Chips Advance Toxicology Testing
FOOD SAFETY AND QUALITY
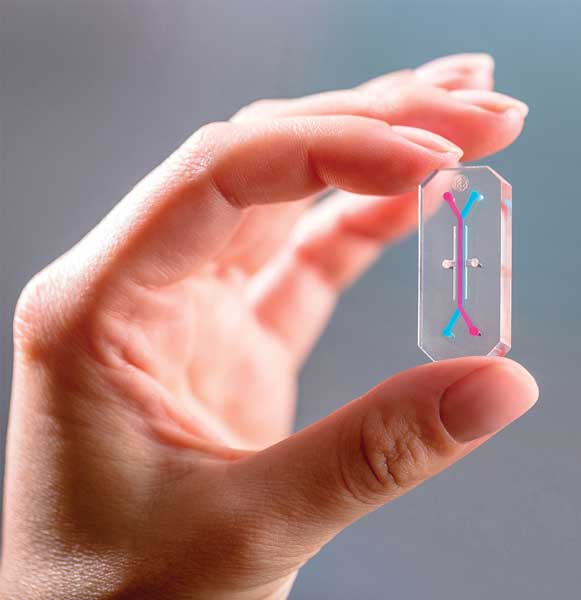 Chips—not potato chips or corn chips, but miniature systems containing living cells—can be used to test the effects of drugs, chemicals, and foods on human physiology and health. These organs-on-chips are transparent, flexible, domino-shaped polymeric systems (about the size of a thumb drive) that contain living cells of human organs. They are part of what Emulate Inc. calls its Human Emulation System.
Chips—not potato chips or corn chips, but miniature systems containing living cells—can be used to test the effects of drugs, chemicals, and foods on human physiology and health. These organs-on-chips are transparent, flexible, domino-shaped polymeric systems (about the size of a thumb drive) that contain living cells of human organs. They are part of what Emulate Inc. calls its Human Emulation System.
According to Suzanne Fitzpatrick, senior advisor for toxicology at the Center for Food Safety and Applied Nutrition (CFSAN) of the U.S. Food and Drug Administration (FDA), organs-on-chips technology has been the focus of a public-private collaboration between the FDA, the Defense Advanced Research Projects Agency, and the National Institutes of Health since 2012, and millions of dollars in grants have been awarded to universities nationwide to advance this research.
Researchers at Harvard University’s Wyss Institute for Biologically Inspired Engineering helped develop the organs-on-chips concept and published a paper on the topic in Science in 2010. Emulate was spun out from Harvard in 2015 to further develop the concept and the technology. The company holds an exclusive worldwide license from Harvard for the technology and related systems. Emulate’s president and chief scientific officer, Geraldine Hamilton, said that the company’s Human Emulation System recreates the natural physiology of specific human tissues and organs and is designed to provide a predictive model of human response to diseases, drugs, chemicals, and foods with greater precision and detail than other preclinical testing methods such as cell culture or animal-based experimental testing. The company has developed working models of the human lung, liver, intestine, skin, and brain; is developing designs for other organ chips for the kidney and heart; and has published several papers with industry partners. Chips have also been made with cells of dog and rat organs for cross-species comparisons.
On April 11, 2017, Emulate and the FDA signed a three-year cooperative research and development agreement (CRADA) to collaborate in evaluating the company’s organs-on-chips technology as a platform for toxicology testing to meet the FDA’s criteria for approval of foods. Hamilton said that Fitzpatrick and her colleagues in the FDA’s Division of Toxicology recognized the progress that had been made at Emulate to commercialize the technology and determined that the timing was right to establish the CRADA to assess the technology and its fit in the regulatory process. The ultimate goal of the collaboration, Fitzpatrick said, is to predict how specific organs will respond to exposure to potential chemical hazards found in foods, cosmetics, and dietary supplements with greater precision than other toxicological testing methods being used.
How the System Works
The Human Emulation System emulates the natural physiology and mechanical forces that cells experience within the human body, Hamilton said, recreating the complex, dynamic state in which living cells function within a real human organ. The system has three components: organ-chips, instrumentation, and software. Each chip contains two micro-engineered hollow channels lined with tens of thousands of living cells and tissues. These microchannels are connected by porous membranes that allow compartmentalization of different cell types and allow cell-to-cell interactions similar to those found in the human body. They also facilitate the regulation of the biochemical environment within the chip. The microchannels allow nutrient media to be tailored to the specific cell type that lives in each channel, Hamilton said. Researchers can sample the outflow from the chip for analysis, much like they would analyze blood for biomarkers that can be indicators of health or disease. Growth factors, hormones, dissolved gases, and small molecules such as salts and nutrients can be precisely re-created and controlled by continuously flowing blood or blood substitutes that bring in fresh nutrients, soluble factors, and dissolved gases while washing away waste. Immune cells such as white blood cells can be added to the system in a manner that mimics the dynamics found in the capillaries. Researchers can introduce medicines, chemicals, and toxins to the chip’s environment to test the organ’s response. The chips are also flexible and transparent.
