Dealing With Antimicrobial Resistance
Newly released IFT Expert Report elucidates the implications of antimicrobial uses throughout the food system. Here’s a summary.
Availability of a variety of efficacious antimicrobials, antibiotics, food antimicrobial agents, sanitizers, disinfectants, and other substances that can control microorganisms is integral to our complex, macrobiologic ecosystem and interdependent food system. Antimicrobials provide for high quality or good physical condition of crops, good health of food animals entering the food chain, and effective sanitation during food processing.

The inherent ability of microorganisms to mutate and adapt to environmental stressors, however, presents an ongoing food safety challenge. Use of antimicrobials, more specifically antibiotics, can create selective pressure that leads to emergence of antimicrobial-resistant microorganisms, potentially undermining the effectiveness of antimicrobial and antibiotic applications throughout the eco- and food systems. The pathways of microbial exchange among the environment, animals, and humans, and resistance selection and dissemination are shown in the diagram on the next page.
To advance the scientific perspective on this issue, the Institute of Food Technologists convened a panel of internationally renowned experts to review the science related to antimicrobial resistance. The panel members worked tirelessly through an initial meeting, scores of conference calls, and innumerable e-mail exchanges and produced the IFT Expert Report, “Antimicrobial Resistance: Implications for the Food System.” The complete report appears in the July 2006 issue of Comprehensive Reviews in Food Science and Food Safety, accessible at http://members.ift.org/IFT/Pubs/CRFSFS/. This article summarizes the report.
Emergence, Dissemination, and Monitoring of Resistance
Resistance may be intrinsic to a microbe (relating to general physiology or anatomy), develop via mutation or other genetic alteration, or occur via temporary adaptation to an Antimicrobial-containing environment (e.g., food-contact surfaces in food manufacturing plants). Bacterial resistance mechanisms are quite diverse, as are the modes of action of antimicrobials (Courvalin, 2005). Antimicrobials may inhibit DNA replication, transcription, and translation or act at the level of the cell wall or membrane.
Bacterial strategies for resisting antimicrobials include impaired uptake, modification or overproduction of the target site, bypass of sensitive steps, absence of enzymes or metabolic pathways, efflux, enzymatic degradation, receptor alteration, and change in membrane permeability (Cloete, 2003; Dever and Dermody, 1991; Russell et al., 1997).
Bacteria may also experience stress adaptation (resistance stemming from exposure to subinhibitory levels of stress that trigger stress-response protein transcription and translation), co-selection (resistance to antimicrobials having unrelated targets, stemming from separate genes transferred together), cross-resistance (resistance to antimicrobials having the same molecular targets), and cross-protection (in which adaptation to one stress is associated with increased resistance to another, unrelated stress).
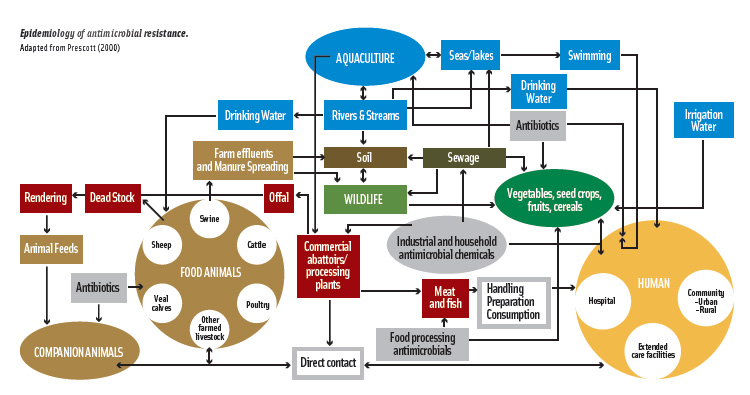 The prevalence and mechanism of resistance among most food-use antimicrobial compounds is often unknown and needs to be advanced. With greater knowledge of these mechanisms, scientists will be better able to predict the potential for cross-resistance with antibiotics.
