO sweetener enhancement, Lindley and coworkers (Holland Sweetener Co.) reported that 2, 4-dihydroxybenzoic acid (DHB) (Figure 4) causes aspartame to taste more sugar-like, but also to be of higher sweetness intensity (Britton et al., 1999). We evaluated DHB in combination with sucrose and were elated to observe a clear sweetness enhancement effect (i.e.,1.3-fold). And later, we observed a weaker effect for fructose and no effect for glucose. We accepted our results as proof of concept for the existence of PAMs for the human sweetener receptor. We did a substantial amount of follow-up work on structural analogues of DHB, but progress by human taste as the assay was slow. However, the human sweetener receptor discovery by Xiaodong Li and coworkers (Li et al., 2002) provided hope that PAM discovery could be markedly accelerated by use of cell-based assays and HTS.
In search of precedent for sweetness enhancement, we found many claims, but were unable to replicate them. Interestingly, however, while not reporting CHO sweetener enhancement, Lindley and coworkers (Holland Sweetener Co.) reported that 2, 4-dihydroxybenzoic acid (DHB) (Figure 4) causes aspartame to taste more sugar-like, but also to be of higher sweetness intensity (Britton et al., 1999). We evaluated DHB in combination with sucrose and were elated to observe a clear sweetness enhancement effect (i.e.,1.3-fold). And later, we observed a weaker effect for fructose and no effect for glucose. We accepted our results as proof of concept for the existence of PAMs for the human sweetener receptor. We did a substantial amount of follow-up work on structural analogues of DHB, but progress by human taste as the assay was slow. However, the human sweetener receptor discovery by Xiaodong Li and coworkers (Li et al., 2002) provided hope that PAM discovery could be markedly accelerated by use of cell-based assays and HTS.
The sweetener receptor is a G Protein-Coupled Receptor (GPCR) of Class C, a small subset of 12 receptors of the super family of GPCRs, which is unique in that its members all possess large extracellular domains, resembling Venus Flytraps (VFDs) in their modes of binding ligands, attached to Transmembrane Domains (TMDs), common to all GPCRs. And in parallel with the human sweetener receptor discovery, reports began to appear on discoveries of PAMs for other Class C GPCRs.
The impetus for this research in the pharmaceutical industry was the expectation of high selectivity and low off-target activity for drugs acting at allosteric rather than orthosteric receptor sites (Werner, 2009). And today, PAMs and/or negative allosteric modulators (NAMs) for all Class C GPCRs are known and a hyperparathyroidism drug known as cinacalcet is on the market (Nagano, 2006). Research in this field has recently been reviewed (Urweyler 2011). It is noteworthy that allosteric modulators of the metabotrophic glutamate, GABA and extracellular calcium Class C GPCRs have uniformly been found to bind at loci in the TMD rather than in the VFD, while the umami receptor, also a class C GPCR, binds its allosteric modulator inosine monophosphate (IMP) in the VFD adjacent to the glutamate binding site (Zhang et al., 2008). Thus, as illustrated in Figure 5, it was not clear at all as to the locus (loci) of allosteric modulatory sites on the sweetener receptor.
The human sweetener receptor discovery made possible HTS of large diverse libraries of organic compounds as the first systematic PAM discovery program, a collaboration of Senomyx with The Coca-Cola Co. This program first led (Servant et al., 2010) to the 3- to 8-fold sucralose-selective enhancer 4-amino-5,6-dimethylthieno[2,3-D]pyrimidin-2(1H)-one (ADTP) and, later (Shigemura et al., 2010), to the 2-fold sucrose-selective enhancer 3-[(4-amino-2,2-dioxido-1H-2,1,3-benzothiadiazin-5-yl) oxy]-2,2-dimethyl-N-propylpropanamide (ADBT) (Figure 4). Preliminary studies on formulations of sucrose with ADBT are consistent with the expectation that PAM formulations would retain the desirable sensory properties of CHO sweeteners. Both ADTP and ADBT have been accepted as GRAS (generally recognized as safe) by the FEMA (Flavor and Extract Manufacturers Assoc.) Expert Panel as artificial flavors, and are available from Firmenich.
