Resistance and Adaptation to Food Antimicrobials, Sanitizers, and Other Process Controls
This IFT Scientific Status Summary discusses the potential for microorganisms to become resistant to antimicrobials and sanitizers used in food processing.
Most antimicrobials used in food manufacture have been in use for about 50 to 100 years. A few antimicrobials, e.g., sulfites and nitrites, have been in use for an even longer period of time. Similarly, sanitizing agents, used to reduce microorganisms on processing equipment, have been in use for nearly 100 years. Concern has been recently raised, however, about the potential for target pathogenic microorganisms to develop resistance to these compounds.
Surprisingly, despite the considerable length of time that food antimicrobials and equipment sanitizers have been used in the food industry, there is little data about the development of microbial resistance to these compounds. This lack of data might be viewed as a good indication that resistance development is probably not a major problem.
Concern remains, however, for three reasons. One concern is the increasing incidence of microorganisms exhibiting resistance to antibiotics used for therapeutic purposes in human and animal medicine. A second concern is the increasing reliance on antimicrobials and sanitizers as primary tools for controlling the outgrowth of pathogens in foods. A third concern is the evidence indicating that tolerance to antimicrobials, sanitizers, and other preservation processes may be generated within microorganisms exposed to certain stresses.
If antimicrobials and sanitizers are to play a major role in effective control of foodborne pathogens, food manufacturers and others within the food industry must know more about the potential for development of resistance among target microorganisms. This Scientific Status Summary explores the potential for such resistance development. In addition, the Summary examines the interrelationship of resistance to antimicrobials with resistance to environmental controls (e.g., sanitizers) used within food manufacturing.
This Summary specifically addresses: (1) antimicrobial and sanitizer functions, (2) mechanisms for the development of resistance and tolerance to antimicrobials and sanitizers, (3) results of studies on resistance development to traditional and novel food antimicrobials and the effect of microbial adaptation and tolerance to these substances, and (4) impact of microbial resistance development on food safety.
 Food Antimicrobials and Sanitizers
Food Antimicrobials and Sanitizers
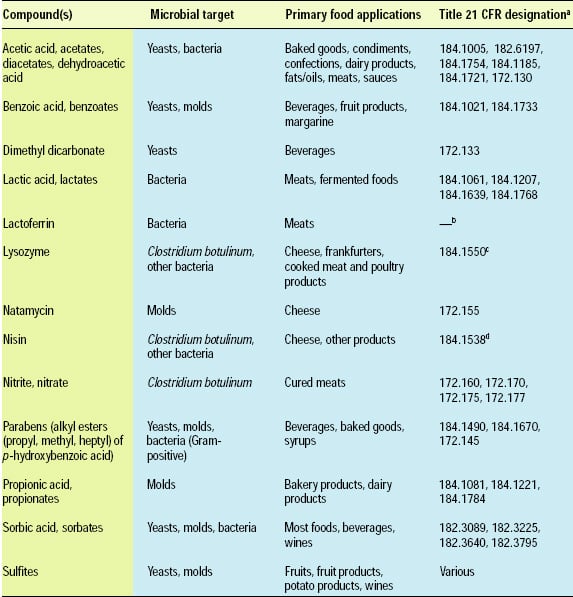
Food antimicrobials are compounds used to extend the lag phase or kill microorganisms. They are different than therapeutic antibiotics (e.g., penicillin, tetracyclines) used to treat human or animal disease. Food antimicrobials are sometimes called “preservatives.” The term “preservative,” however, often includes antioxidants in addition to antimicrobials. “Antimicrobial” is the more specific term in the context of this discussion and, therefore, is used throughout this document. Antimicrobials may be classified as “traditional” or “naturally occurring” (Davidson, 2001). A number of traditional antimicrobials, e.g., acetic acid and benzoic acid, are approved for use in foods by most international regulatory agencies. Antimicrobials approved for use in the United States are listed in Table 1.
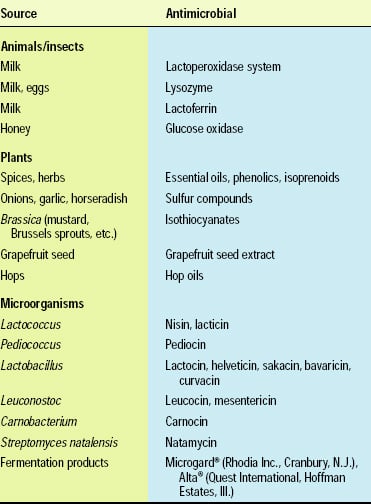 Naturally occurring antimicrobials include compounds that originate from microbial, plant and animal sources. A subgroup of naturally occurring antimicrobials is the bacteriocins, proteins produced by lactic acid bacteria, e.g., Lactococcus, Lactobacillus, and Pedicoccus species, and a few other bacteria. Only a few naturally occurring antimicrobials, such as nisin, natamycin, lactoferrin and lysozyme, have regulatory approval for application to foods (Table 1). Many additional antimicrobials, especially those derived from microorganisms, hold the potential for regulatory approval in the future (Table 2).
Naturally occurring antimicrobials include compounds that originate from microbial, plant and animal sources. A subgroup of naturally occurring antimicrobials is the bacteriocins, proteins produced by lactic acid bacteria, e.g., Lactococcus, Lactobacillus, and Pedicoccus species, and a few other bacteria. Only a few naturally occurring antimicrobials, such as nisin, natamycin, lactoferrin and lysozyme, have regulatory approval for application to foods (Table 1). Many additional antimicrobials, especially those derived from microorganisms, hold the potential for regulatory approval in the future (Table 2).
--- PAGE BREAK ---
Food antimicrobials are traditionally used for their ability to inhibit spoilage microorganisms and, thus, prolong shelf life and preserve food quality. Recently, however, antimicrobials have been used increasingly as primary interventions to inactivate or inhibit the outgrowth of pathogenic microorganisms in foods. Although food antimicrobials have been used for many years, few of these substances are used exclusively to control the growth of specific foodborne pathogens. Examples of those used exclusively to control specific pathogens are nitrite to inhibit the growth of Clostridium botulinum in cured meats, selected organic acid sprays to reduce pathogens on beef carcass surfaces, nisin and lysozyme to inhibit growth of C. botulinum in pasteurized process cheese, and lactate and diacetate to inactivate Listeria monocytogenes in processed meats (FDA, 2000). Generally, these compounds serve as the primary microbial controls among a combination of inhibitors and inhibitory conditions (e.g., low pH and low temperature). Such use of combinations of several microbial controls (multiple interventions) is sometimes called “hurdle technology” (Leistner, 2000; Leistner and Gorris, 1995).
Sanitizers, as defined by the Environmental Protection Agency, are “pesticide products that are intended to disinfect or sanitize, reducing or mitigating growth or development of microbiological organisms including bacteria, fungi or viruses on inanimate surfaces in the household, institutional, and/or commercial environment” (40 Code of Federal Regulations [CFR] 455.10). Sanitizers used by food manufacturers include chlorine and chlorine derivatives, iodine derivatives, quaternary ammonium compounds, acid-anionic sanitizers, hydrogen peroxide, peroxyacetic acid, and acidified sodium chlorite (21 CFR 178.1010). Sanitizers are generally used to inactivate target microorganisms on the food contact surfaces of cleaned food processing and food service equipment. A relatively novel use of these compounds is for the inactivation of microorganisms on raw, unprocessed food products, e.g., meat and poultry carcasses and fresh fruits and vegetables.