The instrument pumps fluid through the microchannels to emulate the shear stress forces arising from blood flow and stretches or flexes the chip to emulate the mechanical forces that cells experience during breathing, for example. Their flexibility aids in re-creating breathing motions or muscle contractions, and their transparency allows researchers to view the organ’s behavior and response at the cellular and molecular level.
The software provides precise control of the organ system, including cell architecture, tissue-to-tissue interfaces, mechanical forces, and biochemical surroundings; apps have been created to collect and analyze the data. The techniques the researchers use to gather results are similar to those customarily used in in vivo and in vitro studies. The chips allow researchers to generate the same types of samples and analysis that an animal study or conventional cell culture system would, such as microscopy and visualization of the cells, measurement of protein markers, and gene expression.
Hamilton said that the Human Emulation System’s precise tuning of extracellular matrix, tissue-tissue interfaces, mechanical microenvironment, chemical surroundings, and immune components results in a biologically accurate model of human physiology. For example, the lung-chip has been used to re-create and quantify key aspects of inflammation and immune response in the lung after bacterial infection and viral infection. The intestine-chip recreates peristalsis-like motions and flow, and its epithelial cells form structural folds within the chip that better re-create the intestinal barrier in the gut than conventional culture systems. The intestine-chip can be colonized by microbial flora and has been used to reproduce the beneficial effects of probiotic bacteria on barrier function in the gut.
Evaluating the System
After the CRADA was signed, Emulate representatives installed the Human Emulation System in the FDA’s Division of Toxicology laboratories located in the CFSAN Office of Applied Research and Safety Assessment. Division director Robert Sprando said that the CRADA research is initially beginning with the liver-chip because the liver is the site of toxin filtration and metabolic breakdown and is particularly susceptible to organ toxicity. Drug-induced liver injury is a leading cause of acute liver failure in the United States and Europe, he said, and liver injury induced by herbal and dietary supplements accounted for 20% of the liver injury cases reported in the United States in 2013–2014.
 The research carried out under the CRADA to evaluate the Human Emulation System is divided into two phases, Sprando said. During phase one, Emulate’s scientists will train FDA scientists in the use, care, and maintenance of the system and on best practices for initiating experiments using the system and will offer technical support. During phase two, the FDA scientists will expose liver cells growing on the liver-chip to chemicals known to produce deleterious effects in the liver and for which extensive scientific literature is available. This will permit the FDA scientists to assess the system response (the ability to predict toxicity) and system performance. Chip-generated data will be compared to data obtained using accepted toxicological testing procedures. The performance evaluation may include, but is not limited to, identification of statistically valid sample sizes, error levels, and confidence intervals (predictability); evaluation of accuracy, precision, repeatability, sensitivity, and specificity; and assessment of the technology’s limitations (system boundaries).
The research carried out under the CRADA to evaluate the Human Emulation System is divided into two phases, Sprando said. During phase one, Emulate’s scientists will train FDA scientists in the use, care, and maintenance of the system and on best practices for initiating experiments using the system and will offer technical support. During phase two, the FDA scientists will expose liver cells growing on the liver-chip to chemicals known to produce deleterious effects in the liver and for which extensive scientific literature is available. This will permit the FDA scientists to assess the system response (the ability to predict toxicity) and system performance. Chip-generated data will be compared to data obtained using accepted toxicological testing procedures. The performance evaluation may include, but is not limited to, identification of statistically valid sample sizes, error levels, and confidence intervals (predictability); evaluation of accuracy, precision, repeatability, sensitivity, and specificity; and assessment of the technology’s limitations (system boundaries).
Sprando said that CFSAN is the FDA lead agency for the Tox21 partnership between the FDA, the U.S. Environmental Protection Agency, the National Institutes of Health’s National Center for Advancing Translational Sciences, and the National Institute of Environmental Health Sciences, all of which take turns as the lead agency. Tox21, short for Toxicology in the 21st Century, was established in 2008 to introduce and develop new in vitro technologies for use by the federal government and its stakeholders. Evaluating Emulate’s Human Emulation System in a regulatory testing environment (i.e., FDA laboratories), he said, provides a unique perspective that other laboratories cannot provide.
Hamilton said that having a Human Emulation System in FDA laboratories will pave the way for qualifying the use of the technology and integrating it within the existing regulatory framework for product testing. The FDA is one of the company’s pivotal collaborators along with the company’s industry partners and academic experts who also collaborate with Emulate, working to enable the organs-on-chips technology to become an industry standard.
Heading into Space
On June 20, 2017, Emulate received a grant for $2 million to use the Human Emulation System to evaluate the effects of space travel on human brain cells, the results of which may provide new insights to understand neurological diseases on Earth. The grant was provided by the National Institutes of Health’s National Center for Advancing Translational Sciences. The National Aeronautics and Space Administration’s Center for the Advancement of Science in Space will coordinate the study on the International Space Station. The brain-chip consists of living human neuronal and vascular endothelial cells, and experiments will be conducted under healthy and inflamed states to assess how space travel affects neuronal function.
 Neil H. Mermelstein, IFT Fellow,
Neil H. Mermelstein, IFT Fellow,
Editor Emeritus of Food Technology
[email protected]


