Formulating and Manufacturing Ice Cream and Other Frozen Desserts
I scream, you scream, we all scream for . . . frozen desserts. Here’s how ice cream and the wide variety of other frozen desserts are formulated and manufactured.
 Frozen Desserts
Frozen Desserts
Standards for frozen desserts differ by country and region but leave much room for variety within each defined product. By modifying the formula of regular ice cream, processors may follow different standards and/or market their products under specific labels. The following are short descriptions of products marketed in the United States.
Ice Cream is a frozen emulsion of air bubbles, ice crystals, milk fat globules, colloidal proteins, and gums suspended within viscous syrup, the continuous phase (Fig. 1). Some components of the continuous phase, especially the lactose (milk sugar), are in the supersaturated state. Under certain conditions, lactose may crystallize in the frozen product, causing a sand-like feeling in the mouth. 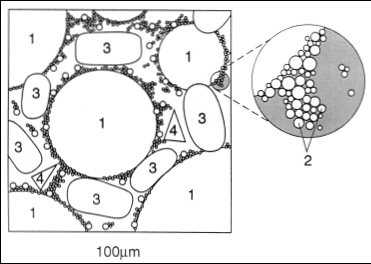
Incorporation of air into ice cream occurs within seconds after entry of the mix into the continuous freezer barrel. As the temperature of the mix drops rapidly in the barrel of the continuous freezer, the air bubbles become entrapped and stabilized within a semi-solid matrix that consists of viscous syrup, ice crystals, and partially churned fat. Properly formed, this matrix can prevent the collapse of air bubbles in the packaged product. Collapse of a significant number of air cells can result in shrinkage within the package and the concurrent loss of quality and acceptability of the product (Dubey and White, 1997).
In the U.S., plain ice cream must contain at least 10% milkfat (MF) and 20% total milk solids (TMS). Additionally, for each 1% that MF content is increased, up to 14%, U.S. processors may reduce the nonfat milk solids (NMS) content by 1% and may also add cocoa or chocolate, fruit, nuts, and confections, plus the additional sweeteners used in each, to displace up to 2% of the MF. Up to 4% of the TMS may be replaced by bulky flavoring agents. The minimum weight of 540 g/L limits the amount of air that can be incorporated, consequently the maximum overrun, to approximately the same volume as the volume of mix. Overrun is the percentage of increase in volume over the volume of mix frozen per unit total volume. For example, when 1 L of mix yields 2 L of ice cream, the overrun is 100%.
Bulky Flavored Ice Cream. This product contains a significant amount of flavoring ingredients, including cocoa, fruit, nuts, confections, or cookies. A reduction in the minimum fat content is permitted as follows: 2.5 times the weight of cocoa solids or 1.4 times the weight of fruit, fruit juices, or nuts. Weights of dehydrated fruits or fruit concentrates may be calculated at their natural levels before being multiplied by 1.4.
Frozen Custard, French Ice Cream. This product contains egg yolk solids constituting not less than 1.4% of the weight of the frozen product nor less than 1.12% for bulky flavored products.
--- PAGE BREAK ---
Reduced-Fat Ice Cream. This product contains 25% less fat than the reference ice cream, about 7.5% fat.
Light, Lite Ice Cream. This product contains 50% less fat (about 5% fat) or 1/3 fewer calories than the reference ice cream, provided that in case of the caloric reduction less than 50% of the calories are derived from fat.
It is difficult to meet the label requirement using calories as the standard. For example, consider two products frozen at 100% overrun. The reference containing 10% MF, 10% NMS, and 17% sweetener would yield 120 kcal/serving, while the lowfat version containing 5% MF, 13% NMS, and 17% sweetener would yield 105 kcal/serving.
Lowfat Ice Cream. This product contains not more than 3 g of MF/4-fl-oz serving that may weigh as little as 60 g. Australia and New Zealand require not more than 3 g fat/100 g of ice cream. In Canada, the similar product with 3–5% fat is labeled as ice milk.
Nonfat, No-Fat Ice Cream. This product contains less than 0.5 g of MF/serving. It may contain no added ingredient that is fat or that contains more than a trivial amount of fat. Australia and New Zealand permit 0.15 g of fat/100 g of ice cream.
Sugar-Free Ice Cream. This product is made without sucrose, glucose, fructose, or corn syrup solids but is not free of lactose. Substitutes for the sugars include polyols, polydextrose, maltodextrin, and high-intensity sweeteners.
Lactose-Free Ice Cream. To prepare this product, lactose must be removed by ultrafiltration and/or enzymatically hydrolyzed to the component monosaccharides glucose and galactose. Since lactose constitutes about 55% of NMS, it must be replaced with some other ingredient if it is removed.
Gelato. This is an Italian-style ice cream that is rich in egg yolk solids and total food solids while containing little air and no stabilizer or emulsifier. Milkfat content may vary widely up to 18%; however, 6% is characteristic.
Mellorine. This product has the general composition of ice cream but with the milkfat replaced in whole or part by vegetable or animal fat. It contains, by Food and Drug Administration Standard of Identity (21 CFR 135.130), not less than 6% fat, 2.7% milk-derived protein, and 40 IU of vitamin A/g of fat.
Frozen Yogurt. There is no federal standard for this frozen dessert in the U.S. However, it commonly is formulated in semblance of light or nonfat ice cream. The property that characterizes the product is fermentation of all or part of the dairy ingredients by the combined actions of Streptococcus salivarius subspecies thermophilus and Lactobacillus delbrueckii subspecies bulgaricus. A minimum of 0.3% acidity, calculated as lactic acid, is required by some regulatory agencies. Many of these bacteria die during the freezing process.
--- PAGE BREAK ---
Fruit Sherbet. This is an intermediate product between water ices and ice cream; it consists of fruit juices, fruit flavorings, about 30% sugar and corn syrup solids, stabilizer, and 2-5% TMS (MF of 1–2% and NMS of 1–4%). The frozen sherbet must weigh at least 720 g/L, compared to a minimum of 540 g/L for ice cream.