Responses of Microorganisms to Antimicrobials and Sanitizers
If a population of microorganisms is exposed to a sufficiently high concentration of an antimicrobial compound, susceptible cells will be killed. However, some cells may possess a degree of natural resistance or they may acquire it later through mutation or genetic exchange and will, therefore, survive and grow (Bower and Daeschel, 1999). To fully understand antimicrobial resistance, one must understand the mechanisms of action and/or the specific targets of an antimicrobial within a microorganism. For example, antibiotics used for therapeutic purposes often have specific target sites in a microbial cell and the development of resistance to these compounds is the result of changes in these target sites. These changes may include inactivation or modification of the antibiotic by enzymes within the cell, absence of or bypassing of an enzymatic or metabolic step targeted by the antibiotic, impaired uptake or efflux of the antibiotic, modification of the antibiotic target site, or overproduction of a target molecule (Russell et al., 1997). Unfortunately, while we know a great deal about the mechanisms of action and resistance to antibiotics used therapeutically, the precise mechanisms and targets of most food antimicrobials and sanitizers remain a mystery. Therefore, we are less able to predict and/or understand potential resistance to these groups of compounds.
The resistance responses of microorganisms to antimicrobials or sanitizers may be innate, apparent, or acquired. Innate resistance is a chromosomally controlled property that is naturally associated with a microorganism. Differences in resistance to antimicrobials occurring among different types, genera, species, and strains of microorganisms under identical environmental conditions and antimicrobial concentrations are most likely controlled innately. Mechanisms of innate resistance may include cellular barriers preventing entry of the antimicrobial (e.g., the outer membrane of Gram-negative bacteria and teichoic acids contained within Gram-positive bacteria), cellular efflux (i.e., mechanisms that pump compounds out of the cell), lack of a biochemical target for antimicrobial attachment or microbial inactivation, and inactivation of antimicrobials by microbial enzymes (Bower and Daeschel, 1999).
--- PAGE BREAK ---
Apparent resistance is related to assay or application conditions. As with any preservation technique, susceptibility to antimicrobials is dependent upon the conditions of the application. The presence of interacting stress conditions (e.g., low pH, high temperatures, high pressure) may increase or decrease the measured resistance of a microorganism. Food composition often has a major influence on the apparent activity of food antimicrobials, especially organic acids. Food pH is the most universally important factor that influences the effectiveness of food antimicrobials. Many food antimicrobials are weak acids. Because these acids are able to penetrate the cytoplasmic membrane of microorganisms more effectively in the protonated form, they are most effective in their undissociated form (Davidson, 2001). Therefore, the pKa (the pH at which 50% of the acid is in the undissociated form) of these compounds is important in selecting a particular compound for a specific application. The lower the pH of a food product, the greater the proportion of acid in an undissociated form, and the greater the antimicrobial activity. Polarity is another important factor that affects apparent activity (Davidson, 2001). Polarity relates both to the ionization of the molecule and the contribution of any hydrocarbon side groups or hydrophobic parent molecules. Antimicrobials must be lipophilic and soluble in the aqueous phase to attach and pass through the cell membrane. Microorganisms exposed to food antimicrobials in lipid-containing food systems will demonstrate apparent increased resistance due to the solubilization or binding of the antimicrobials by the lipids.
Acquired resistance results from genetic changes in the microbial cell through mutation or acquisition of genetic material from plasmids (Russell, 1991). Because antibiotics used for therapeutic purposes generally have specific target sites in microbial cells, they have greater potential to result in mutations and development of acquired resistance. In contrast, antimicrobials used nontherapeutically and sanitizers are generally non-specific, and the development of resistance to these compounds is caused primarily by innate factors (Russell et al., 1997).
Bacterial Stress Responses
Food preservation processes are designed to either inhibit the growth of or inactivate bacteria, depending upon the type and severity of the process used. Thus, food preservation exposes bacteria to both lethal and sub-lethal stresses. Bacteria may have different mechanisms for surviving these external environmental stresses. For example, the formation of endospores in response to stress is a survival strategy for Bacillus and Clostridium species. Bacteria that cannot form endospores undergo other significant physiological changes that enhance their ability to survive environmental stressors. Regardless of the specific microbial strategy, genetic regulatory modification is involved. Common genetic regulatory factors, called Sigma (σ) factors, are frequently involved in enhanced stress resistance. Sigma factors produced in response to a stress bind to core microbial RNA polymerase, conferring different promoter specificities and leading to the production of stress proteins which protect the cell from the stress. RpoS, for example, is a regulatory factor required for transcriptional activation of a large number of genes required for tolerance to environmental stresses, including growth phase-dependent acid tolerance. Rees et al. (1995) found that rpoS-deficient mutants were highly sensitive to food processing conditions compared with non-mutants. Abee and Wouters (1999) prepared a very good review of studies involving stress responses of foodborne bacterial pathogens.
Resistance of Microorganisms to Traditional Antimicrobials
Benzoic acid and its salts were one of the first groups of antimicrobials approved for application to foods in the United States. The primary application of benzoic acid and benzoates is to inhibit yeasts and molds in acidic foods. Differences in microbial resistance to benzoates occur as a result of differences in innate tolerance. Because benzoates are used primarily as antifungal agents, one might conclude that bacteria are generally more resistant to the compounds than fungi. In fact, however, bacteria are quite variable in their resistance to benzoates. Benzoates are used primarily as antifungals because: (1) they function best in the undissociated state, which is the predominant form of the compound at low pH in high acid foods; and (2) fungi are the primary spoilage microorganisms in acidic foods. Therefore, the innate resistance of yeasts and molds to benzoates is of greater concern than that of bacteria. A number of yeasts, including Schizosaccharomyces pombe and Zygosaccharomyces bailii, have been observed to grow in the presence of about 500 μg/mL benzoic acid (Warth, 1985). Other yeasts, including Pichia membranefaciens and Byssochlamys nivea, are also known to be resistant to benzoates (Chipley, 1993).
--- PAGE BREAK ---
Warth (1988) suggested that the mechanism by which yeasts develop resistance to weak acidic antimicrobials, including propionic as well as benzoic acids, is related to membrane permeability and the ability of the cells to continuously pump antimicrobials out of the cell. Some microorganisms on the other hand have innate resistance to benzoates because they metabolize the compounds. These bacteria—Bacillus, Pseudomonas, Corynebacterium, Micrococcus, and the mold Aspergillus—degrade benzoic acid through their β-ketoadipate pathway, in which benzoic acid is converted to succinic acid and acetyl Coenzyme A (Chipley, 1993).
Few studies examine the potential for acquired resistance to benzoic acid. Warth (1988) incubated a variety of yeasts, including Candida krusei, Hansenula anomala, Kluyveromyces fragilis, Kloeckera apiculata, Saccharomyces cerevisiae, Saccharomycodes ludwigii, S. pombe and Z. bailii, in the presence of either 0.25 mM (31 μg/mL) or 2 mM (244 μg/mL) benzoic acid. The minimum inhibitory concentration (MIC) or lowest concentration preventing growth for unexposed cells was significantly lower for cells exposed to these concentrations of benzoic acid than for cells previously exposed to sub-inhibitory concentrations of benzoic acid. Pre-exposure to benzoic acid caused a 1.4 to 2.2 fold increase in MIC, with Z. bailii and S. pombe exhibiting the greatest MIC increases. The proposed resistance mechanism was increased cellular efflux. There was no evidence to indicate any increased resistance due to mutation nor any evidence that the resistance was stable. Further, there is little or no evidence in the literature of acquired bacterial resistance to benzoic acid.