The prevalence and mechanism of resistance among most food-use antimicrobial compounds is often unknown and needs to be advanced. With greater knowledge of these mechanisms, scientists will be better able to predict the potential for cross-resistance with antibiotics.
--- PAGE BREAK ---
The existence of a variety of resistance genes also complicates the issue. It appears that antimicrobial resistance genes are widely disseminated in nature and present in a diversity of microorganisms and niches (Chee-Sanford et al., 2001; Nield et al., 2001; Riesenfeld et al., 2004; Sundin, 2002). Two main factors contribute to the persistence of antimicrobial-resistant microorganisms in the environment: survival of the microbe and maintenance of the resistance genotype. Commensals, such as nonpathogenic Escherichia coli and Enterococcus species, may serve as reservoirs of potential antimicrobial-resistance genes in the environment, from which resistance may be transferred to other commensals or pathogenic bacteria. However, of singular interest are those antibiotic-resistant intestinal bacteria in food animals, such as Salmonella and Campylobacter, that can contaminate foods during slaughter or processing and result in human illness.
Various factors complicate our ability to fully understand the transfer of resistant bacteria through the food chain to human illness causation. These factors include unique resistance genes among various foodborne pathogens; aspects of animal production and distribution prior to slaughter; processing practices; retail food preparation, distribution, and storage; consumer food preparation practices; varying susceptibility to pathogens among different subpopulations; and varying medical practices and treatment options.
Several countries and communities have surveillance programs to measure resistance trends, but harmonization is needed before international comparisons can be made and resistance trends elucidated. In the United States, the National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS)—a collaborative effort of the Centers for Disease Control and Prevention, Food and Drug Administration, and U.S. Dept. of Agriculture—monitors changes in susceptibilities of zoonotic pathogens (Salmonella, E. coli, Campylobacter, and Enterococcus) in humans, animals, and animal products.
NARMS data are beginning to reveal resistance trends, which are not consistent in any one direction. Other surveillance programs showed increasing resistance trends during the past 20–25 years, whereas other sources reveal decreasing resistance trends, particularly in the past 6–7 years.
The history of the epidemiology of Salmonella, for example, shows that clones, including multiple-drug-resistant (MDR) clones, spread worldwide and then lost predominance. Some clones of Salmonella Typhimurium DT104, which possess a penta-resistance gene cassette (genetically linked resistance to ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracycline), have spread widely and resulted in foodborne disease outbreaks. It appears that the prevalence of S. Typhimurium DT104 and/or the penta-resistant S. Typhimurium may have peaked in 1996 and declined since then.
Notably, trends in the prevalence of resistance among microorganisms do not necessarily reflect trends in the incidences of either foodborne illness or resistant infections, which in many cases have declined in recent years. Additionally, it is difficult to correlate antibiotic resistance among foodborne pathogens with antibiotic uses on the farm. An increased incidence of illness in any given year may or may not parallel increased use of antibiotics potentially selecting for resistant microorganisms. Therefore, it is difficult to compare year-to-year resistance trend data with disease prevalence and corresponding changes in annual use of a specific antibiotic or class of antibiotics.
--- PAGE BREAK ---
Impacts of Antimicrobial Use and Resistance
Antimicrobial resistance can have adverse effects on human health, food manufacturing, the environment, trade, and the economy.
• Human Health. Antibiotic resistance among foodborne pathogens may create an increased burden on human health in different ways. For example, resistant pathogens contaminating food animals have the potential to reach humans; human use of antibiotics may increase the risk of acquiring an infection with an antimicrobial-resistant pathogen; human infection with a resistant microbe may limit illness treatment options (in the uncommon instances of foodborne illness in which antibiotic use is warranted); and antibiotic-resistant foodborne pathogens may develop increased virulence.