Ices, Water Ice. This product contains fruit juice and/or fruit flavoring, nutritive sweetener, stabilizer, and color, with or without added fruit acid and water. No dairy or egg products are contained. It is commonly frozen quiescently on sticks. A formula suitable for making 1,200 L of mix for ice on sticks contains 1,000 L of water, 192 kg of sugar, 48 kg of corn syrup solids, 4.8 kg of stabilizer, 10 L of citric acid, and 2.6 L of flavoring. Content of flavoring varies considerably among flavors and suppliers.
Novelties. These are uniquely and creatively produced and packaged individual servings of frozen desserts made from any type of mix and hard-frozen. They include bars with or without sticks, sandwiches (e.g., ice cream plus wafers), combinations of flavors, special shapes, multiple categories of mix (e.g., sherbet and ice cream), and coatings (e.g., chocolate and nuts).
Soft Serve. This is any frozen dessert sold as drawn from the freezer without hardening.
Other Products. Several other terms describe selected ice cream products: aufait means that the ice cream contains or has layers of viscous fruit; bisque, a bakery product; confection, candy; neopolitan, multiple flavors in a package; pudding, high fat, fruits, nuts, spices, eggs; and variegated, ripples or swirls of flavored syrups.
Flavor Categories. U.S. standards identify three categories of flavoring: in Category I, only pure extracts and flavorings are contained; in Category II, pure extracts and flavors are dominant over synthetic components; and in Category III, artificial flavor is dominant over natural flavoring components. Labels must reflect these categories; e.g., when vanilla is the flavoring, labels of products in Categories I, II, and III would read “vanilla,” “vanilla flavored,” and “artificially flavored vanilla,” respectively.
Ingredients
Since ice cream is the frozen dessert with the greatest consumption in the U.S., the rest of this article will focus on ingredients used in the formulation of ice cream. Of course, these ingredients have applications in many other frozen desserts.
Although the ingredients of ice cream must supply minimal amounts of milk solids and food solids per unit of volume, the sources of milk solids can vary widely. For example, ice cream can be made using highly perishable cream and concentrated milk or from highly stable butter or anhydrous milk fat plus nonfat dry milk. Of course, these and other forms of milk solids can be mixed in numerous combinations. Likewise, numerous types of sweeteners are available to suit specific product profiles. Ingredients for stabilization and emulsification are several, and formulas vary in proportions of each as well as in total concentrations.
U.S. standards for ice cream permit the use of certain ingredients and specifically limit the use of others. Most milk and nonfermented milk products are permitted, including the liquid and dry forms. However, amounts of whey solids or modified whey solids are limited to 25% of the NMS content. Whey may have been treated to reduce the lactose and/or mineral content. Caseinates and/or hydrolyzed milk proteins may be added for functional purposes to mixes containing at least 20% TMS. Limits apply to acidities of sweet cream buttermilk and modified skim milk.
--- PAGE BREAK ---
Milkfat. The MF emulsion is composed of about 96% triacylglycerides plus mono- and diacylglycerides and other fat-soluble components, especially vitamin A. MF contains almost 400 different fatty acids (Jensen, 2002) and uniquely contains 11.8 and 4.6 moles each of butyric and caproic acids, respectively, per 100 moles of total fatty acids. A broad melting range of MF from –40 to +40°C is caused by the distribution of the fatty acids in the glycerides, where all of the butyric acid and 93% of the caproic acid are esterified on the third carbon of the glycerol molecule. The natural fat globule membrane is high in the emulsifying phospholipids, especially lecithin.
MF provides richness of flavor, carries fat-soluble flavors, and lubricates the mouth as well as the barrel of the freezer. Importantly, it partially coalesces during freezing to form a structure that helps to stabilize the foam structure and to give body (resistance) to the ice cream. Relatively high cost, high caloric density, and the tendency to reduce the whipping ability of the mix are primary factors limiting the amounts of fat that should be used in ice cream.
To be a satisfactory substitute for MF in ice cream, a vegetable fat must have a bland flavor and contain an intermediate liquid:solid fat ratio during freezing.
Nonfat Milk Solids. The nonfat portion of milk is composed of approximately 55% lactose, 37% protein, 8% minerals, and small amounts of vitamins, acids, and enzymes. The amount of NMS in ice cream mixes ranges from 6 to 14%, and usually varies inversely with the fat content. This ratio of the major components is significantly affected by the addition of whey solids or their substitution for NMS in the formula. Whey solids (WS) characteristically contain about 77% lactose, 13.4% protein, 8.6% minerals, and 1% fat. Compared with NMS in the formula, the higher lactose content in WS increases the potential for crystallization of this sugar in the frozen product. Furthermore, the comparatively high concentrations of lactose and minerals cause the freezing point to be lowered. Finally, protein content is decreased. These are the major reasons for the limitation of 25% substitution of WS for NMS. These effects can be offset by using de-lactosed and de-mineralized whey solids, as found in whey protein isolate. The main incentive for using whey solids in ice cream is low cost.
Buttermilk solids can be a superior substitute for NMS, especially in mixes made with butter, butter oil, or anhydrous MF as the source of fat. Buttermilk contains a higher concentration of fat globule membrane phospholipids than does skim milk. Thus, it reduces the need for emulsifiers in regular mixes and is desired in mixes containing no emulsifiers.
Lactose. Lactose can be undesirable in the diets of persons whose bodies produce insufficient ß-D-galactosidase to hydrolyze the lactose. Therefore, it may be desirable to remove it by ultrafiltration and diafiltration or to hydrolyze it enzymatically. The freezing point can be maintained by removing 50% of the lactose, a disaccharide, using ultrafiltration, followed by hyrolysis of the remaining lactose to the monosaccharides glucose and galactose. The resulting frozen dessert can be labeled lactose free.