Sorbic acid has been used as an antimicrobial in foods in the United States since the 1940s when it was patented for use in foods and on packaging to retard spoilage by molds (Sofos and Busta, 1993). Innate resistance to sorbate is demonstrated by bacteria, including catalase-negative lactic acid bacteria, Sporolactobacillus, some Pseudomonas, yeasts (including Brettanomyces, Candida, Saccharomyces, Torulopsis, and Z. bailii), and molds (including Aspergillus, Fusarium, Geotrichum, Mucor, and Penicillium) (Sofos and Busta, 1993; Warth, 1985). As with benzoic acid, some microorganisms can metabolize sorbic acid. Molds isolated from cheese, including seven Penicillium species, exhibited growth in the presence of and degradation of 0.3 to 1.2% sorbate (Finol et al., 1982). Penicillium puberulum and Penicillium cyclopium were the most resistant species evaluated. Marth et al. (1966) demonstrated that Penicillium species isolated from cheese produced 1,3 pentadiene, which has a kerosene off-odor, from sorbic acid. Sorbic acid is also degraded by Mucor species to 4-hexenol and by Geotrichum species to 4-hexenoic acid and ethyl sorbate (Liewen and Marth, 1985). High numbers of lactic acid bacteria can produce ethyl sorbate, 2,4-hexadien-1-ol, 1-ethoxyhexa-2,4 diene, 5-hexadien-1-ol, and 2-ethoxyhexa-3,5 diene in sorbic acid-treated red wine (Liewen and Marth, 1985). The 2,4 hexadien-1-ol metabolic product can cause “geranium” type off-odors in wines and fermented vegetables (Liewen and Marth, 1985; Sofos and Busta, 1993).
As for benzoic acid, there is little evidence of acquired resistance to sorbic acid. Warth (1977) observed that Z. bailii grown in the presence of sorbic acid acquired resistance to subsequent exposure to the compound. Schroeder and Bullerman (1985) found little or no increase in the resistance of Penicillium digitatum or Penicillium italicum when exposed to increasing concentrations of sorbic acid. Bills et al. (1982) also investigated acquired resistance to sorbic acid using the osmotolerant yeast, Saccharomyces rouxii. The yeast was pre-conditioned by growth in the presence of 0.1% sorbic acid for four transfers. Preexposure significantly increased resistance of cells subsequently exposed to 0.1% sorbic acid, as evidenced by shorter lag times and/or shorter time to stationary phase.
To combat the effects of sorbic and other organic acids, yeasts have several mechanisms by which they can develop resistance. One mechanism for acquired resistance that has been demonstrated among yeasts is the triggering of an inducible, energy-requiring system that increases sorbic acid efflux (Bills et al., 1982; Warth, 1977). However, resistance of yeasts to sorbic acid and other weak acids probably involves more than one system (Brul and Coote, 1999). The mechanism by which organic acids inhibit microorganisms involves passage of the undissociated form of the acid across the cell membrane lipid bilayer. Once inside the cell, the acid dissociates because the cell interior has a higher pH than the exterior. Protons generated from intracellular dissociation of the organic acid then acidify the cytoplasm and must be extruded to the exterior. Yeasts use the enzyme, H+-ATPase, along with energy in the form of ATP to remove excess protons from the cell. Inhibition and/or inactivation may be due to eventual loss of cellular energy or inactivation of critical cellular functions due to low intracellular pH.
Another mechanism used to prevent depletion of energy pools involves the induction of a membrane protein that can decrease the activity of the ATPase to conserve energy (Brul and Coote, 1999). In addition, exposure of S. cerevisiae to sorbic acid can strongly induce a membrane protein ATP-binding cassette transporter (Pdr12), which is a “multidrug resistance pump” that confers resistance by mediating energy-dependent extrusion of anions (Piper et al., 1998). Mutants without the transporter are hypersensitive to sorbic, benzoic, and propionic acids. One problem with extruding anions and protons is the potential for recombination in the extracellular medium, thus allowing them to reenter the cell. To prevent the futile cycle allowing the acid back into the cell, adapted yeasts apparently reduce diffusion and passage of the weak acids into the cell, most likely by altering cell membrane structures (Brul and Coote, 1999). Similar mechanisms likely also exist for bacteria that are capable of developing resistance to sorbic or other organic acids. Considering the length of time that sorbic and benzoic acids have been applied to food products it would seem, however, that the development of acquired resistance by spoilage and pathogenic microorganisms is very rare or non-existent.
--- PAGE BREAK ---
Pre-exposure to sub-inhibitory concentrations of other food antimicrobials has demonstrated varying resistance responses by microorganisms. For example, Moir and Eyles (1992) compared the effectiveness of methyl paraben and potassium sorbate on the growth of four psychrotrophic foodborne bacteria—Aeromonas hydrophila, L. monocytogenes, Pseudomonas putida and Yersinia enterocolitica. They observed little or no adaptation when cells were exposed to sub-inhibitory concentrations of antimicrobials. Bargiota et al. (1987) examined the relationship between lipid composition of S. aureus and resistance to parabens. Differences in total lipid, phospholipids, and fatty acids were found for S. aureus strains that were relatively resistant and a strain that was sensitive to parabens. The paraben-resistant strain had a higher percentage of total lipid, higher relative percentage of phosphatidyl glycerol, and decreased cyclopropane fatty acids compared with the sensitive strains. Bargiota et al. suggested that these changes could influence membrane fluidity and, therefore, adsorption of the parabens to the membrane. Juneja and Davidson (1993) altered the lipid composition of L. monocytogenes by growth in the presence of added fatty acids (C14:0, C18:0, and C18:1). Growth of L. monocytogenes in the presence of exogenously added C14:0 or C18:0 fatty acids increased resistance of the cells to parabens. Growth in the presence of C18:1, however, increased sensitivity to the antimicrobial agents. Thus, a correlation exists between lipid composition of the L. monocytogenes cell membrane and susceptibility to antimicrobial compounds.
Resistance of Microorganisms to Naturally Occurring Antimicrobials
The probable reason for this is the similarity in form and/or ability to kill target cells that some of these compounds have to medically important antibiotics. Because of the similarities, it has been suggested that use of microbiologically derived antimicrobials in foods may result in the development of acquired resistance to the compounds themselves or possibly cross resistance to antibiotics used in human medicine. In contrast to antibiotics used for therapeutic purposes, microbiologically derived antimicrobials generally have a much narrower spectrum of activity, i.e., affecting limited types of target microorganisms, and often having different mechanisms which may reduce chances for acquired resistance.
Two microbiologically derived antimicrobials that have been studied for their impact on the development of acquired resistance are natamycin and nisin. Natamycin, formerly called pimaricin, is an antifungal produced by Streptomyces natalensis that is effective against nearly all molds and yeasts but which has little or no effect on bacteria. Natamycin has no medical uses; however, it is used primarily as an antifungal agent on cheese. De Boer and Stolk-Horsthuis (1977) investigated the potential for development of resistance to natamycin among fungi. They reported no evidence of resistant fungi in cheese warehouses where natamycin was used for periods of up to several years. They also attempted to induce tolerance in 26 strains of fungi by transferring each culture 25-31 times in media containing concentrations of natamycin equal to and greater than the MIC. Following multiple transfers, the MIC increased in only 8 of 26 strains by a maximum of 4 μg/mL. De Boer and Stolk-Horsthuis concluded that lack of increased resistance among fungi was due to the lethal (as opposed to static) activity of the compound along with the compound’s instability over time.