--- PAGE BREAK ---
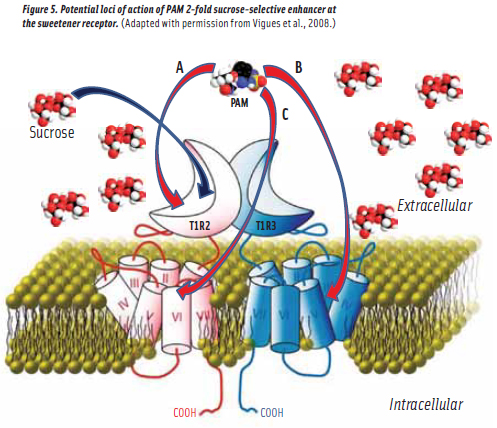 The question now returns as to loci of action of ADTP and ADBT on the sweetener receptor (Figure 5). Do they act at a locus in the T1R2 VFD as is the case of IMP in the umami receptor (i.e.,Pathway A)? Or do they bind in the TMD as in the glutamate, GABA and calcium Class C GPCRs (i.e.,Pathways B or C)? It is noteworthy that aspartame and cyclamate, two sweeteners synergistic with each other, have been determined to bind in the T1R2 VFD and the T1R3 TMD, respectively (Xu et al., 2004). This synergism seems best interpreted as a PAM effect, induced by cyclamate acting as an aspartame-selective PAM while also acting as a sweetener (DuBois, 2004). And thus, there is precedent to expect T1R3 TMD binding for CHO sweetener PAMs since CHO sweeteners, just like aspartame, bind at T1R2 VFD sites. However, in 2010, Senomyx scientists (Zhang et al., 2010) demonstrated that ADTP binds in the T1R2 VFD (Figure 6) and it is thought that sucrose and ADBT, most probably, bind at the same locus.
The question now returns as to loci of action of ADTP and ADBT on the sweetener receptor (Figure 5). Do they act at a locus in the T1R2 VFD as is the case of IMP in the umami receptor (i.e.,Pathway A)? Or do they bind in the TMD as in the glutamate, GABA and calcium Class C GPCRs (i.e.,Pathways B or C)? It is noteworthy that aspartame and cyclamate, two sweeteners synergistic with each other, have been determined to bind in the T1R2 VFD and the T1R3 TMD, respectively (Xu et al., 2004). This synergism seems best interpreted as a PAM effect, induced by cyclamate acting as an aspartame-selective PAM while also acting as a sweetener (DuBois, 2004). And thus, there is precedent to expect T1R3 TMD binding for CHO sweetener PAMs since CHO sweeteners, just like aspartame, bind at T1R2 VFD sites. However, in 2010, Senomyx scientists (Zhang et al., 2010) demonstrated that ADTP binds in the T1R2 VFD (Figure 6) and it is thought that sucrose and ADBT, most probably, bind at the same locus. 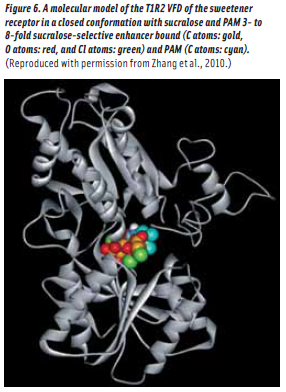
The Future of PAMs
In summary, proof of concept for positive allosteric modulation of the sweetener receptor is now in place and, while it is too soon to tell, sweetener PAM technology just might be a “breakthrough technology” enabling reducedcalorie foods and beverages with qualities of sweetness not possible with HP sweeteners.
Yet there is much we do not know. For example, what is the maximal enhancement effect possible? GPCR PAMs with >100-fold enhancements have been reported (Ma et al., 2009); however, what level of enhancement is possible with the sweetener receptor activated by a CHO sweetener? Also, will it be possible to identify PAMs active against all sweeteners? And given consumer interest in natural flavors, will it be possible to find commercially viable natural enhancers?