Proteins. Along with their high nutritional value and favorable effect on flavor, milk proteins contribute desirable physical properties to ice cream. As good foaming agents, they are vital to the incorporation of air. They provide strength to the lamellae of the air cells (Turan et al., 1999). Native milk protein consists of about 80% micellar casein and 20% whey proteins that are mostly globular. These proteins can be isolated and processed for their particular functional attributes. A large supply of whey proteins is available as a by-product of cheese manufacture. This highly nutritious protein provides an economical additive to frozen desserts. Sodium caseinate is available in the world market at a low cost relative to milk protein from normal sources. However, federal standards in the U.S. require that caseinates be added only after the requirement of 20% total milk solids has been met for regular ice cream.
--- PAGE BREAK ---
The type of milk protein can affect the stability of the air cells in ice cream. Sodium caseinate contributes to aeration and emulsification of ice cream mixes, but it does not function the same as micellar casein (Goff et al., 1989). Although it increases the stability of air cells, it may render the emulsion so stable that adequate churning does not occur in the freezer. Segall and Goff (1999) showed that the membranes of fat globules stabilized by whey protein isolate were more susceptible to destabilization than those formed from sodium caseinate or casein micelles.
As with fat, proteins have little effect on freezing point except as they displace water. Milk proteins are hydrated, and the degree of hydration increases during high-temperature pasteurization because of the unfolding of protein structures. Therefore, high heat treatment may be used to minimize or eliminate the need for stabilizers in frozen desserts. Freeze-concentration of proteins in ice cream greatly increases the viscosity of the unfrozen phase, and this has a great effect on ice crystallization, ice crystal stability, and solute mobility (Flores and Goff, 1999).
Minerals. The minerals of ice cream, being mostly dissolved, reduce the freezing point, thus affecting the energy needed to freeze the product, rate of melting, and ease of dipping. They also contribute to the flavor and nutritional value. Nutritionally important calcium and phosphorus in ice cream are derived almost entirely from the NMS. Since NMS content can range from about 6% to 14% and 1 g of NMS contains 14 mg of calcium and 11 mg of phosphorus, a 70-g serving of ice cream can supply 58–135 mg of calcium and about 45–105 mg of phosphorus.
Fortunately, milk contains little copper or iron, the two minerals that catalyze oxidation. Since ice cream is often stored for months, it is imperative that contamination of any of the ingredients with copper or iron be prevented.
Sweeteners. The obvious function of sweeteners in ice cream is to sweeten; however, they do much more than that. Since their total concentration may range from as low as 13% in soft-serve ice cream to 30% or more in ices and sherbets, it is apparent that they displace a lot of water. Furthermore, since they are dissolved, they are the major ingredients that lower the freezing point of the mix. For reference, 15% sucrose or 7.5% of dextrose (glucose) by weight lowers the freezing point of a pure solution by nearly 1.2°C. Polysaccharides also displace water; however, depending on their chain length, they sweeten and lower the freezing point much less than do mono- and disaccharides. Sweeteners usually are the least expensive source of total solids in ice cream.
As sweetener concentration is increased, viscosity of the mix and firmness of the frozen product increase. When the sweetener content is about 16% and higher, ice cream tends to become too soft or too dense and chewy, depending on the type of sweetener used in excess.
--- PAGE BREAK ---
Sucrose has been the major sweetener used in ice cream, and the amount normally used is 14–16%. Since there is no chemical test for sweetness and this taste sensation varies among individuals, it is common practice to compare the sweetness of other sugars to that of sucrose, which is assigned a value of 100 (relative sweetness). Most of the natural sweeteners used, other than sucrose, are derivatives of starches, especially corn starch.
Corn sweeteners used in the industry are in three forms, viz., crystalline corn sugars (dextrose and fructose), dried corn syrup (corn syrup solids or glucose solids), and liquid corn syrups. Dry products are also available that have been agglomerated to produce powders with high wettability and little dust. Maltodextrins, mildly hydrolyzed products of starch, are also available in dried form but are sparingly sweet. They are used primarily as bulking agents.
Degree of hydrolysis of starch is measured in dextrose equivalents (DE). Dextrose, the product of completely hydrolyzed starch, has a DE of 100 and a relative sweetness of 74. Conversion of dextrose to fructose increases the relative sweetness to 173. Maltose, the glucose–glucose disaccharide, has a relative sweetness of 32. Breaking of the 1,4-glucosidic bonds of starch produces multiple polymers of various lengths depending on the number and locations of the bonds hydrolyzed.
Corn syrups are those starch hydrolysates that have 20 to about 70% of the glucosidic linkages broken. Their classification is based on degree of conversion: low conversion is 28– 38 DE; regular conversion, 39–48 DE; intermediate conversion, 49–58 DE; and high conversion, 59–68 DE. The ratio of higher- to lower-molecular-weight fractions can be estimated from the DE of the syrup. Ice cream manufacturers usually use liquid or dry corn syrup products with a DE of 28–42. Maltodextrins range in DE from 4 to 20. They are most useful in the production of lowfat frozen desserts, in which they contribute greatly to body. The medium-molecular-weight saccharides (dextrins) are effective stabilizers and slow the formation of large ice crystals, thus improving heat-shock resistance. High-maltose syrups reduce the effect of dextrose on freezing point. Optimization of DE and concentration of corn sweeteners is required for the most beneficial effects.
Sugar Alcohols. Sugar alcohols are used to replace conventional sweeteners in sugar-free frozen desserts. Because they have a much lower glycemic index than sugar and corn sweeteners, they provide solutions for formulating frozen desserts for insulin-dependent diabetics. Included in this group of mono- and disaccharide sugar alcohols (the polyols) are sorbitol, mannitol, xylitol, erythritol, lactitol, maltitol, isomalt, and some related hydrogenated starch hydrolysates (Dalzell, 1996; Nabors, 2001, 2002). Sorbitol is the main polyol used in ice cream.
Functionally, the polyols add bulk, body, and sweetness to ice cream. They vary in relative sweetness, freezing-point depression, solubility, heat of solution (cooling effect), stability, laxation potential, cost, and caloric content. In the U.S., self-determination of the caloric content is permitted, and some of the values acknowledged by FDA are 2.6 kcal/g for sorbitol, 1.6 kcal/g for mannitol, 2.4 kcal/g for xylitol, 2.1 kcal/g for maltitol, 2 kcal/g for lactitol, and 2 kcal/g for isomalt.