Nisin is a polypeptide composed of 34 amino acids that is produced by certain strains of Lactococcus lactis ssp. lactis. Nisin has a narrow spectrum of activity affecting primarily vegetative cells and spores of Gram-positive bacteria. Susceptible strains are found among lactic acid bacteria, Bacillus, Clostridium, Listeria, and Streptococcus. The peptide alone generally does not inhibit Gram-negative bacteria, yeasts, or molds. The mechanism of antimicrobial action of nisin against vegetative cells includes binding to the anionic phospholipids of the cell membrane and insertion into the membrane, resulting in pore formation. Disruption of the cytoplasmic membrane causes efflux of intracellular components and eventual depletion of the proton motive force (PMF; Crandall and Montville, 1998). Microorganisms exhibiting resistance to nisin may inactivate the peptide via enzymatic action or they may alter their membrane susceptibility (Montville et al., 2001). Streptococcus thermophilus, Lactobacillus plantarum, and certain Bacillus species that produce the enzyme nisinase neutralize the antimicrobial activity of the polypeptide (Hoover and Hurst, 1993). In addition, spontaneous nisin resistant mutants, including L. monocytogenes, C. botulinum, Bacillus species, and S. aureus, could occur via exposure of wild-type strains to nisin or transfer of strains in media containing increasing concentrations of nisin (Harris et al., 1991; Mazzotta et al., 1997; Ming and Daeschel, 1993; Montville et al., 2001). L. monocytogenes resistant mutants, which are stable, may occur at a rate of 1 in 106 to 108 (Harris et al., 1991; Ming and Daeschel, 1993) or even lower (Schillinger et al., 1998). Crandall and Montville (1998) observed that nisin resistant strains of L. monocytogenes (NisR) had altered phospholipid composition, including decreased anionic phospholipid (cardiolipin and phosphatidylglycerol) and increased phosphatidyletha-nolamine in the cell membrane resulting in a decreased net negative charge that could hinder binding of cationic compounds such as nisin. In addition, the cell membranes of NisR strains exhibited increased long chain fatty acids and reduced ratios of C15/C17 fatty acids, suggesting reduced fluidity and stabilization caused by reduced effect on PMF (Ming and Daeschel, 1993; Mazzotta and Montville, 1997). These and other changes suggest an alteration of the cytoplasmic membrane to prevent access by nisin.
--- PAGE BREAK ---
The obvious implication of the emergence of pathogenic microorganisms resistant to bacteriocins is the potential hazard in foods that are preserved exclusively by a single compound. To overcome this potential hazard, some researchers suggest using combinations of bacteriocins or combinations of bacteriocins with other antimicrobials or preservation methods (Mulet-Powell et al., 1998; Schillinger et al., 1998). In theory, combinations of bacteriocins could be successfully applied if the mechanisms of action of the bacteriocins were different. However, even this strategy must be validated for each combination. Crandall and Montville (1998) demonstrated that L. monocytogenes ATCC 700302 was both nisin- and pediocin-resistant. Cross resistance among bacteriocins, however, is variable. Rasch and Knøchel (1998) found no cross resistance between nisin- and pediocin-resistant strains of L. monocytogenes but they did observe pediocin and bavaricin cross resistance.
Because microorganisms in foods are often exposed to some variation of acidic environmental conditions, it is interesting to speculate whether acid adaptation of a microorganism could alter its sensitivity to bacteriocins. Van Schaik et al. (1999) investigated acid adaptation at pH 5.5 and bacteriocin sensitivity and found that acid adapted L. monocytogenes was more resistant to nisin and lacticin 3147. The difference in resistance between the acid adapted and non-adapted cells was more noticeable with nisin than with lacticin 3147. The potential for this change in resistance in a food system remains uncertain, however, because the experiment was done in a microbiological medium (tryptic soy broth supplemented with 0.6% yeast extract).
The most important question concerning the potential for microorganisms to acquire resistance to bacteriocins is whether such resistance conveys a natural advantage over non-resistant strains in food systems. Mazzotta et al. (2000) demonstrated that nisin-resistant strains of L. monocytogenes and C. botulinum were not as resistant as wild-type strains to other traditional food antimicrobials including sodium chloride, sodium nitrite and potassium sorbate. Dykes and Hastings (1998) observed that leucocin- and sakacin-resistant L. monocytogenes B73 had a reduced growth rate in a microbiological growth medium (brain heart infusion broth) without bacteriocin than bacteriocin-sensitive strains. In addition, NisR strains failed to compete with bacteriocin-sensitive strains when grown in mixed populations, even at a 1:1 ratio. Dykes and Hastings concluded that the bacteriocin-resistant phenotype of L. monocytogenes B73 was not likely to become stable in natural populations. Gravesen et al. (2002) also observed that pediocin-resistant L. monocytogenes frequently exhibited a reduced growth rate and extended lag phase in a microbiological broth medium compared to wild-type cells. However, nisin-resistant L. monocytogenes strains had fewer and less pronounced growth rate reductions. Interestingly, pediocin- and nisin-resistant strains were no more stress susceptible (pH, salt, low temperature) than sensitive strains, and they grew equally well in a model sausage system as the parent strains. Mazzotta and Montville (1999) demonstrated that nisin-resistant C. botulinum 169B spores have similar heat resistance patterns as wild type spores. Therefore, while acquired resistance to a single bacteriocin does not appear to automatically confer resistance to other antimicrobials or preservative treatments nor any natural advantage for a population in the absence of the inhibitor, more research in food systems is definitely warranted. For a more extensive review on naturally occurring antimicrobials, readers are referred to Sofos et al. (1998).
Resistance of Microorganisms to Sanitizers
Certain microorganisms, e.g., bacterial spores and Cryptosporidium, have innate chlorine resistance. Microorganisms may also develop acquired resistance following exposure to chlorine. Conditions that may lead to the development of resistance include application of sub-lethal concentrations of chlorine used in error or, more likely, neutralization of the compound during use. For example, the antimicrobial activity of hypochlorites is significantly reduced by organic matter and high pH (Cords and Dychdala, 1993). Mokgatla et al. (1998) studied the chlorine resistance of Salmonella isolates obtained during various stages of poultry processing. They observed that some were resistant to hypochlorous acid (defined as growth in the presence of 72 μg/mL hypochlorous acid). The frequency of isolates exhibiting chlorine resistance differed depending upon the processing line location, but resistant strains occurred at most sites, including scalding, plucking, and final packaged product. The mechanisms of chlorine resistance identified for Salmonella include catalase production, decreased activity of membrane-bound dehydrogenases, and decreased DNA damage (Mokgatla et al., 2002). These alterations would result in reduced hydroxy radicals and singlet oxygen, both of which react with hypochlorous acid (the active form of hypochlorite sanitizers) to cause cellular inactivation and improve DNA repair. Mokgatla et al. (1998) initially recommended that in poultry processing, concentrations of chlorine sufficient to ensure that Salmonella are eradicated and not subjected to sub-lethal concentrations must be used. It is now common practice to also use sanitizers, such as chlorine, in the wash water for fresh and minimally processed fruits and vegetables (Beuchat, 1996; Cherry, 1999). Chlorine and other sanitizers, however, do not necessarily eliminate pathogens from these products (Zhuang et al., 1995), which could potentially lead to the development of resistant strains.
