Simulating Digestion
FOOD SAFETY & QUALITY
Various attempts have been made over the past several decades to simulate the human gastrointestinal (GI) tract. The University of Massachusetts at Amherst and the University of California at Davis are among the schools conducting research on modeling of the human GI tract, and TNO Quality of Life (www.tno.nl) in the Netherlands and the Institute for Food Research (www.ifr.ac.uk) in the United Kingdom are among the research groups that have developed commercially available artificial GI tracts.
 A Brief Look at Digestion
A Brief Look at Digestion
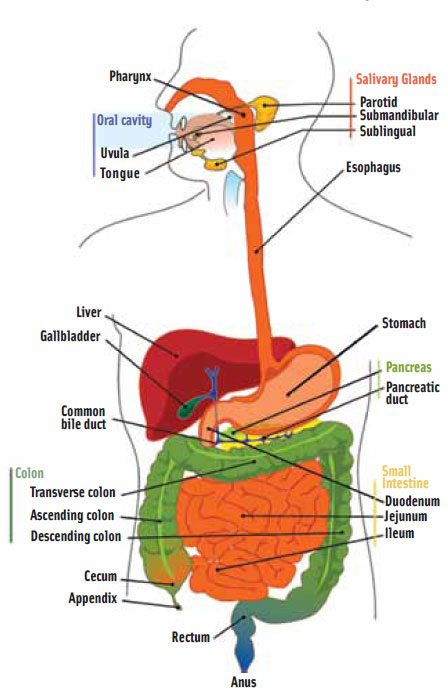
The human digestive system consists of the mouth, esophagus, stomach, small and large intestines, rectum, and anus. During digestion, food breaks down both mechanically and chemically to allow absorption of nutrients. Enzyme-containing digestive juices chemically break down proteins, carbohydrates, and fats while muscle contractions (peristalsis) throughout the GI tract propel food through the tract and aid in the digestion of food and absorption of nutrients.
In the mouth, food is chewed to reduce its particle size and mixed with enzyme-containing saliva. The mixture passes through the esophagus to the stomach, where it mixes with gastric juices and acid then empties into the small intestine, where it mixes with digestive juices from the pancreas and liver (bile). The digested nutrients are absorbed through the intestinal walls and transported throughout the body while undigested products pass into the colon for microbial fermentation and water absorption and subsequently exit from the body.
Research at UMass
Increasing interest in designing functional foods has stimulated food scientists to find out what artificial GI tract systems had been developed elsewhere, mainly in the pharmaceutical area, according to David Julian McClements, Professor of Food Science at the University of Massachusetts at Amherst. Food scientists, realizing that many of the systems used in the pharmaceutical industry are unsuitable for foods because foods have greater compositional and structural complexity than drugs, have put much work into understanding what important phenomena occur in the GI tract and how they could be modeled more accurately in vitro.
Food scientists, he says, have tried to develop systems based on knowledge of pharmaceuticals, nutrition, and medicine that more accurately represent the composition of the fluids in the different regions of the GI tract, the transit times, and the mechanical forces involved. They have used new techniques, such as confocal microscopy, particle size analysis, zeta-potential measurements, and interfacial rheology and tension, to take a more fundamental physicochemical approach to understanding the behavior of foods through simulated GI tracts.
--- PAGE BREAK ---
The human body, McClements says, is very complex and varies from person to person, depending on age, sex, health status, and type of meal, so it is difficult to accurately simulate the digestion process. Scientists need better knowledge of these variations for many different food systems, he says, and to develop standardized methods so that data from one lab can be compared to data from another since each researcher typically uses different methods. As part of his research, McClements has extensively reviewed the scientific literature on artificial digestive systems (see sidebar).
Some large food companies have used in vitro systems to screen different formulations for their effects on digestion of food components, he says. This information is being used to design functional foods for specific health benefits (e.g., satiety). Food and food ingredient companies can use these systems as a tool to rapidly screen many formulations so that a few promising candidates can be tested in more detail using animal and human studies. These systems are also being used as a means of understanding fundamental physicochemical mechanisms, McClements concludes.
