High-Pressure Sterilization of Foods
Processing of foods using multiple high-pressure pulses and achieving an end temperature above 105°C under pressure produces sterility with minimal impact on flavor, texture, and color.
In the quest for better quality of shelf-stable, low-acid foods, a number of emerging technologies have been considered (FDA, 2000). Of these technologies, only high pressure has proven to be effective in eliminating all spores and enzymes while retaining a quality level equal to or better than frozen. 
Advances in equipment design and construction have brought down the processing costs to be competitive with retorting and freezing. High-pressure processing will now deliver the ultimate convenience for consumers: shelf-stable gourmet meals, which can be prepared in less than two minutes. High-pressure-sterilized main meal entrees like macaroni and cheese, chicken ala king, salmon fettuccine, ravioli, and beef stroganoff all have a freshly prepared flavor, texture, and color. High-pressure-processed vegetables have a crispy texture. Soups, sauces, and stews no longer have scorched flavors nor soft-textured vegetables and pasta, as seen with retort-processed products and frozen foods.
High-pressure processing is already being used commercially for pasteurization of such products as guacamole, meats, and seafood but not yet for sterilization. This article presents results of High-pressure sterilization studies conducted by the authors individually and in concert.
Previous Studies
There have been several published research papers claiming to have achieved sterility in low-acid foods using high pressure. Hayakawa et al. (1994) applied a pressure of 600 MPa (1 MPa = 145.038 psi) with an end temperature (temperature during pressurization, which includes adiabatic heating) of 70°C for 5 min, then reduced the pressure to ambient pressure and repeated this process 5 more times. The authors tested a Bacillus stearothermophilus spore concentration of 106/g in an enriched medium. Treated samples were immediately subjected to microbiological testing in which the treated samples were plated on nutrient agar medium and aged for 7 days at 55°C. The results indicated no survivors, and electron microscope observations led researchers to believe that all the spores had disintegrated.
Sojka and Ludwig (1997) also tested pulsed high pressure, with the first pressure set at 60 MPa and an initial temperature (prior to pressurization) of 70°C for 1 min, followed by 500 MPa for 1 min; this cycle was repeated 10 more times. The researchers believed that the germination of spores was initiated during the first pressurization and the resulting vegetative cells were subsequently destroyed with the second pressurization. This test was conducted on a Bacillus subtilis ATCC 9372 spore load of 108/g in a germination medium. Treated samples were microbiologically tested immediately after high pressure testing, including a 10-day incubation period at 37°C in test tubes. At the end of the incubation period, the samples were visually assessed for signs of growth. No growth was observed.
Wilson and Baker (1995) in International Patent Filing WO 97/21361 and U.S. patent 6,086,936 claimed to achieve sterility with two processes using a 106/g spore load of B. subtilis, B. stearothermophilus, and Clostridium sporogenes in a meat emulsion. Initial process conditions were 621 MPa, 85°C for 30 min; and second conditions were 621 MPa, 98°C for 5 min. Microbiological testing was conducted immediately after pressure testing and included a 7-day incubation period at 37°C. The test conditions for all three studies were duplicated, except that processed samples were given a 30-day incubation period at ambient temperature before being subjected to microbiological tests. In all cases, growth was observed. To ensure that true sterility has been achieved, a 30-day incubation period is required to allow surviving spores and cells to repair and grow.
--- PAGE BREAK ---
Current Studies
Meyer (2000) reported achieving sterility in low-acid foods using pulsed high pressure in conjunction with heat. An initial temperature (prior to pressurizing) of 90°C combined with 690 MPa for 1 min was followed by an ambient pressure pause for 1 min, then another 690 MPa pressurization for 1 min was applied. By this treatment, sterility was achieved in macaroni and cheese with a 106/g spore load of C. sporogenes PA 3679. The test was repeated with a 106/g spore load of Bacillus cereus, and sterility was also obtained.