--- PAGE BREAK ---
Development of resistance to other compounds commonly used as sanitizers in food processing environments may also be possible. Pickett and Murano (1996) exposed L. monocytogenes to sub-lethal concentrations of an acidic anionic sanitizer, a chlorine-based sanitizer, an iodophor, a quaternary ammonium agent, lactic acid, citric acid, and propionic acid before challenging the cells to minimum inhibitory concentrations of each. Except for the acid anionic (phosphoric acid/dodecylbenzene sulfonic acid) sanitizer, there was no difference in susceptibility among the cells. L. monocytogenes developed resistance to the MIC of the acidic anionic (500 μg/ mL) when challenged after an initial shock of 350 μg/mL with the same sanitizer. While citric acid did not produce resistant cells at the test pH of 2.8, when the pre-exposure pH was raised to 5.0, the acid yielded cells that survived exposure to the MIC (0.4%). Pickett and Murano suggested that pre-exposure of the cells to the dissociated form of the acid, but not the undissociated form, caused L. monocytogenes to become resistant. Strains of L. monocytogenes isolated from poultry were shown to be resistant (MIC = 16 μg/mL) to the quaternary ammonium sanitizer, benzalkonium chloride (Lemaître et al., 1998). Resistance was plasmid-borne and could be transferred to other Listeria and S. aureus. The plasmid genes may code for an energy-dependent efflux system. These authors suggested that use of a single type of sanitizer may allow for selection and persistence of resistant strains.
When microorganisms are attached to a surface or are part of a bio-film, they are more resistant to antimicrobials than are freely suspended (planktonic) cells (Bower and Daeschel, 1999; Frank and Koffi, 1990; Oh and Marshall, 1996). The increased resistance may be caused by a lack of adequate contact of the antimicrobial with the microorganisms (Frank and Koffi, 1990) or to adsorption of the antimicrobial to the biofilm glycocalyx (a complex extracellular polysaccharide material) (Cross, 1990). Although data are lacking, it is also possible that resistance to sanitizers could be acquired by microorganisms in bio-films through exposure to sublethal levels of the compounds.
Some studies suggest a relationship between microbial resistance to sanitizers and microbial resistance to antibiotics used therapeutically. For example, Russell and Day (1996) noted that cross resistance exists between biocides and antibiotics, and Russell (1997) reported that antibiotic-resistant S. aureus and Staphylococcus epidermidis developed plasmid-mediated resistance to chlorhexidine and quaternary ammonium compounds. Russell (1997) reported that methicillin-resistant S. aureus (MRSA) were significantly more tant to both povidine-iodine and hypochlorite than methicillin-sensitive strains. Moken et al. (1997) suggested that microorganisms may become resistant to antibiotics through exposure to pine oil disinfectants. Moken et al. exposed Escherichia coli to pine oil disinfectant in a microbiological disk assay and isolated resistant mutants. Pine oil-resistant mutants were also more resistant to the medically important antibiotics tetracycline, ampicillin and chloramphenicol than the parent strain. These mutants overexpressed a gene that triggered an increase in the general antimicrobial efflux pump. The mutants did not have cross resistance to hydrogen peroxide, hypochlorite, or a quaternary ammonium compound. The authors suggested that constant use of disinfectants in the home may increase the development of resistance to antibiotics used therapeutically.
There is no evidence that proper use of sanitizers in food manufacturing will lead to development of resistant microorganisms. However, with increasing reliance on and use of sanitizers on food handling equipment, in food processing environments, and on raw products, the potential for emergence of such resistant microorganisms does exist. Therefore, the potential for development of resistant strains must continue to be evaluated.
Resistance of Microorganisms to Other Processing Conditions
It has been shown repeatedly in laboratory situations that bacteria can become resistant to certain environmental factors under conditions that would normally be considered lethal to the organism (Bower and Daeschel, 1999). For example, E. coli O157:H7, Salmonella Typhimurium, and L. monocytogenes can become more acid resistant and possibly more resistant to other stresses (e.g., heat, osmotic pressure), if subjected to relatively mild acidity before exposure to more acidic conditions (Brudzinski and Harrison, 1998; Buchanan and Edelson, 1999a; Garren et al., 1998; Leyer and Johnson, 1992, 1993; Leyer et al., 1995; Mazzotta, 2001; O’Driscoll et al., 1996; Ravishankar and Harrison 1999; Wilde et al., 2000). Developed resistance is referred to as tolerance, adaptation, or habituation depending upon how the microorganism is exposed to the stress and the physiological conditions that lead to enhanced survival (Buchanan and Edelson, 1999b). In addition, production of acidic conditions by the microorganism itself can produce acid tolerance. For example, growth of E. coli in an acidogenic broth (acid generating) (e.g., tryptic soy broth [TSB] + glucose) produced cells that expressed an acid resistance response while cells grown in a nonacidogenic broth (TSB without glucose) did not (Buchanan and Edelson, 1999b).
Whether this occurs in actual food environments during processing situations or in the product itself is intriguing, but largely unanswered. If pH resistance is the issue, then pH levels typically found in foods of concern should be evaluated. Ravishankar and Harrison (1999) found that L. monocytogenes exhibited an acid tolerance response when it was acid adapted to pH 5.5 with lactic acid and then challenged in acidified skim milk at pH 3.5 and 4.0. When the challenge pH of 4.5 was used, however, there was no adaptive acid tolerance response. Because the pH of 4.5 is more closely related to pH levels that might occur in fermented products made from skim milk, results based on this pH may be the most meaningful. In apple, orange, and white grape juices, Mazzotta (2001) found that the thermal resistance of E. coli O157:H7, Salmonella, and L. monocytogenes increased after acid adaptation. However, Mazzotta also pointed out that the typical pasteurization process applied to these types of fruit juices provides sufficient thermal inactivation of these pathogens, regardless of whether or not they have enhanced thermal resistance due to acid adaptation.
--- PAGE BREAK ---
Many studies of acid shock and acid adaption of bacteria have been conducted. Most studies have evaluated these responses over a relatively short period of time (typically a few hours). Within foods and on food contact surfaces, adaptive alteration of cells might be of more concern if the resistance is sustained by the adapted cells. The greater the degree of severity of the antimicrobial challenge, the less likely it will be for the microorganism to survive for extended periods, even if it is adapted.
Numerous product and antimicrobial combinations are possible in foods. Under certain scenarios, there may be reason for concern when considering whether or not bacteria can acquire some degree of resistance to a particular antimicrobial. Direct acidification of a food or food ingredient may shock microflora so they become more acid resistant. Fermentation of foods, however, may lead to somewhat different situations. Lactic acid bacteria can lower the pH of a substrate gradually over time, likely resulting in a pH gradient rather than a sharp change in pH as would be expected with direct acidification. Leyer et al. (1995) reported an acid adaptive response in E. coli O157:H7 which enhanced its survival in fermented sausage (pH 5.6).
The use of acidic antimicrobial sprays on the surfaces of meat carcasses has become very common (Dickson, 1995). One could ask if bacteria on the meat surface become more acid resistant when they are exposed to a low concentration of a weak acid (e.g., <3%) solution. Whether or not the exposure of microorganisms to acids on substrates such as meat is sufficient to result in acquired resistance is largely unknown at this time. One study by Van Netten et al. (1998), however, demonstrated a lack of increased resistance to lactic acid for E. coli O157:H7, S. Typhimurium, S. aureus, and C. jejuni when acid adapted cells were inoculated on pork bellies and treated with 2% lactic acid as a sanitizer. However, most other studies related to acquired stress response have been done either in laboratory culture media with lower than typically encountered concentrations of antimicrobials, or in foods under conditions which might be only marginally similar to actual situations.
In a study using laboratory culture broth, Duffy et al. (2000) reported that E. coli O157:H7, which was adapted to acid under mild acidic conditions, acquired cross protection to heat in an acidic environment. They concluded that this might have implications for fermented meat production and similar processes, which are also given a thermal process. However, since this study was done in culture broth, it does not fully mimic what could occur in the more complex food matrix.