Sorbitol, produced by hydrogenation of glucose, is 0.6 times as sweet as sucrose, while mannitol, produced by hydrogenation of fructose, is 0.5 times as sweet as sucrose. Sorbitol is hygroscopic, while mannitol is not. Both cool the mouth because of their negative heat of solution. Being monosaccharides, they depress the freezing point twice as much as an equal weight of sucrose. The laxation thresholds, in g/day, are considered to be 50 for sorbitol and 20 for mannitol.
Maltitol, produced by hydrogenation of maltose, provides similar freezing-point depression and sweetness as sucrose and does not have the cooling effect of sorbitol and mannitol. Its laxation threshold is set at 100 g/day before a warning label must be used.
--- PAGE BREAK ---
Nonnutritive Sweeteners. Sugar-free ice creams can be made using combinations of high-potency, “nonnutritive,” sweeteners and bulking agents. Whereas it is easy to find a high-potency sweetener to replace the sweetness of sucrose and corn syrup solids, it may be difficult to find suitable ingredients to depress the freezing point and to build total solids while contributing few calories. Polydextrose is a low-calorie bulking agent that can be used at a significant concentration without greatly affecting viscosity, but it contributes little to freezing-point depression.
The most-common high-potency sweeteners (Dalzell, 1996; Nabors, 2001, 2002) are described below. Some are digested, and thus caloric, but are often considered nonnutritive because so little is required to adequately sweeten.
Saccharin is approximately 300 times as sweet as sucrose. It can withstand long periods of storage as well as heat and is the least expensive of the nonnutritive sweeteners.
Aspartame is the methyl ester of two amino acids, L-aspartic acid and L-phenylalanine, both of which occur naturally in foods. It is digested in the same manner as other amino acids. However, it contributes few calories to a serving of ice cream because, being 200 times as sweet as sucrose, little of it is needed to sweeten. Aspartame shows sweetness synergy with several other sweeteners, e.g., saccharin and acesulfame K, and enhances some flavors. At high temperature in dry environments, aspartame undergoes hydrolysis and loss of sweetness, but this is not an issue in the normal pasteurization of ice cream mixes. Persons with the rare hereditary disease phenylketonuria (PKU)—about 1 in 15,000—must control their intake of phenylalanine. Therefore, products containing aspartame must carry a warning statement on the label.
Neotame, the product of hydrogenation of aspartame and 3,3-dimethylbutyraldehyde, is 7,000–13,000 times as sweet as sucrose and 30–60 times as sweet as aspartame. This heat-stable, non-nutritive sweetener was approved by FDA in 2002 (Prakash et al., 2002). The addition of dimethylbutyraldehyde to the molecule essentially blocks the hydrolysis of the aspartic acid–phenylalanine dipeptide, reducing the availability of phenylalanine. Therefore, there is no requirement for a warning label to alert persons with phenylketonuria (Anonymous, 2002).
Acesulfame potassium (acesulfame K) is a nonnutritive organic salt containing sulfur and nitrogen. It is 150–200 times as sweet as sucrose, is stable and water soluble, does not decompose on heating, and has a synergistic sweetening effect with aspartame, cyclamate, and several nutritive sweeteners. Mixtures of acesulfame K and aspartame can provide a sweetness profile similar to that of sucrose.
Sucralose is the generic name of a high-intensity, noncaloric sweetener made by selectively substituting three chlorine atoms for three hydroxyl groups of sucrose. It looks and tastes like sugar but is, on the average, 600 times sweeter. It remains stable during pasteurization and at pH ranges common to frozen desserts (pH 3–7).
Fat Replacers. High-quality frozen desserts containing 5–6% fat can be produced without fat replacers, but mixes containing less than 4–5% fat usually require additional ingredients specifically chosen for their fat-replacing properties. No single fat replacer is satisfactory for all applications.
--- PAGE BREAK ---
No single fat replacer is satisfactory for all applications. However, combinations of them can compensate for the loss of several functions of fat. Chief among their deficiencies are reduced creamy mouthfeel, loss of the milkfat flavor, and lack of the ability to carry fat-soluble flavors.
Emulsifiers function in lowfat frozen desserts by promoting the fine distribution of air bubbles and ice crystals. Structured lipids are short- and long-chain fatty acid triacylglycerols combined to mimic fat but provide only about 5 kcal/g. One such product, salatrim (Danisco’s, Benefat®), consists of acetate, propionate, and butyrate short-chain fatty acids with long-chain stearate.
Fatty acid polyesters have fat-like properties but are not digested by humans. Olestra (Procter & Gamble Co.’s Olean®), a sucrose-based fatty acid polyester with 8–10 fatty acids esterified to sucrose hydroxyl groups, is one such product.
Protein-based fat replacers, such as CP Kelco’s Simplesse®, are typically derived from whey protein concentrate. They are thermally aggregated under shear, producing particles 0.5–2 µm in diameter that can promote a creamy textural sensation. Size of the particulates is an important determinant of mouthfeel.
Maltodextrins, discussed previously, have been widely used as bulking agents in reduced-fat ice creams. Those with a DE of 5–10 have the best fat-replacing properties. Having lost the granular structure of starch, they are soluble and can form macromolecular networks that impart creaminess to lowfat frozen desserts.
Methylcellulose and hydroxypropyl methylcellulose are surface-active polymers that form films in solution then gel upon heating. Microcrystalline cellulose and carboxymethyl cellulose (CMC) can be blended (e.g., FMC BioPolymer’s Avicel®) to provide colloidal particles. They require high shear and homogenization for proper dispersion and functionality. These cellulosic additives improve the perception of creaminess in lowfat ice creams. The rate of use is 0.2–0.8%.