TNO’s Intestinal Models
TNO has developed an artificial GI tract, the TNO-Intestinal Model (TIM). The multicompartment, dynamic computer-controlled system has been extensively validated based on data from human and animal nutritional research and has been used for studying, among other things, availability of nutrients for absorption, interactions between nutritional and functional food compounds, effects of food processing on the nutritional and functional quality stability of probiotics, and efficacy of prebiotics in the upper and lower GI tract.
Two versions are available. The TIM-1 system consists of compartments representing the stomach and the small intestine. It simulates such parameters as body temperature; flow of saliva, gastric and pancreatic juice, including digestive enzymes, and bile; peristalsis for mixing and gastrointestinal transit times; regulation of gastric and intestinal pH; and continuous removal of digested lipophilic and hydrophilic compounds. It can mimic specific conditions related to age (infant, adult, elderly), type of meal (e.g., liquid, solid, high- or low-fat), and health or disease status.
The TIM-2 system simulates the conditions in the large intestine (colon). This includes pH, anaerobiosis, and gradual intake of predigested meal compounds coming from the small intestine. The compartments contain human microflora at the same density and complexity, but other beneficial or pathogenic bacteria can be added, and the system allows removal of fermentation products from the lumen. The fermentation properties of dietary compounds such as fibers, probiotics, prebiotics, and antioxidants can be investigated by analysis of metabolites such as short-chain fatty acids and ammonia, and/or composition and metabolic activity of the colon microflora.
TNO started developing the TIM system in 1992. The initial development was the gastric compartment in 1992, followed by the small intestine in 1993 and the large intestine in 1995. The TIM system was made commercially available for contract research in 1994 and since 1995 has been leased to various universities and food and pharmaceutical companies. In addition to their use by such pharmaceutical companies as GSK and AstraZeneca, the TIM systems are being used for novel and functional food studies by such major international food, feed, and food ingredient companies as Nestlé, Danone, Chr. Hansen, Adisseo, and smaller companies. In early 2009, TNO installed a TIM system at Rutgers University for collaboration on botanicals research, and in early 2010 TNO installed TIM systems at Seventh Wave Labs, a company in Missouri that performs contract research for pharmaceutical companies.
--- PAGE BREAK ---
Over the years, according to TNO’s Rob Havenaar, Senior Scientist, Gastrointestinal Research, the TIM system has been continuously improved. The most recent development is the TIM-Carbo system, which measures the digestibility of carbohydrates and the intestinal uptake of monosaccharides from food products—as single ingredients or in complex food matrices—and predicts the human glycemic response. The system can be used by food companies as a rapid and highly predictive tool to support their development of new carbohydrate food products, he says.
Havenaar, co-inventor of the TIM system with TNO’s Mans Minekus, says that unlike other in vitro GI models, the TIM system provides the most advanced dynamic simulation of the GI tract. It can accurately mimic the specific dynamic GI conditions of infants, adults, and the elderly under healthy conditions as well as GI disease conditions. This offers the unique opportunity to study novel and functional foods under realistic GI conditions without ethical constraints on a time- and cost-efficient basis, he says. Samples can be taken from different parts of the GI tract system at realistic transit times. In this way, he says, the system can perform studies that can’t be done in humans, resulting in mechanistic understanding of and claim support for novel food products.
A major challenge ahead, Havenaar says, is to develop a realistic mucosal transport membrane inside the TIM system. The TIM system uses a dialysis membrane for intestinal absorption to simulate the luminal GI conditions. But because of the lack of a realistic mucosal layer for intestinal transport, TIM experiments are combined with off-line assays with cultured intestinal cell lines.
According to Havenaar, the system has been used in the food industry for studies on the nutritional quality of novel and fortified food products by studying the digestion and availability for absorption of different types of food products, ranging from proteins to n-3 fatty acids and from fat-soluble vitamins to minerals and trace elements. The system has also been used for studies on the development and claim support of functional foods such as probiotics, prebiotics, bioactive peptides, lipase inhibitors, fat and cholesterol binders, and antioxidants and for safety analysis such as potential food allergens, lack of availability of potential toxic compounds such as heavy metals, and gene stability of genetically modified products. These studies, Havenaar says, have been described in more than 60 peer-reviewed publications.