Several studies involving acid adapted microbes in heated and or dried meat products have been conducted. In many of these, acid adaptation did not offer any enhanced protection to other environmental stresses to which the microorganism was subsequently exposed in an actual food product. Calicioglu et al. (2002) investigated the inactivation of acid adapted and unadapted E. coli O157:H7 during the processing of beef jerky. They found that the survival rate of E. coli O157:H7 varied depending on the marinade formulation used in making the product. Depending on the formulation, there was either no significant effect on the survival rate between the acid adapted and non-adapted cells or the populations of acid adapted cells actually decreased at a more rapid rate than the non-adapted populations. In considering their results and those of Calicioglu et al. (1997, 2001), and Riordan et al. (2000), Calicioglu et al. (2002) offered the suggestion that heating foods with a lowered pH and a low aw may present a combination of preservation effects that overcomes any cross protection benefit provided by acid adaptation.
In addition to looking at the possibility of L. monocytogenes becoming more acid resistant through an acid tolerance response, Ravishankar and Harrison (1999) also conducted experiments to determine if acid adaptation of the microorganism by cross protection enhanced survival in the presence of an activated lactoperoxidase system, a naturally occurring antimicrobial system in milk. They observed that the survival rates were similar for the acid adapted and non-adapted cells at pH 4.5 both in the presence and absence of an activated lactoperoxidase system, indicating that no cross protection was afforded the acid adapted cells. In contrast, Leyer and Johnson (1993) reported cross protection to an activated lactoperoxidase system with acid adapted S. Typhimurium in a laboratory culture medium. The activity of the lactoperoxidase system can vary depending on the medium, a possible explanation for the contrasting results. Another possible explanation for the difference in the studies may be the greater degree of acid tolerance exhibited by Salmonella than Listeria. The lactoperoxidase system may also be a less effective antimicrobial toward Salmonella compared with Listeria.
--- PAGE BREAK ---
Leyer and Johnson (1993) also reported that acid adapted S. Typhimurium offered cross protection against heat, salt, and selected surface active agents. O’Driscoll et al. (1996) found that acid adapted L. monocytogenes also exhibited increased resistance to heat, cold, salt, selected surface active agents, and ethanol. However, Leyer and Johnson (1997) reported that when populations of S. Typhimurium were acid adapted at a pH of 5.0 to 5.8 the sensitivity of the cells to hypochlorous acid and iodine increased. They also investigated the mechanism of the inactivation by hypochlorous acid and concluded that, whether the cells were acid adapted or not, the mechanism involved changes in membrane permeability, inability to maintain or restore energy charge, and probably oxidation of essential cellular components. They proposed that acid pre-treatment in a food plant sanitation program may thus enhance the efficiency of halogen sanitizers.
Using a L. monocytogenes isolate from a food processing plant drain, Taormina and Beuchat (2001) investigated the survival and heat resistance of the microorganism after exposure to alkaline pH (up to pH 12.0) and after exposure to chlorine (up to 6.0 mg of free chlorine per liter). The alkaline stress enhanced the resistance of L. monocytogenes to thermal processing conditions of 56 and 59°C. In contrast, exposure of the cells to chlorine resulted in populations that were more sensitive to heating at 56°C. Based on these results, there are differences in the cross protection offered after exposure to alkaline pH depending on whether an alkaline- or chlorine-based sanitizer is used in sanitation routines.
Conclusions
Because evidence exists that microorganisms can acquire varying levels of resistance or tolerance to environmental stresses, there is some concern that this might provide protection for foodborne pathogens against antimicrobials and preservation processes. Development of resistance to manufacturing and processing treatments could occur at numerous points in a food production system and could influence preservation treatment efficacy. However, before decisions can be made with confidence concerning the development of tolerance or resistance among foodborne pathogens, it is critical to acquire data that is relevant to real food processing situations. In addition, conditions most representative of those in actual products or food processing situations must be included in experimental protocol. For example, as was noted by Leyer and Johnson (1993), the physiological state of foodborne pathogens used in challenge studies in food and in evaluating Hazard Analysis Critical Control Point programs is an important consideration because the effectiveness of control measures may vary with varied microbial physiological states.
A major problem with use of antimicrobial combinations, however, is the general lack of knowledge about food antimicrobial mechanisms. Without this information, antimicrobials cannot be applied effectively to achieve synergistic interactions. More research is needed to determine: (1) the frequency and mechanisms of resistance to food antimicrobials and process stresses in food systems, (2) mechanisms of action of traditional and naturally occurring food antimicrobials, and (3) application strategies to minimize tolerance or resistance development. Simple methods for overcoming the potential for development of acquired resistance include using appropriate antimicrobials, avoiding the use of sub-lethal concentrations of antimicrobials, using combinations of antimicrobials for environmental or process controls (such as hurdle technology) and using combinations of antimicrobials that have different mechanisms. Although data concerning development of resistance to food antimicrobials is scarce, antimicrobial resistance does not appear to be a phenomenon that would have a major negative impact on public health.
by P. Michael Davidson and Mark A. Harrison
Author Davidson is Professor, Dept. of Food Science and Technology, University of Tennessee, 2509 River Dr., Knoxville, TN 37996-4539. Author Harrison is Professor, Dept. of Food Science and Technology, University of Georgia, Athens, GA 30602. Both authors are Professional Members of IFT.
References
Abee, T. and Wouters, J.A. 1999. Microbial stress response in minimal processing. Intl. J. Food Microbiol. 50: 65-91.
Bargiota, E., E. Rico-Muñoz and P.M. Davidson. 1987. Lethal effect of methyl and propyl parabens as related to Staphylococcus aureus lipid composition. Intl J. Food Microbiol. 4: 257-266.
Beuchat, L.R. 1996. Pathogenic microorganisms associated with fresh produce. J. Food Protect. 59: 204-216.
Bills, S., Restaino, L., and Lenovich, L.M. 1982. Growth response of an osmotolerant sorbate-resistant yeast, Saccharomyces rouxii at different sucrose and sorbate levels. J. Food Protect. 45: 1120-1124.
Bower, C.K. and Daeschel, M.A. 1999. Resistance responses of microorganisms in food environments. Intl. J. Food Microbiol. 50: 33-44.
Brudzinski, L. and Harrison, M.A. 1998. Influence of incubation conditions on survival and acid tolerance response of Escherichia coli O157:H7 and non-157:H7 isolates exposed to acetic acid. J. Food Protect. 61: 542-546.
Brul, S. and Coote, P. 1999. Preservative agents in foods. Mode of action and microbial resistance mechanisms. Intl. J. Food Microbiol. 50: 1-17.
Buchanan, R.L. and Edelson, S.G. 1999a. Effect of pH-dependent, stationary phase acid resistance on the thermal tolerance of Escherichia coli O157:H7. Food Microbiol. 16: 447-458.
Buchanan, R.L. and Edelson, S.G. 1999b. pH-dependent stationary-phase acid resistance response of enterohemorrhagic Escherichia coli in the presence of various acidulants. J. Food Protect. 62: 211-218.
Calicioglu, M., Faith, N.G., Buege, D.R., and Luchansky, J.B. 1997. Viability of Escherichia coli O157:H7 in fermented semidry low-temperature cooked beef summer sausage. J. Food Protect. 60: 1158-1162.