Further testing using a variety of pressures, initial temperatures, multiple pulses, and pulse/pause times found that the most critical variables for achieving sterility are:
1. Two or more pulses.
2. End temperature (temperature achieved during pressurization, or the initial temperature plus the adiabatic temperature less any sidewall cooling) of 105°C or greater.
3. Uniform initial temperature of the product prior to pressurizing (no cold spots).
4. Initial spore load and type.
The period of time at each pressure pulse or the period of time at the pause period (zero pressure) between pulses had little effect except with a marginal (low) end temperature (below 105°C); the same held true for more than two pulses.
The primary variables affecting quality are:
1. Initial temperature (temperature immediately prior to pressurizing).
2. Time period to achieve initial temperature (preheating step).
3. Vessel pressurization time.
4. Initial product quality.
5. Time period to depressurize.
6. Time period to cool after depressurization.
By using a higher pressure level with a lower initial temperature, a better-quality product can be achieved. This will minimize the preheating and post-processing heat damage. Pressure-induced thermal processing protects the product against excessive heat damage during pressurization. The fluid used in the pressure vessel has its own adiabatic heating properties which influence the end temperature of the food (Balasubramaniam, 1999). Typical fluids used in pressure vessels for the sterilization of foods include glycerol, edible oils, and water/edible oil emulsions.
Fig. 1 depicts a typical double-pulse process and lists the process variables. The pressure is indicated on the left axis and is expressed as mega Pascals; the temperature is on the right axis and is expressed in degrees Celsius; and the base axis is the time expressed in seconds.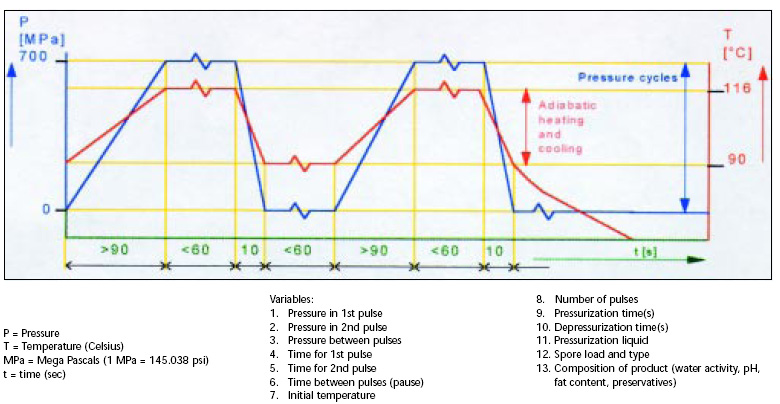
--- PAGE BREAK ---
After numerous studies in which the time was held constant and the pressure and initial temperature were varied, a trend was observed and a lethality chart was drawn (Fig. 2). A 1-min pulse was followed by a 30-sec ambient pressure pause and a 1-min final pulse. The spore load was at a minimum of 106/g of food product. The food product had 10% or less fat, a water activity above 0.82, and a pH above 4.5. It appears that there is always sterility at 621 MPa or higher, if the end temperature is at 105°C or higher. There is a gray zone where sterility is achieved, but not all the time. This is believed to be primarily due to the various spore loads and types, the vessel pressurizing fluid, and the food composition. C. sporogenes PA 3679, B. subtilis, B. cereus, and B. stearothermophilus were used as spore loads. B. subtilis was found to be the most-pressure-resistant organism.