Polydextrose (e.g., Danisco Sweeteners’ Litesse®) is a randomly bonded melt-condensation polymer of dextrose with lesser amounts of sorbitol and citric acid. It resists breakdown by human digestive enzymes, yielding only 1 kcal/g, and qualifying as 90% dietary fiber. It functions primarily as a bulking agent that raises the viscosity of the product’s unfrozen phase, thus contributing to desirable mouthfeel. At equivalent solids substitution levels, a 60:40 combination of polydextrose and sorbitol provides the same freezing-point depression as sucrose.
Some blends of gums form microscopic spherical particles that mimic the rheology and mouthfeel characteristics of emulsified fat. These blends may contain guar, locust bean, and xanthan gums; carrageenan; sodium carboxymethyl cellulose; and microcrystalline cellulose.Schmidt et al. (1993) reported that in light-type ice cream the protein-based alternatives produced products more similar to ice cream than did the carbohydrate-based products. This was due in part to the better emulsification and whipping imparted by the proteins, as well as the colloidal nature of the microparticulated proteins.
--- PAGE BREAK ---
Stabilizers. Stabilizers are added to ice cream for the primary purpose of affecting the texture. The consumer usually desires a velvety-smooth product with a moderate rate of melt, limited coldness, and a uniform distribution of particulates. Stabilizers contribute to these properties by absorbing water (therefore swelling) and limiting its migration, increasing viscosity, adsorbing to air cell lamellae, and, in some instances, forming a gel-like structure. At high concentration, they interact and become entangled with each other, greatly modifying the rheological characteristics of solutions. Stiffness added by stabilizers to ice cream exiting the freezer barrel promotes easy cut-off of extruded products and efficient packaging. The major contribution to quality is the retardation of growth of crystals of ice and lactose.
Overuse of stabilizers can cause excessive mix viscosity, a heavy or soggy body, and undesirable melting characteristics. As ice freezes out of solution in frozen desserts, stabilizer concentration, which may be only 0.25–1.0% in the unfrozen mix, increases many-fold in the unfrozen milieu. Most polysaccharides are incompatible with milk proteins in solution, which leads to further localized concentrations. Some stabilizers can form a cryo-gel and entrap ice crystals within the gel (Goff et al., 1999; Regand and Goff, 2002, 2003; Patmore et al., 2003). Phase separation of polysaccharides and proteins appears also to be related to this gel formation.
Stabilizers have little (Caldwell et al., 1992) or no (Sutton and Wilcox, 1998a, b) impact on size of ice crystals exiting the freezer, and the impact is limited during hardening (Flores and Goff, 1999a,b). Stabilizers have no effect on the freezing properties of an ice cream mix, e.g., freezing-point depression (Budiaman and Fennema, 1987a, b), amount of freezable water or enthalpy of melting (Sahagian and Goff, 1995), or heterogeneous nucleation (Muhr et al., 1986, and thus may not affect initial ice crystallization processes.
However, stabilizers limit the rate of growth of ice crystals during recrystallization, i.e., the melting of small ice crystals, migration of that water, and refreezing onto other crystals (Donhowe and Hartel, 1996a, b; Regand and Goff, 2002, 2003). They may modify the ice crystal serum interface by (1) adsorbing onto the surface of the crystal (Sutton and Wilcox, 1998a, b), (2) limiting the rate of water diffusion to the surfaces of crystals, or (3) limiting the rates at which solutes and macromolecules can diffuse away from the surfaces of ice crystals (Goff et al., 1993).
The stabilizing ingredients most used in frozen dairy foods are guar gum, locust bean gum (carob bean gum), CMC, sodium and propylene glycol alginates, xanthan gum, gelatin, and carrageenan. Pectin is useful in combination with the gums in sherbet and ices. Each has a particular effect on body, texture, meltdown, and stability in storage. Therefore, to gain synergism in function, proprietary manufacturers combine them with emulsifiers. Components selected vary with the composition of the mixes and the outcomes expected from their use. Attributes desired in blends are high rate of dispersion, dissolution at selected temperatures, minimal dust formation, and reasonable cost for the intended product.
Except for gelatin (a protein), these hydrocolloids are polysaccharides, polymers of sugar residues, the functionality of which can be modified by changing their chemical structure. Most of the hydrocolloids used are incompatible in solution with milk proteins, especially with casein micelles (Schorsch et al., 2000) and thus will cause a phase separation known as wheying off. Carrageenan is used in low concentration in most blends to retard such separation.
--- PAGE BREAK ---
Emulsifiers. Homogenization of the ice cream mix reduces the diameter of fat globules from as large as 10 µm or more to less than 2 µm and causes large changes in the characteristics of the emulsified fat. Although the amount of natural emulsifier in an ice cream mix is fully adequate to stabilize the newly formed globules, the rate of agglomeration of these globules is usually too low to provide the most desirable ice cream structure. When emulsifiers are added, the native globule membrane is substantially displaced by the emulsifiers and adsorbed proteins, and the surface area on the fat globules is increased markedly. The extent of adsorption of these molecules depends on their concentrations and individual physical properties (Krog, 1988).
The emulsifiers used in ice cream are primarily mono- and diacylglycerides and the sorbitan esters, especially polyoxyethylene sorbitan monooleate (polysorbate 80) (Marshall et al., 2003). They are supplied to processors as blends with stabilizers.
Goff and Jordan (1989) summarized the mechanism of action of emulsifiers as follows: “They lower the fat/water interfacial tension in the mix, resulting in protein displacement from the fat globule surface, which in turn reduces the stability of the fat globule to partial coalescence that occurs during the whipping and freezing process, leading to the formation of a fat structure in the frozen product that contributes greatly to texture and meltdown properties.” In addition, emulsifiers promote nucleation of fat during aging of mixes, thus reducing the time needed to age mixes before freezing. Their positive effects on ice cream structure decrease tendencies for shrinkage and lower the rate of melting.
The type and concentration of emulsifier used are unique for each mix formula and type of product. For example, it is important to obtain a high degree of stiffness and dryness in making extruded products such as ice cream bars. In making this product, a high amount of agglomeration (churning) of the fat should occur during freezing. Therefore, the surfaces of the fat globules must have a relatively high concentration of emulsifier and a small amount of adsorbed protein.