The extent to which antibiotic use in food animals produces clinically important antibiotic-resistant infections in humans is unknown. There is evidence that points to but does not prove that antibiotic use in food animals poses a human health threat. There are very few data regarding food animal-to-human transfer of antimicrobial resistance to indicate more frequent or severe infections or increased morbidity and mortality.
To date, there is little evidence of a human health impact from use of antibiotics in plant production. Similarly, ingestion of antibiotic-resistant bacteria from aquaculture and contact with animals, including pets, appears to have no significant adverse impact on human health.
It appears that household use of antibacterial cleaning and hygiene products does not present an adverse human health impact. A year-long study found that use of triclosan in hand soap did not lead to resistant microbes on hands or in drains (Aiello et al., 2005). Another study investigated use of triclosan at commercial levels in a simulated drain environment and determined that emergence of antibiotic resistance through use of triclosan in kitchens is highly improbable (McBain et al., 2003). Furthermore, use of triclosan or PCMX in industrial environments has not led to emergence of bacterial tolerance or resistance (Lear et al., 2002).
• Food Manufacturing. Food product modifications, such as changes in formulation or processing conditions, may lead to sublethal stressing of microbes. Surviving microorganisms may have increased resistance or virulence. Some antimicrobial treatments may lead to dominance of acid-resistant pathogens. For example, spraying meat carcasses with organic acids may select for survival of acid-tolerant E. coli O157:H7. It is notable, however, that decontamination treatments are effective in reducing microbial contamination of carcasses and in helping meat processors meet performance standards.
The impact on human health of pathogen resistance to food antimicrobials is not fully understood. Advancing our understanding of resistance mechanisms and impact of resistance, however, will help elucidate why some combinations and sequences of antimicrobial interventions result in synergistic “multiple hurdle” effects while others cause stress-hardening or adaptation. Although some studies have suggested that in certain situations (e.g., use at sublethal levels, overuse, presence of biofilms, and existence of cross-resistance mechanisms) the potential exists for negative public health impact, resistance to food antimicrobials is not considered a major public health concern because the resistance mechanisms are often temporary adaptations.
To date, the use in foods of chemical and biological antimicrobials and physical preservation systems has been remarkably successful in providing safe foods and has not been compromised by the occurrence of resistant microorganisms. Furthermore, bacterial adaptation (temporary resistance) to food preservatives and sanitizers is generally a transient state rather than genetically based.
--- PAGE BREAK ---
When resistance to antimicrobials does occur, however, it is of little practical relevance to the food industry because the antimicrobial concentrations used in food manufacturing are well above the low levels to which bacteria exhibit resistance. However, the ability of some sanitizers and disinfectants to induce MDR-pumps in microbes, conferring antibiotic resistance (e.g., to penicillin), is of some concern.
In contrast to antibiotics, which inhibit a specific biosynthetic cellular target, most biocides used by food manufacturers attack multiple, concentration-dependent targets, causing major cell wall and membrane damage in a short period of time (Russell, 2003). Thus, mutations resulting in acquired resistance to biocides are much less likely to occur than mutations resulting in antibiotic resistance.
• The Environment. Overall, there is a general lack of knowledge and agreement about the frequency and extent of occurrence, fate, and effects associated with antimicrobials entering the environment. As a result, it is difficult to assess the environmental impact of the use of antimicrobials. Although there are concerns with antibiotics entering the animal production environment through manure or other waste streams, more information is needed to better understand the situation and implement appropriate strategies. Current evidence suggests that it is not likely that antimicrobials in manure will pose any direct risk to soil microbiota. However, it is not yet possible to exclude other indirect effects on soil microbiota and ecosystems, such as alterations in biodiversity.
• Trade. As noted in a report by the Government Accountability Office (GAO, 2004), there are differences among key U.S. trading partners in acceptable uses of antibiotics, such as those permissible for growth promotion. The U.S., Australia, Canada, Japan, and South Korea allow the use in animals of some antibiotics from classes important in human medicine, but the European Union prohibits such use. GAO also reported that, to date, antimicrobial resistance associated with use of antibiotics in animals has not significantly affected U.S. trade in meat products. GAO indicates that this issue may be a factor in the future, given the EU’s phasing out by 2006 the use of all antibiotics for growth promotion.