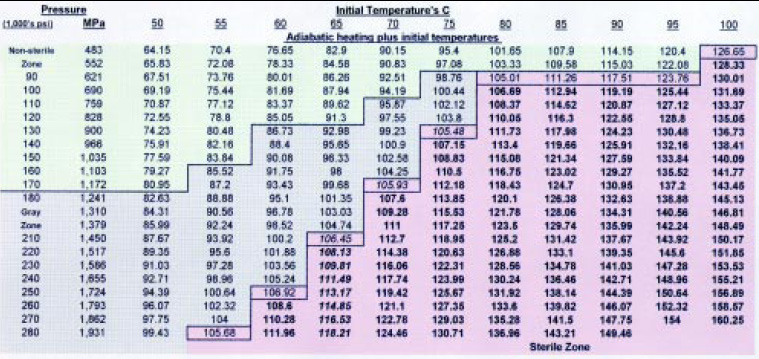 From this lethality chart, the high pressure process can be customized based on the heat sensitivity of the food product. The initial temperature can be lowered as the pressure is increased, so that the same sterilizing end temperature (105°C or greater) due to instant adiabatic heating is achieved. At lower initial temperatures, heat-sensitive products can be processed without heat damage. As the pressure increases, the cost of the High-pressure vessel and processing costs rise. Table 1 presents guidelines for producing the best product for each food category.
From this lethality chart, the high pressure process can be customized based on the heat sensitivity of the food product. The initial temperature can be lowered as the pressure is increased, so that the same sterilizing end temperature (105°C or greater) due to instant adiabatic heating is achieved. At lower initial temperatures, heat-sensitive products can be processed without heat damage. As the pressure increases, the cost of the High-pressure vessel and processing costs rise. Table 1 presents guidelines for producing the best product for each food category.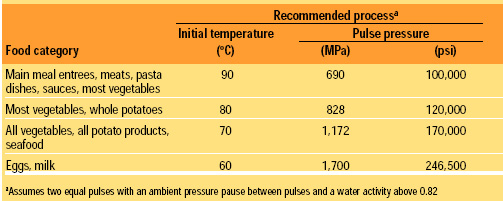
Most food products can be sterilized using 90°C at 690 MPa and two pulses. Main meal entrees, such as macaroni and cheese, tuna noodle casserole, beef stroganoff, and pasta dishes, are unaffected by the high-pressure process. Pasta retains its al dente texture. Vegetables such as carrots, celery, onions, green beans, and cauliflower will have nearly raw-product properties with a crispy texture. By using 60°C at 966 MPa and two pulses, asparagus comes out crispy; potatoes have a “freshly cooked” flavor and texture; and eggs are as if they were “freshly cooked” to medium consistency.
Critical Control Points
To ensure that a given product is sterilized, certain variables need to be measured and recorded, much like in conventional retorting. These critical control points for a batch process are as follows:
1. Temperature in the high-pressure vessel chamber prior to processing. This will ensure that the initial temperature is at the proper target temperature prior to introducing the prepackaged foods.
2. Product temperature and uniformity of temperature throughout the product. The product must be at the initial homogeneous target temperature, and there must be no cold spots; otherwise the product will not achieve the temperature during pressurization to achieve sterility.
3. Ratio of pressurizing fluid to product in the vessel chamber. Since the specific heat of the pressurizing fluid differs from that of the food product, the ratio of food product to pressurizing fluid must be kept the same to obtain repeatable results. The same pressurizing fluid must be used for all calibrations and processing to be consistent. A new type of fluid may change the final temperature, since it might have different adiabatic heat properties which will influence the final temperature of the product. Thus, it needs to be tested to measure its impact on the adiabatic heat of the food.
4. Package integrity (materials and seals). Prior to loading into the high-pressure vessel, the package must be checked to ensure that there is a hermetic seal.
--- PAGE BREAK ---
5. Pressurization time. The time it takes to pressurize affects the amount of sidewall heat loss and the ultimate end temperature. To ensure a consistent process, the pressurization time needs to be reproducible for each cycle.
6. Peak pulse pressure. Since the peak pressure provides the adiabatic heating to achieve the appropriate end temperature, it is critical that the peak pulse pressure be recorded for each cycle.
7. Product temperature during the peak pulse pressure. Besides two complete pulses, the end temperature achieved during the peak pulse pressure is the most critical variable in achieving sterility. This temperature must be measured in several locations in the vessel and recorded for each cycle.
8. Pulse time. The time at the peak of the pulse and during the ambient pressure pause in both cycles needs to be measured and recorded to provide uniformity in the process and ensure a margin of safety.