Calicioglu, M., Faith, N.G., Buege, D.R., and Luchansky, J.B. 2001. Validation of a manufacturing process for fermented, semidry Turkish Soudjouk to control Escherichia coli O157:H7. J. Food. Protect. 64: 1156-1161.
Calicioglu, M., Sofos, J.N., Samelis, J., Kendall, P.A., and Smith, G.C. 2002. Inactivation of acid-adapted and nonadapted Escherichia coli O157:H7 during drying and storage of beef jerky treated with different marinades. J. Food Protect. 65: 1394-1405.
CFR. 2001. Code of Federal Regulations. National Archives and Records Administration. U.S. Government Printing Office. (www.acces.gpo.gov/cgi-bin/cfrassemble.cgi?title=200121)
Cherry, J.P. 1999. Improving the safety of fresh produce with antimicrobials. Food Technol. 53(11): 54-57, 59.
Chipley, J.R. 1993. Sodium benzoate and benzoic acid. In “Antimicrobials in Foods,” ed. P.M. Davidson and A.L. Branen, 2nd ed., pp. 11-48. Marcel Dekker, Inc., N.Y.
Cords, B.R. and Dychdala, G.R. 1993. In “Antimicrobials in Foods,” ed. P.M. Davidson and A.L. Branen, 2nd ed., pp. 469-537. Marcel Dekker, Inc., N.Y.
Crandall, A.D. and Montville, T.J. 1998. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl. Environ. Microbiol. 64: 231-237.
Cross, A.S. 1990. The biological significance of bacterial encapsulation. In “Current Topics in Microbiology and Immunology: Bacterial Capsules,” ed. K. Jann and B. Kann, Vol. 150, pp. 86-95, Springer-Verlag, Berlin.
Davidson, P.M. 2001. Chemical preservatives and natural antimicrobial compounds. In “Food Microbiology: Fundamentals and Frontiers,” ed. M.P. Doyle, L.R. Beuchat, and T.J. Montville, 2nd ed., pp. 593-627. ASM Press, Washington, D.C.
Davidson, P.M. and Branen, A.L. 1993. “Antimicrobials in Foods,” 2nd ed. Marcel Dekker, Inc. N.Y.
De Boer, E. and Stolk-Horsthuis, M. 1977. Sensitivity to natamycin (pimaricin) of fungi isolated in cheese warehouses. J. Food Protect. 40: 533-536
Dickson, J.S. 1995. Susceptibility of preevisceration washed beef carcasses to contamination by Escherichia coli O157:H7 and salmonellae. J. Food Protect. 58: 1060-1068.
Duffy, G., Riordan, D.C.R., Sheridan, J.J., Call, J.E., Whiting, R.C., Blair, I.S., and McDowell, D.A. 2000. Effect of pH on survival, thermotolerance, and verotoxin production of Escherichia coli O157:H7 during simulated fermentation and storage. J. Food Protect. 63: 12-18.
Dykes, G.A. and Hastings, J.W. 1998. Fitness costs associated with class IIa bacteriocin resistance in Listeria monocytogenes B73. Lett. Appl. Microbiol. 26: 5-8.
FDA. 1998. Direct food substances affirmed as generally recognized as safe; Egg white lysozyme. Food and Drug Administration, Washington, D.C. Fed. Reg. 63: 12421-12426.
FDA. 2000. Sodium diacetate, sodium acetate, sodium lactate and potassium lactate; Use as food additives. Food and Drug Administration, Washington, D.C. Fed.Reg. 65: 17128-17129.
FDA/CFSAN. 2001a. Agency response letter, GRAS Notice No. GRN 000067, October. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Washington, D.C.
FDA/CFSAN. 2001b. Agency response letter, GRAS Notice No. GRN 000064, April. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Washington, D.C.
FDA/CFSAN. 2001c. Agency response letter, GRAS Notice No. GRN 000065, April. Food and Drug Administration/ Center for Food Safety and Applied Nutrition, Washington, D.C.
Finol, M.L., Marth, E.H., and Lindsay, R.C. 1982. Depletion of sorbate from different media during growth of Penicillium species. J. Food Protect. 45: 398-404.
Frank, J. F. and Koffi, R.A. 1990. Surface-adherent growth of Listeria monocytogenes is associated with increased resistance to surfactant sanitizers and heat. J. Food Protect. 53: 550-554.
Garren, D.M., Harrison, M.A., and Russell, S.M. 1998. Acid tolerance and acid shock response of Escherichia coli O157:H7 and non-O157:H7 isolates provide cross protection to sodium lactate and sodium chloride. J.Food Protect. 61: 158-161.
Gravesen, A. Jydegaard Axelsen A.-M., Mendes da Silva, J.,Hansen, T.B., and Knøchel, S. 2002. Frequency of bacteriocin resistance development and associated fitness costs in Listeria monocytogenes. Appl. Environ. Microbiol. 68: 756-764.
Harris, L.J., Fleming, H.P., and Klaenhammer, T.R. 1991. Sensitivity and resistance of Listeria monocytogenes ATCC 19115, Scott A and UAL500 to nisin. J. Food Protect. 54: 836-840.
Hoover, D.G. and Hurst, A. 1993. Nisin. In “Antimicrobials in Foods,” ed. P.M. Davidson and A.L. Branen, 2nd ed., pp. 369-394. Marcel Dekker, N.Y.
Juneja, V.K. and P.M. Davidson. 1993. Influence of altered fatty acid composition on resistance of Listeria monocytogenes to antimicrobials. J. Food Protect. 56: 302-305.
Leistner, L. 2000. Basic aspects of food preservation by hurdle technology. Intl. J. Food Microbiol. 55: 181-186.
Leistner, L. and Gorris, L.G.M. 1995. Food preservation by hurdle technology. Trends Food Sci. Technol. 6: 41-46.
Lemaître, J.-P., Echchannaoui, H., Michant, G., Divies, and Rousset. 1998. Plasmid-mediated resistance to antimicrobials among Listeriae. J. Food Protect. 61: 1459-1464.
Leyer, G.J. and Johnson, E.A. 1992. Acid adaptation promotes survival of Salmonella spp. in cheese. Appl. Environ. Microbiol. 58: 2075-2080.
Leyer, G. J. and Johnson, E.A. 1993. Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Appl. Environ. Microbiol. 59:1842-1847.
Leyer, G.J. and Johnson, E.A. 1997. Acid adaptation sensitizes Salmonella Typhimurium to hypochlorous acid. Appl. Environ. Microbiol. 63: 461-467.
Leyer, G.J., Wang, L.-L., and Johnson, E.A. 1995. Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Appl. Environ. Microbiol. 61: 3752-3755.
Liewen, M.B. and Marth, E.H. 1985. Growth and inhibition of microorganisms in the presence of sorbic acid: A review. J. Food Protect. 48: 364-375.
Marth, E.H., Capp, C.M., Hasenzahl, L., Jackson, H.W., and Hussong, R.V. 1966. Degradation of potassium sorbate by Penicillium species. J. Dairy Sci. 49: 1197-1205.
Mazzotta, A.S. 2001. Thermal inactivation of stationary-phase and acid-adapted Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes in fruit juices. J.Food Protect. 64: 315-320.
Mazzotta, A.S. and Montville, T.J. 1997. Nisin induces changes in membrane fatty acid composition of Listeria monocytogenes nisin-resistant strains at 10EC and 30EC. J. Appl. Microbiol. 82: 32-38.
Mazzotta, A.S. and Montville, T.J. 1999. Characterization of fatty acid composition, spore germination, and thermal resistance in a nisin-resistant mutant of Clostridium botulinum 169B and in the wild-type strain. Appl. Environ. Microbiol. 65: 659-664.