Undoubtedly, there is a great deal to be learned about PAMs and the sweetener receptor. Only then will the value of PAM technology be clear. However, given consumer interest in great-tasting sweet foods and beverages with lower calories, PAM technology will be an active area of research for years to come. This is already evident on the basis of recent patent activities of many companies, including Cadbury Adams USA (Bingley et al., 2007), Givaudan (Slack et al., 2007), Nutrinova (Krohn & Zinke, 2009), Redpoint Bio Corp. (Salemme, 2010), and Symrise (Ley et al., 2008).
Grant E. DuBois, Ph.D., a Member of IFT, is Research Fellow, Global Research Department, The Coca-Cola Co., P.O. Box 1734, Atlanta, GA 30301 ([email protected]).
References
Bingley, C.A., Olcese, G., and Darnell, K.C. 2007. Taste potentiator compositions and beverages containing same, U.S. Patent App. No. US 2007/0054023 A1 (March 8, 2007).
Britton, S.J., Fry J.C., Lindley, M.G., Marshall, S. 1999. PCT Patent App. No. WO 99/15032 (April 1, 1999).
Crammer, B. and Ikan, R. 1987. Progress in the chemistry and properties of rebaudiosides. In Developments in Sweeteners, Vol. 3, pp. 45-64. Chapman and Hall. London.
DuBois, G.E., DeSimone, J., and Lyall, V. 2008. Chemistry of gustatory stimuli, In The Senses: A Comprehensive Reference, Vol. IV: Olfaction and Taste, ed. D. Smith and S. Firestein, pp. 27-74, Elsevier. London. DuBois, G.E. 2008. Sweeteners and sweetness modulators: Requirements for commercial viability. Chapter 29 in Sweetness and Sweeteners: Biology, Chemistry and Psychophysics. ed. D.K. Weerasinghe and G.E. DuBois, ACS Symposium Series 979, American Chemical Society, pp. 444-462. Washington, DC.
DuBois, G.E. 2011. Validity of early indirect models of taste active sites and advances in new taste technologies enabled by improved models. Flavour and Fragrance Journal. In press.
DuBois, G.E., 2004. Unraveling the biochemistry of sweet and umami tastes. Proc. Natl. Acad. Sci. USA 101(39): 13972-13973.
Helgren, F.J. 1957. Sweetening composition and method of producing the same. U.S. Patent 2,803,551 (Aug. 20, 1957).
Krohn, M. and Zinke, H. 2009. Oligopeptides for use as taste modulators. PCT Patent App. WO 2009/098016 A1 (Aug. 13, 2009).
Kuhn, C., Bufe, B., Batram, C., and Meyerhof, W. 2010. Oligomerization of TAS2R bitter taste receptors. Chemical Senses 35: 395-406.
Ley, J.P., Blings, M., Paetz, S., Kindel, G., Freiherr, K., Krammer, G.E., and Bertram, H.J. 2008. Enhancers for sweet taste from the world of non-volatiles: polyphenols as taste modifiers. In Sweetness and Sweeteners: Biology, Chemistry, and Psychophysics, ACS Symposium Series 979, ed. D.K. Weerasinghe and G.E. DuBois, American Chemical Society, pp. 400-409. Washington, DC.
Li, X., Staszewski, L., Xu, H., Durick, K., Zoller, M., and Adler, E. 2002. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA. 99: 4692-4696.
Ma, L., Seager, M., Wittmann, M., Jacobson, M., Bickel, D., Burno, M., Jones, K., Graufelds V.K., Xu, G., Pearson, M., McCampbell, A., Gaspar, R., Shughrue, P., Danziger, A., Regan, C., Flick, R., Pascarella, D., Garson, S., Doran, S., Kreatsoulas, C., Veng, L., Lindsley, C.W., Shipe, W., Kuduk, S., Sur, C., Kinney, G., Seabrook, G.R., Ray, W.J. 2009. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc. Natl. Acad. Sci. USA 106: 15950-15955.
Miyano, M., Yamashita, H., Sakurai, T., Nakajima, K., Ito, K., Misaka, T., Ishimaru, Y., Abe, K., and Asakura, T. 2010. Surface plasmon resonance analysis on interactions of food components with a taste epithelial cell model. J. Agric. Food Chem. 58(22): 11870-11875.