Flavorings. To consumers, flavor is the most important of all of the positive attributes of frozen dairy desserts. It can be varied in so many ways that the most desirable formula becomes a great challenge to the imagination of product developers. Traditional vanilla, chocolate, and strawberry remain high on the list of preferred flavors, but even within these flavors there are many variations. In addition, processors can choose a variety of fruits, fruit extracts, nuts, bakery inclusions, spices, liqueurs, and sweeteners. Some manufacturers have flavor lists consisting of 500 or more flavor formulas.
The two important characteristics of flavor are type and intensity. Whereas delicate flavors are easily blended and not often objectionable in high concentrations, harsh flavors may be objectionable even at low concentrations. Flavors should be only intense enough to be recognized easily and to present a delicate, pleasing taste.
--- PAGE BREAK ---
Even though mix composition, ingredient quality, process variables, freezing and storage conditions, and age of product affect flavor, important defects are too much or too little flavoring, unnatural or atypical flavoring, and too much or too little sweetness. Flavorings also can affect appearance; examples of problems include lack or excess of particles, particles too large or too small, uneven distribution of particles or variegate, ribbon that is too thick or thin, and wrong ingredient or color.
The contribution of milk solids to flavor is substantial. Milkfat provides creaminess, and the churning that occurs during freezing causes fat to impart a sense of smoothness to the consumer’s mouth. NMS contribute cooked and slightly salty flavors to ice cream because of the release of sulfhydryl groups during pasteurization and the natural milk salts contained in these solids, respectively.
The procedure for addition of flavorings to frozen desserts varies among the liquid, syrup, semisolid and solid forms, and the type of freezer. Liquid flavorings are added to mixes just prior to freezing. Most other forms of flavorings are added to the batch freezer just prior to completion of the freezing process. Product exiting the continuous freezer receives fruits, nuts, and other solid or semisolid flavorings as it passes through an ingredient feeder. Swirls or ribbons of syrups are added to the flowing semi-frozen ice cream by positive-type “variegating” pumps.
Performance of the freezer and related equipment varies depending on the mix composition and flavor chosen. In particular, chocolate ice cream mixes whip more slowly than mixes of most other flavors. Citrates or phosphates (0.1%) may be added to chocolate mixes to reduce the viscosity and whipping time.
Some flavorings are shelf stable, with varying “use by” times. These include vanilla extract, vanillin, and vanilla flavorings; cocoa; nuts; bakery products; confections; canned syrups; and aseptically processed acidic fruit preparations. However, once the packages of canned and aseptically processed ingredients are opened, they have a limited shelf life even when refrigerated. Frozen fruits must be used soon after hawing. Fresh fruits are seldom used in today’s frozen desserts industry.
For further discussion of specific flavorings and their uses in frozen desserts, see Marshall et al. (2003).
Manufacturing
Processing operations for ice cream and similar frozen desserts are diagrammed in Fig. 2. They can be divided into two distinct stages: mix manufacture and freezing operations. Ice cream mix manufacture consists of combination and blending of ingredients, batch or continuous pasteurization, homogenization, and mix aging. 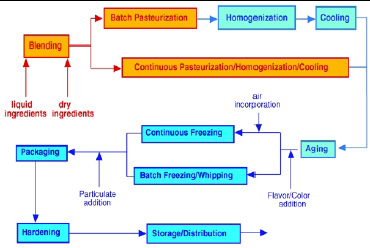
--- PAGE BREAK ---
In blending, high-shear blenders are normally used for efficient powder or solids incorporation, enabling a wide range of ingredients and product consistencies. This is especially important for production of novel, high-viscosity mixes containing, for example, fat replacers. Mix tanks are now designed for load cell installation and employ robust agitation from high motor torque. This enables precise mix batching and blending.
Vat pasteurization is still common in many ice cream operations, but batch pasteurizers are now typically conical-bottomed, domed-top tanks with total sweep agitation. This improves heat transfer and energy efficiency, as well as mixing (Marshall et al., 2003). Advances in high-temperature, short-time plate pasteurizers are normally focused around control rather than construction.
Much of the milk processing industry has moved toward low-pressure-valve homogenizers (e.g., Gaulin Micro-Gap) for energy efficiency, but these are known to wear quickly with the solids ingredients normally used in ice cream formulations. Thus, they are of limited use in ice cream processing. At the other end of the scale, there has also been much interest in ultra-high-pressure homogenization, to create more fat surface area and “make the fat go further,” so to speak, in terms of functionality.
Freezing is a two-step process, first under high shear (in scraped-surface freezers) and second under quiescent conditions (in hardening tunnels). In a continuous scraped-surface freezer, numerous processes take place that ultimately influence the overall quality of the ice cream (Russell et al., 1999; Sakurai et al., 1996). One of the most important of these, of course, is freezing water into ice (Hartel, 1996). At the same time as ice is being formed, there is also air incorporation, leading to development of air cells and the desired overrun. In addition, destabilization of the fat emulsion (partial coalescence) takes place during freezing, promoting incorporation and stabilization of the air cells (Kokubo et al., 1996, 1998; Goff, 1997). All of these processes take place simultaneously in the minute or less that ice cream spends in the dynamic freezing step. Draw temperatures are typically –5 to –6°C, and at these temperatures in conventional mixes about 50% of the water will be frozen.
Tremendous improvements in freezer design allow for exact control of output parameters through sophisticated automation. Modern programmable freezers can be linked to the ingredient feeder for exact dosing of particulate ingredients and to the filler machine to permit it to control the mix flow rate and thereby adjust the rate of production to the rate of fill.
Air handling is the first major area of change in modern ice cream freezers. They are typically supplied with compressed, sterile-filtered air through a regulator or a mass flow meter. Overrun control is precise and variable from 10 to 150%.
Freezer manufacturers vary in their approach to controlling the overrun, stiffness, and draw temperature of frozen products. One method is to control the speed of the mix pump, while using an air mass controller to meter the desired percentage of overrun air. The freezer maintains the desired mix flow rate by adjusting the speed of the mix pump. Based on the mix flow rate, the freezer sends a signal to the air mass controller to meter the proper amount of air to provide the desired percentage of overrun. The stiffness (viscosity)—and therefore indirectly the output temperature—is measured by monitoring the dasher motor load required to rotate the dasher assembly.