• The Economy. GAO determined that a ban or partial ban on antibiotics in food animal production would increase costs to producers, who would bear the greatest financial burden, decrease production, and increase retail prices to consumers. For example, it estimated that eliminating antibiotic use in pork production would increase producer costs from $2.76 to an estimated $6.05 per animal, which translates to increased consumer costs for pork from $180 million to $700 million per year. Economic assessment of the consequences of use of antibiotics in human medicine is essentially nonexistent, owing to the diversity and breadth of the issues.
Risk Assessment and Management
Qualitative and quantitative risk assessments are now being applied to address the complexity of resistance selection, transfer through the food chain, and human health consequences. For many antibiotics, such as tylosin, tilmicosin, and virginiamycin used in food animals and for which a risk assessment has been conducted, the estimated risk to human health is small. Fluoroquinolone use in water to treat poultry disease, however, was deemed by FDA as an unacceptable risk to humans, and its approval was withdrawn. FDA now requires new animal drug sponsors to satisfy microbial food safety criteria for antibiotic products by submitting evidence outlined in regulatory guidance that appropriate use conditions are ensured.
Given the different resistance mechanisms, conditions selecting for resistance, and dissemination patterns of resistant microorganisms, a single approach to addressing the resistance issue is not possible. Instead, thorough, science-based risk assessments focused on individual microorganisms exposed to specific agents under specific conditions of use should guide selection of risk-management actions to minimize unintended consequences.
--- PAGE BREAK ---
Risk-management strategies to minimize and contain antibiotic-resistant foodborne bacteria are in place all along the food chain, but can be improved. The strategies that have been implemented include use of various antibiotic alternatives, implementation of judicious or prudent guidelines for use of antibiotics, and implementation of national resistance monitoring programs.
While prudent use of antibiotics should be practiced to limit resistance selection and maintain maximum benefit, responsible use is not necessarily reduced use—antimicrobials offer valuable benefits when used appropriately. Responsible use involves prescribing antimicrobial therapy only when it is beneficial to the patient, targets therapy to desired pathogens and use of appropriate drugs, and confines treatment duration. Prudent-use guidelines for food antimicrobial agents and sanitizers need to be developed, validated, and implemented. Additionally, effective alternatives to antibiotics should be explored.
The key points of influence that food scientists have in preventing the spread of antibiotic-resistant and antibiotic-sensitive pathogenic microorganisms in foods are preventing them from entering the food supply, and if present, inactivating them or preventing their growth. The use of multiple hurdles in food manufacturing is likely to combat resistance to singular food safety interventions. Surveillance programs and food attribution models should be explored as means for measuring the effectiveness of the food industry’s microbiological interventions. Currently available data indicate that microbial interventions are equally effective for antimicrobial-susceptible and antimicrobial-resistant microorganisms. Further studies are needed to confirm this.
The Regulatory Environment
The regulatory environment is geared toward protecting the public from additional risk without consideration of benefits, hence the emphasis on risk assessment. Within the current U.S. regulatory framework, it is not possible for regulatory agencies to judge between the benefits of antibiotic use to livestock producers and risks to the public. Therefore, regulators must reject any practice that appears to produce an apparent risk, unless a demonstrated higher risk would occur upon rejection of the practice. There is evidence that there are significant human health benefits from antibiotic use to prevent subclinical disease in food animals and reduce levels of Salmonella and Campylobacter contamination of poultry carcasses.