IFR’s Dynamic Gastric Model
After more than 15 years of studies designed to understand and then simulate the human digestive process, the Institute of Food Research (IFR) developed a computer-controlled artificial stomach called the Dynamic Gastric Model (DGM). It was first used commercially in 2008. The system processes real foods and simulates physical mixing, transit, and breakdown forces (including flow, shear, and hydration); automatically adjusts for gastric residence time, acid and enzyme additions (quantity and rate), and physical processing; and provides such data as residence time, emptying profiles, pH gradients, gastric additions/flow rates, and other factors, accurately replicating food breakdown and mixing in the stomach.
To date, access to the DGM has been solely through contract research services provided by IFR’s virtual company—the Model Gut Group (www.modelgut.com) —or through academic collaborations with IFR. According to Richard Faulks, retired from IFR and now Senior Scientist for Model Gut, most of the major global food companies that have active R&D programs have used the DGM to study such things as food processing and breakdown, nutrient release, bioactive release, generation of bioactive or allergenic components, probiotic and pathogen survival, and satiety.
--- PAGE BREAK ---
Faulks, who was co-inventor of the system with Leatherhead’s Martin Wickham when both were at IFR, says that the current emphasis is to use models to assess the satiating capacity of foods and new formulations. How a food or food ingredient company can best utilize these systems, he says, depends on the questions that the company asks and whether it is possible to interpret the data from the model in terms of physiological responses. Major challenges ahead, he adds, include developing a realistic chew model and a dynamic duodenal model, currently being explored at Birmingham University.
IFR has used the DGM to evaluate, among other things, persistence of allergenic proteins and peptides in the upper GI tract; survival of probiotics in the upper GI tract, with various media; lipid digestibility and the quality of the emulsion interface; lipid digestibility and emulsion stability in the stomach; glycemic index; effects of viscosity on gastric emptying; and mass transfer of lipophilics from food to lipid phases during digestion.
Faulks says that the food industry was initially the major customer, but over the past year pharmaceutical work has increased for two main reasons: some difficulty in establishing the relationships between the properties of a food and its physiological impact, especially in a mixed diet, and general curtailment of R&D spending. The pharmaceutical industry has many products that have been licensed at great expense and that can be reformulated under the same license, provided the new product performs as well or better. Thus, a realistic in vitro test is a good starting point, offering a potential income stream based on existing drugs rather than on new drugs in the pipeline.
IFR intends to produce a commercial version of the DGM for sale in the near future. The new version will have modifications that enhance its use for pharmaceutical applications.
UC Davis’s Human Gastric Simulator
R. Paul Singh, Professor of Food Engineering at the University of California at Davis, says that the IFR and TNO systems account for a number of biochemical reactions that occur in vivo and the opportunity to introduce various acids, enzymes, and other gastric and intestinal fluids makes these units useful in digestion studies. With recent advances in other noninvasive techniques, such as magnetic resonance imaging, he says, researchers now have a better capability to validate the results obtained from these in vitro systems. While these units have obvious applications in the pharmaceutical area, their value is further enhanced in being able to study food and drug interactions.
The complexities of the human GI tract challenge researchers to develop improved versions of in vitro systems, he says. Although researchers can conduct animal trials and some noninvasive studies with humans, the need for improved in vitro systems is crucial to develop the next generation of foods for health.
--- PAGE BREAK ---
Mechanical forces along with chemical and enzymatic reactions play an important role in digesting foods, Singh says. The structure of a food material, either created in nature or manufactured in a processing operation, has a major influence on how that food is digested. Since the release of nutrients, allergens, or other bioactives is dictated by the mechanical and chemical forces acting on the chewed food as it travels through the GI tract, understanding the role of critical forces that maintain a specific food structure and how they may be overcome in the gastric environment is necessary in studies related to bioaccessibility of nutrients embedded within that structure. Controlling the targeted release of nutrients or bioactives in a desired region of the GI tract for maximum efficacy is therefore a challenging area of research for the future.