Mazzotta, A.S., Crandall, A.D., and Montville, T.J. 1997. Nisin resistance in Clostridium botulinum spores and vegetative cells. Appl. Environ. Microbiol. 63: 2654-2659.
Mazzotta, A.S., Modi, K.D., and Montville, T.J. 2000. Nisin resistant (NisR) Listeria monocytogenes and NisR Clostridium botulinum are not resistant to common food preservatives. J. Food Sci. 65: 888-890.
Ming, X. and M.A. Daeschel. 1993. Nisin-resistance of foodborne bacteria and the specific resistance responses of Listeria monocytogenes Scott A. J. Food Protect. 56: 944-948.
Moir, C.J. and Eyles, M.J. 1992. Inhibition, injury and inactivation of four psychrotrophic foodborne bacteria by the preservatives methyl p-hydroxybenzoate and potassium sorbate. J. Food Protect. 55: 360.
Moken, M.C., McMurry, L.M., and Levy, S.B. 1997. Selection of multiple-antibiotic-resistant (mar) mutants of Escherichia coli by using the disinfectant pine oil: Roles of the mar and acrAB loci. Antimicrob. Agents Chemother. 41: 2770-2772.
Mokgatla, R.M., Brözel, V.S., and Gouws, P.A. 1998. Isolation of Salmonella resistant to hypochlorous acid from a poultry abattoir. Lett. Appl. Microbiol. 27: 379-382.
Mokgatla, R.M., Gouws and P.A., and Brözel, V.S. 2002. Mechanisms contributing to hypochlorous acid resistance of a Salmonella isolate from a poultry-processing plant. J. Appl. Microbiol. 92: 566-573.
Montville, T.J., Winkowski, K., and Chikindas, M.L. 2001. Biologically based preservation systems. In “Food Microbiology: Fundamentals and Frontiers,” 2nd ed., pp. 629-647. ed. M.P. Doyle, L.R. Beuchat, and T.J. Montville. American Society for Microbiology, Washington, DC.
Mulet-Powell, N., Lacoste-Armynot, A.M., Vinas, M., and Simeon de Buochberg, M. 1998. Interactions between pairs of bacteriocins from lactic bacteria. J. Food Protect. 61: 1210-1212.
O’Driscoll, B., Gahan, C.G.M., and Hill, C. 1996. Adaptive acid tolerance response in Listeria monocytogenes: Isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl. Environ. Microbiol. 62: 1693-1698.
Oh, D. and Marshall, D.L. 1996. Monolaurin and acetic acid inactivation of Listeria monocytogenes attached to stainless steel. J. Food Protect. 59: 249-252.
Pickett, E.L. and Murano, E.A. 1996. Sensitivity of Listeria-monocytogenes to sanitizers after exposure to a chemical shock. J. Food Protect. 59: 374-378.
Piper, P., Mahe, Y., Thompson, S., Pandjaitan, R., Holyoak, C., Egner, R., Muehlbauer, M., Coote, P., and Kuchler, K. 1998. The Pdr12 ABC transporter is required for the development of weak organic acid resistance in yeast. EMBO J. 17: 4257-4265.
Rasch, M. and Knøchel, S. 1998. Variations in tolerance of Listeria monocytogenes to nisin, pediocin PA-1 and bavaricin A. Lett. Appl. Microbiol. 27: 275-278.
Ravishankar, S. and Harrison, M.A. 1999. Acid adaptation of Listeria monocytogenes strains does not offer cross-protection against an activated lactoperoxidase system. J. Food Protect. 62: 670-673.
Rees, C.E.D., Dodd, C.E.R., Gibson, P.T., Booth, I.R., and Stewart, G.S.A.B. 1995. The significance of bacteria in stationary phase to food microbiology. Intl. J. Food Microbiol. 28: 263-275.
Riordan, D.C., Duffy, G., Sheridan, J.J., Whiting, R.C., Blair, I.S., and McDowell, D.A. 2000. Effect of acid adaptation, product pH, and heating on survival of Escherichia coli O157:H7 in pepperoni. Appl. Environ. Microbiol. 66: 1726-1729.
Russell, A.D. 1991. Mechanisms of bacterial resistance to non-antibiotics: Food additives and food and pharmaceutical preservatives. J. Appl. Bacteriol. 71: 191-201.
Russell, A.D. 1997. Plasmids and bacterial resistance to biocides. J. Appl. Microbiol. 83: 155-165.
Russell, A.D. and Day, M.J. 1996. Antibiotic and biocide resistance in bacteria. Microbios 85: 45-46.
Russell, A.D., Furr, J.R., and Maillard J.-Y. 1997. Microbial susceptibility and resistance to biocides. ASM News 63: 481-487.
Schillinger, U., Chung, H.-S., Keppler, K., and Holzapfel, W.H. 1998. Use of bacteriocinogenic lactic acid bacteria to inhibit spontaneous nisin-resistant mutants of Listeria monocytogenes Scott A. J. Appl. Microbiol. 85: 657-663.
Schroeder, L.L. and Bullerman, L.B. 1985. Potential for development of tolerance by Penicillium digitatum and Penicillium italicum after repeated exposure to potassium sorbate. Appl. Environ. Microbiol. 50: 919-923.
Sofos, J.N. and Busta, F.F. 1993. Sorbic acid and sorbates. In “Antimicrobials in Foods,” ed. P.M. Davidson and A.L. Branen, 2nd ed., pp. 49-94. Marcel Dekker, N.Y.
Sofos, J.N., Beuchat, L.R., Davidson, P.M., and Johnson, E.A. 1998. Naturally occurring antimicrobials in food. Task Force Report No. 132, 103 pp., Council for Agricultural Science and Technology, Ames, Iowa.
Taormina, P.J. and Beuchat, L.R. 2001. Survival and heat resistance of Listeria monocytogenes after exposure to alkali and chlorine. Appl. Environ. Microbiol. 67: 2555-2563.
Van Netten, P., Valentijn, A., Mossel, D.A.A., and Huis in’t Veld, J.H.J. 1998. The survival and growth of acid-adapted mesophilic pathogens that contaminate meat after lactic acid decontamination. J. Appl. Microbiol. 84: 559-567.
van Schaik, W., Gahan, C.G.M., and Hill, C. 1999. Acid-adapted Listeria monocytogenes displays enhanced tolerance against the lantibiotics nisin and lacticin 3147. J. Food Protect. 62: 536-539.
Warth, A.D. 1977. Mechanism of resistance of Saccharomyces bailii to benzoic, sorbic and other weak acids used as food preservatives. J. Appl. Bacteriol. 43: 215-230.
Warth, A.D. 1985. Resistance of yeast species to benzoic and sorbic acids and to sulfur dioxide. J. Food Protect. 48: 564-569.
Warth, A.D. 1988. Effect of benzoic acid on growth yield of yeasts differing in their resistance to preservatives. Appl. Environ. Microbiol. 54: 2091-2095.
Wilde, S., Jorgensen, F., Campbell, A., Rowbury, R., and Humphrey, T. 2000. Growth of Salmonella enterica serovar Enteritidis PT4 in media containing glucose results in enhanced RpoS-independent heat and acid tolerance but does not affect the ability to survive air-drying on surfaces. Food Microbiol. 17: 679-686.
Zhuang, R.Y., Beuchat L.R., and Angulo, F.J. 1995. Fate of Salmonella montevideo on and in raw tomatoes as affected by temperature and treatment with chlorine. Appl. Environ. Microbiol. 61: 2127-2131.