In the future, the public health benefit as well as risks of losing the efficacy of existing and future antimicrobials must be considered. More specifically, it has been estimated that at least 40,000 illness-days per year are prevented by continued use of virginiamycin to reduce bacterial illness in poultry (Cox and Popken, 2004). Similar results have been reported for enrofloxacin and macrolide use in poultry (Cox and Popken, 2006). Thus, in such situations the risk of antibiotic use to control subclinical disease is more than compensated for with a human health benefit.
In Europe, the elimination of the use of antibiotics for feed efficiency and growth promotion—which was done on the basis of the precautionary principle—resulted in increased intestinal disease in animals, concomitant use of more therapeutic antibiotics, and an increase in resistance. For example, while the total use of antibiotics in animals in Denmark decreased 30% between 1997 (before the ban) and 2004, there was a 41% increase in therapeutic uses between 1999 (after ban) and 2004. Although the prevalence of resistant strains decreased for some antibiotics in some animals, prevalence increased for other antibiotics, bacteria, and animals.
Regulatory targeting of specific antibiotic-resistant foodborne pathogens may not be the most successful or cost-effective means to reduce overall foodborne illness. A Hazard Analysis Critical Control Point approach applied throughout the food system is the most-effective measure to control pathogens and reduce foodborne illness. Most of the interventions applied at critical control points are equally effective in controlling microbes, regardless of their resistance to antibiotics. Thus, applying interventions to control foodborne pathogens in general, rather than focusing specifically on antibiotic-resistant strains would have the greatest impact in reducing overall foodborne illness.
--- PAGE BREAK ---
IFT Expert Panel on Antimicrobial Resistance
Michael P. Doyle (Chair), Regents Professor and Director, Center for Food Safety, University of Georgia, Griffin.
Francis (Frank) Busta, Director, National Center for Food Protection and Defense, Professor Emeritus, Food Microbiology and Emeritus Head, Dept. of Food Science & Nutrition, University of Minnesota, St. Paul.
Bruce R. Cords, Vice President, Food Safety and Public Health, Ecolab, Inc.
P. Michael Davidson, Professor, Dept. of Food Science & Technology, University of Tennessee, Knoxville.
John Hawke, Associate Professor, Dept. of PBS, Louisiana State University, Baton Rouge.
H. Scott Hurd, Director, WHO Collaborating Center for Risk Assessment and Hazard Identification in Foods of Animal Origin, Iowa State University, Ames.
Richard E. Isaacson, Professor and Chair, Dept. of Veterinary and Biomedical Sciences, University of Minnesota, St. Paul.
Karl Matthews, Associate Professor, Dept. of Food Science, Rutgers University, New Brunswick, N.J.
John Maurer, Associate Professor, Dept. of Avian Medicine, University of Georgia, Athens.
Jianghong Meng, Associate Professor, Dept. of Nutrition & Food Science, University of Maryland, College Park.
Thomas J. Montville, Professor, Food Microbiology, Dept. of Food Science, Rutgers University, New Brunswick, N.J.
Thomas R. Shryock, Senior Microbiology Technical Advisor, Elanco Animal Health, Greenfield, Ind.
John N. Sofos, Professor, Dept. of Animal Sciences, Colorado State University, Ft. Collins.
Anne K. Vidaver, Professor and Head, Dept. of Plant Pathology, University of Nebraska, Lincoln.
Lyle Vogel, Director, Scientific Activities Division, American Veterinary Medical Association, Schaumburg, Ill.
IFT Staff Contributors to the Expert Report
Jennifer Cleveland McEntire, Research Scientist, Dept. of Science and Technology Projects, Institute of Food Technologists, Washington, D.C.
Rosetta Newsome, Director, Science and Communications, Institute of Food Technologists, Chicago, Ill.
Fred Shank, Vice President, Science, Communications, and Government Relations, Institute of Food Technologists, Washington, D.C.
The contributions of the expert panelists represent their individual scientific perspective and not necessarily that of their employers.