Singh and his coworker M.J. Ferrua therefore used computational fluid dynamics to develop a 3-dimensional model of the shape and motility pattern of the stomach wall during digestion and characterize the fluid dynamics of gastric contents. Subsequently, Singh and his coworker Fanbin Kong developed an artificial stomach they call the Human Gastric Simulator (HGS), for studying gastric digestion of foods.
The HGS consists of a latex vessel that simulates the stomach and a series of rollers on belts that are driven by motor and pulleys to create a continuous contraction of the latex wall. It also incorporates gastric secretion, emptying systems, and temperature control that enable accurate simulation of the digestion process for detailed investigation of changes in physical chemical properties of ingested foods.
Singh says that the HGS, with its unique system of wall movement, is able to achieve greater contraction of the lower part of the vessel. The antral contractions—contractions at the antrum, the bottom of the stomach—create a retropulsive jet for improved mixing and disintegration of food samples. Studies are now underway to characterize the flow patterns created in the vessel due to peristaltic movement of the walls.
By providing a reasonably realistic set of conditions that mimic the human digestion process, Singh says, the HGS can be used to study some of the changes in food constituents that occur during digestion and the influence of physiological conditions, including acid and enzyme secretion and contraction forces, on disintegration kinetics of foods and nutrient release.
--- PAGE BREAK ---
For an interesting discussion of the relationship between cooking and digestion, see the presentation by Nestle’s Heribert Watzke at www.ted.com/talks/heribert_watzke_the_brain_in_your_gut.html.
Other Artificial Systems
A number of other systems have been developed to simulate various functions of the human body:
• Electronic Noses. These instruments detect and measure volatile organic compounds and can be used for screening aromas. They are said to be able to identify 40,000 molecules that cause off-odors. Alpha M.O.S. (www.alpha-mos.com) offers its FOX, Gemini, and other instruments; Electronic Sensor Technology (www.estcal.com) offers its zNose instruments; and Syft Technologies Ltd. (www.syft.com) offers its Voice200 instrument.
• Electronic Tongues. These instruments are designed for measuring dissolved compounds and taste substances in liquids and are said to accurately identify the five tastes (bitter, salty, sweet, sour, and umami) while distinguishing pungency, spiciness, and astringency. Alpha M.O.S. offers its Astree instrument. Intelligent Sensor Technology (www.insent.co.jp) offers its Taste Sensing System TS-5000Z, which features artificial lipid-based taste sensors to evaluate sourness, saltiness, bitterness, sweetness, umami, astringency, and kokumi (richness) of foods and beverages. It uses artificial lipid membranes to imitate the taste reception mechanism of living organisms and is said to be able to determine both initial taste and aftertaste.
• Electronic Eye. The IRIS Visual Analyzer is offered by Alpha M.O.S. for scrutiny of complex food products.
Recent Papers on Artificial Digestion Models
Ferrua, M.J. and Singh, R.P. 2010. Modeling the fluid dynamics in a human stomach to gain insight of food digestion. J. Food Sci. 75(7): R151–R162.
Hur, S.J., Lim, B.O., Decker, E.A., and McClements, D.J. 2010. In vitro human digestion models for food applications. Food Chem., in press.
Kong, F. and Singh, R.P. 2010. A human gastric simulator (HGS) to study food digestion in human stomach. J. Food Sci. 75(9): E627–E635.
McAllister, M. 2010. Dynamic dissolution: A step closer to predictive dissolution testing? Molec. Pharmaceutics 7(5): 1374–1387.
McClements, D.J. and Li, Y. 2010. Review of in vitro digestion models for rapid dcreening of emulsion-based systems. Published online at www.rsc.org/foodfunction, by the Royal Society of Chemistry, September 24.
Neil H. Mermelstein, a Fellow of IFT, is Editor Emeritus of Food Technology
[email protected]