9. Number of pulses. Along with the end temperature, this is a very critical factor in achieving sterility. It must always be measured and recorded.
10. Depressurization time. The depressurization time must be the same for reproducibility in the process. Like the pressurization time, the depressurization time will affect the loss of heat through the side walls of the vessel and can affect the end temperature, especially on the second pulse.
Also, the product must be within operating standards prior to processing (pH, water activity, fat content). For approval of a high-pressure sterilization process by both the Food and Drug Administration and the U.S. Dept. of Agriculture, sterility must be verified with the actual product and equipment to provide proof that sterility is achieved; this is the same standard that is used in commercial canning thermal process verification.
Sterilization Production Cost
The cost to modify an existing line and produce product is estimated to be $0.0455/lb. The assumptions for this estimate are listed in Table 2. We anticipate that the cost of the vessels will decrease over time and the cycle time will be reduced. A cost of about $0.03/lb is possible with expected future improvements.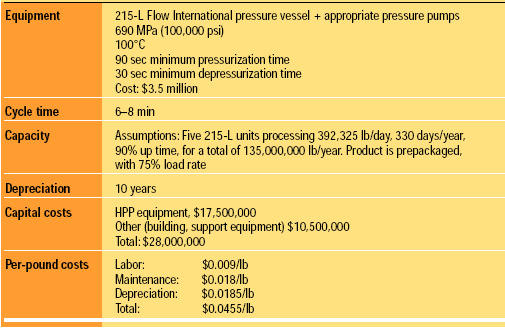
 The costs were estimated by engineers from Unilever and Basic American Foods. Both came up with the same costs independently, applied to different product lines and based on actual operational data. Flow International provided numbers from the actual performance of their 215-L unit.
The costs were estimated by engineers from Unilever and Basic American Foods. Both came up with the same costs independently, applied to different product lines and based on actual operational data. Flow International provided numbers from the actual performance of their 215-L unit.
by RICHARD S. MEYER, KERN L. COOPER, DIETRICH KNORR, AND HUUB L.M. LELIEVELD
Author Meyer is President, Washington Farms, Inc., 3813 E. 80th St., Tacoma, WA 98443. Author Cooper is Manager, Technology Development, Basic American Foods, Inc., 415 W. Collins Rd., Blackfoot, ID 83221-5642. Author Knorr, Secretary General of the European Federation of Food Scientists & Technologists (EFFoST), is Professor, Food Technology, and Department Head, Food Biotechnology and Food Process Engineering, Berlin University of Technology, Koenig-Luise-Strasse 22, D-14195 Berlin, Germany. And author Lelieveld, President of EFFoST, is Senior Technologist, Food Processing, Unilever Research Vlaardingen, P.O. Box 114, 3130 AC Vlaardingen, The Netherlands. Send reprint requests to author Meyer.
Edited by Neil H. Mermelstein, Senior Editor
References
Balasubramanian, V.M. 1999. High pressure processing. 1999 annual report. Natl. Center for Food Safety and Technology, Summit-Argo, Ill.
FDA. 2000. High pressure processing. In “Kinetics of Microbial Inactivation for Alternative Food Processing Technologies.” FDA Center for Food Safety and Applied Nutrition report, June 2. Food and Drug Admin., Summit-Argo, Ill.
Hayakawa, I., Kanno, T., Yoshiyama, K., and Fujio, Y. 1994. Oscillatory compared with continuous high pressure sterilization on Bacillus stearothermophilus spores. J. Food Sci. 59: 164-167.
Meyer, R. 2000. Ultra high pressure, high temperature food preservation process. U.S. patent 6,017,572.
Sojka, B. and Ludwig, H. 1997. Effects of rapid pressure changes on the inactivation of Bacillus subtilus spore. Pharmazeutische Industrie 59: 436-438.
Wilson, M.J. and Baker, R. 1997. High temperature/ultra high pressure sterilization of low acid foods. Patent Cooperation Treaty WO 97/21361 and U.S. patent 6,086,936.