Nagano, N. 2006. Pharmacological and clinical properties of calcimimetics: calcium receptor activators that afford an innovative approach to controlling hyperparathyroidism. Pharmacology & Therapeutics 109: 339-365.
Nelson, G., Hoon, M.A., Chandrashekar J., Zhang, Y., Ryba, N.J.P., Zuker, C.S. 2001. Mammalian sweet taste receptors. Cell 106: 381-390.
Prakash, I., DuBois, G.E., Clos, J.F., Wilkens, K.L., Fosdick, L.E. 2008. Development of rebiana, a natural non-caloric sweetener. Food Chem. Toxicol. 46 (Supplement 7S): 75-82.
Prakash, I., DuBois, G.E., Jella, P., King, G.A., San Miguel, R.I., Sepcic, K.H., Weerasinghe, D.K., White, NR. 2007. U.S. Patent App. 20070128311 (June 7, 2007).
Salemme, F.R., Long, D., Palmer, R.K., Brennan, F.X., and Sprous, D. 2010. Rebaudioside C and its stereoisomers as natural product sweetness enhancers. PCT Patent App. WO 2010/135378 A1 (November 25, 2010).
Servant, G., Tachdjian, C., Tang, X., Werner, S., Zhang, F., Li, X., Kamdar, P., Petrovic, G., Ditschun, T., Java, A., Brust, P., Brune, N., DuBois, G.E., Zoller, M., and Karanewsky, D.S. 2010. Positive allosteric modulators of the human sweet taste receptor enhance sweet taste. Proc. Natl. Acad. Sci. USA, 107: 4746-4751.
Shallenberger, R.S. 1993. Taste Chemistry, Blackie Academic & Professional. London.
Shigemura, R., Podgurski, C., Kitisin, B., Ward, J., and Alatorre, A. 2010. Compositions comprising sweetness enhancers and methods of making them, PCT Patent App. No. WO2010/014813 A2 (February 4, 2010).
Slack, J.P., Brockhoff, A., Batram, C., Menzel, S., Sonnabend, C., Born, S., Galindo, M.M., Kohl, S., Thalmann, S., Ostopovici-Halip, L., Simons, C.T., Ungureanu, I., Duineveld, K., Bologna, C.G., Behrens, M., Furrer, S., Oprea, T.I., and Meyerhof, W. 2010. Modulation of bitter taste perception by a small molecule hTAS2R antagonist. Current Biology 7: 1104-1109.
Slack, J.P. and Evans-Pennimpede, J.E. 2009. Methods of identifying modulators of the bitter taste receptor TAS2R44. PCT Patent App. WO 2009/149577 A1 (December 17, 2009).
Slack, J.P., Simons, C.T., and Hansen, C.A. 2007. Method relating to sweetness enhancement. PCT Patent App. WO 2007/121604 A2 (November 1, 2007).
Ungureanu, I.M. and Van Ommeren, E. 2010. Off-taste masking. PCT Patent App. WO 2010/100158 A1 (September 10, 2010).
Urweyler, S. 2011. Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol. Rev. 63: 59-126.
Vigues, S., Dotson, C.D., and Munger, S.D. 2008. The receptor basis of sweet taste in mammals. Results and Problems in Cell Differentiation, Springer-Verlag, Berlin.
Werner, M. 2009. A new type of drug target: an emerging class of medicines works its magic by targeting unusual sites on biological molecules. Scientific American, Aug., 70-76.
Xu, H., Staszewski, L., Tang, H., Adler, E., Zoller, M., and Li, X. 2004. Differential functional roles of T1R subunits in the heteromeric taste receptors. Proc. Natl. Acad. Sci. USA. 101: 14258-14263.
Zhang, F., Klebansky, B., Fine, R.M., Xu, H., Pronin, A., Liu, H., Tachdjian, C., and Li, X. 2008. Molecular mechanism for the umami taste synergism. Proc. Nat’l. Acad. Sci. USA. 105(52): 20930-20934.
Zhang, F., Klebansky, B., Fine, R.M., Liu, H., Xu, H., Servant, G., Zoller, M., Tachdjian, C., and Li, X. 2010. Molecular mechanism of the sweet taste enhancers, Proc. Nat’l. Acad. Sci. USA, 107: 4752-4757.