Michael P. Doyle, Chair of the Expert Panel on Antimicrobial Resistance, is Regents Professor and Director, Center for Food Safety, University of Georgia, Griffin. Rosetta Newsome contributed to the development of this summary. Send reprint requests to her at [email protected].
www.ift.org
Members Only: Read more about antimicrobial resistance online at www.ift.org. Type the keywords into our search box at the upper left side of our home page.
References
Aiello, A.E., Marshall, B., Levy, S.B., Della-Latta, P., Lin, S.X., and Larson, E. 2005. Antibacterial cleaning products and drug resistance. Emerg. Infect. Dis. [serial on the Internet] 11(10). www.cdc.gov/ncidod/EID/vol11no10/04-1276.htm.
Chee-Sanford, J.C., Aminov, R.I., Krapac, I.J., Garrigues-Jeanjean, N., and Mackie, R.I. 2001. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl. Environ. Microbiol. 67: 1494-1502.
Cloete, T.E. 2003. Resistance mechanisms of bacteria to antimicrobial compounds. Intl. Biodeter. Biodegrad. 51: 277-282.
Courvalin, P. 2005. Antimicrobial drug resistance: Prediction is very difficult, especially about the future. Emerg. Infect. Dis. 11(10). www.cdc.gov/ncidod/EID/vol11no10/05-1014.htm.
Cox, L.A. Jr. and Popken, D.A. 2004. Quantifying human health risks from virginiamycin used in chickens. Risk Anal. 24: 271-288.
Cox, L.A. Jr. and Popken, D.A. 2006. Quantifying potential human health of animal antibiotic use: Enrofloxacin and macrolides in chickens. Risk Anal. 26(1): 135-146.
Dever, L.A. and Dermody, T.S. 1991. Mechanisms of bacterial resistance to antibiotics. Arch. Intern. Med. 151: 886-895.
GAO. 2004. Antibiotic resistance. Federal agencies need to better focus efforts to address risk to humans from antibiotic use in animals. GAO-04-490. U.S. General Accounting Office, Washington, D.C. www.gao.gov/new.items/d04490.pdf.
Lear, J.C., Maillard, J.Y., Dettmar, P.W., Goddard, P.A., and Russell, A.D. 2002. Chloroxylenol-and triclosan-tolerant bacteria from industrial sources. J. Ind. Microbiol. Biotechnol. 29: 238-242.
Linton, A.H. 1977. Antibiotic resistance: The present situation reviewed. Vet. Rec. 100: 354-360.
McBain, A.J., Bartolo, R.G., Catrenich, C.E., Charbonneau, D., Ledder, R.G., Price, B.B., and Gilbert, P. 2003. Exposure of sink drain microcosms to triclosan: Population dynamics and antimicrobial susceptibility. Appl. Environ. Microbiol. 69: 5433-5442.
Nield, B.S., Holmes, A.J., Gillings, M.R., Recchia, G.D., Mabbutt, B.C., Nevalainen, K.M., and Stokes, H.W. 2001. Recovery of new integron classes from environmental DNA. FEMS Microbiol. Lett. 195: 59-65.
Prescott, J. 2000. Antimicrobial drugs: Miracle drugs or pig feed? Adv. Pork Production 11: 37. www.banffpork.ca/proc/2000pdf/Chap05-Prescott.pdf.
Riesenfeld, C.S., Goodman, R.M., and Handelsman, J. 2004. Uncultured soil bacteria are a reservoir of new antibiotic resistance genes. Environ. Microbiol. 6: 981-989.
Russell, A.D. 2003. Biocide use and antibiotic resistance: The relevance of laboratory findings to clinical and environmental situations. Lancet Infect. Dis. 3: 794-803.
Russell, A.D., Furr, J.R., and Maillard, J.Y. 1997. Microbial susceptibility and resistance to biocides. ASM News 63: 481-487.
Sundin, G.W. 2002. Distinct recent lineages of the strA-strB streptomycin-resistance genes in clinical and environmental bacteria. Curr. Microbiol. 45: 63-69.