--- PAGE BREAK ---
Back pressure on the freezing cylinder (“cylinder pressure”) is important for proper freezer performance. Many commercial continuous freezers utilize mix and product pumps. The mix is fed to the freezing cylinder through a mix pump, and the semi-frozen product discharges from the freezing cylinder through the product pump, the speed of which is controlled to maintain a constant pressure in the freezing cylinder. With the two-pump arrangement, the first pump works against only the cylinder pressure, while the discharge pump works against the difference between the downstream line pressures from ingredient feeding and packaging machines and the cylinder pressure. Thus, the two-pump system isolates the freezing cylinder from external pressure changes and tends to yield more constant overrun (Marshall et al., 2003).
Dashers function in ice cream freezers to carry the sharp blades that scrape ice from cylinder walls, to agitate the mix and air, forming a finely divided foam, and to partially destabilize the fat to help stabilize the foam. Dashers come in many different types, from solid (high-displacement) dashers to open (low-displacement) dashers with beaters contained within the dasher assembly.
High-displacement dashers, those with a solid core that rotate at a high speed, tend to produce a stiffer product than the open dasher, which is driven more slowly. These solid dashers displace about 80% of the volume in the freezer barrel, have a high speed of rotation, and tend to produce ice creams with minute ice crystals (Russell et al., 1999). However, these products tend to be highly destabilized, so that they have a slow melting rate. This type of dasher action is desirable for producing extruded products, like ice cream bars that are to be enrobed in chocolate. Here, product shape must be maintained long enough for effective hardening, and shape must be maintained when the bar is covered with the warm chocolate.
However, the combination of solid dasher with a small annular space between the dasher and the freezer cylinder wall limits the volume of mix in the chamber. As the surface-to-volume ratio increases, so do chances of freeze-up within the cylinder. By increasing the diameter of the freezing cylinder and reducing the displacement of the dasher to 30–50% —essentially converting to an open (hollow) dasher—the freezer becomes much less sensitive to variations in refrigerant supply. Mix tends to act as a buffer against physical changes within the system, and the temperature and overrun uniformity of the output is increased. However, less destabilization is likely to occur in such freezers, so that the ice cream tends toward wetness in appearance and quickness of melt. In addition, the ice crystals in ice cream made with an open dasher tend to be larger as the average residence time within the barrel is increased.
One approach to decreasing air cell sizes is to install a pre-aeration device ahead of the freezing cylinder. The dasher in the barrel cannot spin fast enough to divide the mass of air injected with the mix until the mix viscosity increases during freezing. Since viscosity increase due to freezing typically starts about one-third of the distance from the entrance end of the freezing chamber in a continuous freezer, most of the small air cells are formed in the distal two-thirds of the chamber. By substantially dispersing the air before the mix enters the cylinder, air cells are made smaller and more uniform. Consequently, more and smaller ice crystals are formed, and the exiting product appears dryer than when no pre-aeration is done. Small air cells are more stable than large ones, rendering ice cream made in this way comparatively less susceptible to shrinkage in the container. Some types of novelty products, such as stickless bars, must be extruded from the freezer in very stiff form. In addition, since lowfat and nonfat products need to have very small air cells, pre-aeration is a desirable treatment for these products (Marshall et al., 2003).
--- PAGE BREAK ---
Considerable attention has recently been given to low-temperature extrusion freezing of ice cream following freezing in a conventional scraped-surface freezer, and several such units are in commercial use or development. In this system, the ice cream exiting a continuous freezer at –5 to –6°C is passed through a twin-screw extruder and cooled further to about –15°C. The product remains pumpable even at this low temperature (and higher ice content) because the shear effects in the extruder prevent ice crystal accretion. The smaller ice crystals, while allowing adequate flow of ice cream from the extruder, also provide smoother texture and greater resistance to ice recrystallization (development of large ice crystals) during storage (Wildmoser and Windhab, 2001).
The design of the extruder must be such that the impact of the process on the dispersed air and fat phases is either minimized or accounted for by either formulation changes or changes in operation of the scraped-surface freezer, or both. For example, Bolliger et al. (2000) showed that-low temperature extrusion generally enhanced fat destabilization, although the additional mechanical shearing minimized the size of the resulting fat agglomerates. As a result, reduced emulsifier levels were required in the mix to achieve desirable structural and textural characteristics. Air bubble sizes were also smaller with low-temperature extrusion, as a result of further comminution of the air by mechanical shearing. Furthermore, a combination of reduced ice crystal size, reduced air bubble size, and controlled fat destabilization led to greatly enhanced smoothness in the ice cream.
In addition to these quality characteristic advantages, low-temperature extrusion greatly reduces and may even eliminate the need for specific static hardening processes. However, the design challenges of such extrusion processing are now aimed at the post-process handling of the product, i.e., particulate addition and packaging, both of which are rendered more difficult by extreme viscosity. While development work continues along these lines, current applications of low-temperature extrusion center around novelty product manufacture, where advantages are more obvious.
Although much of the focus in North America is on packaged ice cream, novelty or impulse products are exciting and and are attracting consumer attention. In many European countries, novelty products dominate the ice cream market. Sophisticated and ingenious processing technologies for manufacture of hand-held impulse products are now available. High-quality production of 3-dimensional molded products with high-definition details is possible with new molding processes. Extruded or post-molded products can be shaped with deep-cooled tools (Larsen, 2001). Advances in hardening systems for novelty and packaged ice cream products have focused on improving rates of heat transfer for fast freezing, product quality, energy efficiency, and cleanability (Marshall et al., 2003). Spiral tunnels can be shut down, defrosted, cleaned, and prepared again for operation in minimal time.
What’s Ahead
Developments in sweeteners, fat replacers, and bulking agents have provided many options for formulating new products with little or no fat, sugar, or lactose. Technological advances in the modification and concentration of milk and milk products are providing new functional ingredients for frozen desserts. As selected genetic traits of milk-producing animals are manipulated using biotechnological tools, we can expect additional opportunities to formulate frozen desserts advantageously, especially to modify their fatty acid profiles.
Some of the processing advances to watch for in the future include high-pressure homogenization for pre-flocculation and fat structuring, pre-aeration for controlled foaming, and low-temperature extrusion processing for dynamic, low-shear freezing. With a decoupling of these processes, ice cream manufacture could occur without continuous freezers, allowing for the development of a whole host of “outside-the-box” products that have previously not been possible.
--- PAGE BREAK ---
Ice Cream Short Courses and Seminars
May 14–16, 2003
Second International Symposium on Ice Cream, “A Global Endoscopy into the Future of Ice Cream,” Thessaloniki, Greece. International Dairy Federation. For information, visit www.aua.gr/ndcg.
November 12–14, 2003
Tharp & Young On Ice Cream–East Short Course, Airport Marriott Hotel, Orlando, Fla. For information, visit www.onicecream.com/3day_east.html.
November 25–27, 2003
Inter-Ice, European Ice Cream Conference, ZDS, Central College of the German Confectionery Industry, Solingen, Germany. For information, visit www.zds-solingen.de/zds-seminars/jpe2003.htm.
December 3–5, 2003
Tharp & Young On Ice Cream–West Short Course, Embassy Suites, Las Vegas, Nev. For information, visit www.onicecream.com/3day_west.html.
December 4–6, 2003
Premium Ice Cream Project Course, University of Wisconsin, Madison. For more information, visit www.wisc.edu/foodsci or call 608-263-2008.
December 8–12, 2003
Ice Cream Technology Course, Dept. of Food Science, University of Guelph, Guelph, Ontario, Canada. For information, visit www.foodsci.uoguelph.ca/dairyedu/iccourse.html or call 519-824-4120x54737.
January 5–15, 2004
Ice Cream Short Course, The Pennsylvania State University, University Park. For information, visit http://conferences.cas.psu.edu/IceCream/icsc.html or call 814-863-2959.
January 2004
Frozen Dairy Desserts Manufacturing Short Course, Dairy Products Technology Center, California Polytechnic State University, San Luis Obispo, cosponsored by the University of California, Davis. For information, call 805-756-6097.
by Robert T. Marshall and Douglas Goff
Author Marshall, an Emeritus Professional Member of IFT, is Professor Emeritus, Dept. of Food Science, University of Missouri, Columbia, MO 65211. Author Goff, a Professional Member of IFT, is Professor of Food Science, University of Guelph, Guelph, Ontario, N1G 2W1, Canada. They, along with Richard Hartel of the University of Wisconsin-Madison, edited the forthcoming 6th edition of W.S. Arbuckle’s text, Ice Cream. Send reprint requests for this article to author Marshall.
References
Anonymous. 2002. Neotame. A scientific overview. NutraSweet Co., Chicago, Ill.
Bolliger, S., Kornbrust, B., Goff, H.D. Tharp, B.W., and Windhab, E.J. 2000. Influence of emulsifiers on ice cream produced by conventional freezing and low temperature extrusion processing. Intl. Dairy J. 10: 497-504.
Budiaman, E.R. and Fennema, O.R. 1987a. Linear rate of water crystallization as influenced by temperature of hydrocolloid suspensions. J. Dairy Sci. 70: 534-546.
Budiaman, E. R. and Fennema, O.R. 1987b. Linear rate of water crystallization as influenced by viscosity of hydrocolloid suspensions. J. Dairy Sci. 70: 547-554.
Caldwell, K.B., Goff, H.D., and Stanley, D.W. 1992. A low temperature scanning electron microscopy study of ice cream. II. Influence of selected ingredients and processes. Food Structure 11: 11-23.
Dalzell, J.M. 1996. “Ingredients Handbook: Sweeteners.” Leatherhead Food RA, Leatherhead, Surrey, UK.
Donhowe, D.P. and Hartel, R.W. 1996a. Recrystallization of ice in ice cream during controlled accelerated storage. Intl. Dairy J. 6: 1191-1208.
Donhowe, D.P. and Hartel, R.W. 1996b. Recrystallization of ice during bulk storage of ice cream. Intl. Dairy J. 6: 1209-1221.
Dubey, U.K. and White, C.H. 1997. Ice cream shrinkage. J. Dairy Sci. 80: 3439-3444.
Flores, A. A. and H. D. Goff. 1999a. Ice crystal size distributions in dynamically frozen model solutions and ice cream as affected by stabilizers. J. Dairy Sci. 82:1399-1407.
Flores, A. A. and H. D. Goff. 1999b. Recrystallization in ice cream after constant and cycling temperature storage conditions as affected by stabilizers. J. Dairy Sci. 82:1408-1415.
Goff, H.D. 1997. Colloidal aspects of ice cream—A review. Intl. Dairy J. 7: 363-373.
Goff, H.D. and Jordan, W.D. 1989. Action of emulsifiers in promoting fat destabilization during the manufacture of ice cream. J. Dairy Sci. 72: 18-29
Goff, H.D., Kinsella, J.E., and Jordan, W.K. 1989. Influence of various milk protein isolates on ice cream emulsion stability. J. Dairy Sci 72: 385-397.
Goff, H.D., Caldwell, K.B., Stanley, D.W., and Maurice, T.J. 1993. The influence of polysaccharides on the glass transition in frozen sucrose solution and ice cream. J. Dairy Sci. 76: 1268-1277.
Goff, H.D., Ferdinando, D., and Schorsch, C. 1999. Fluorescence microscopy to study galactomannan structure in frozen sucrose and milk protein solutions. Food Hydrocoll. 13: 353-364.
Hartel, R.W. 1996. Ice crystallization during the manufacture of ice cream. Trends Food Sci. Technol. 7: 315.
Jensen, R.G. 2002. The composition of bovine milk lipids: January 1996 to December 2000. Invited review. J. Dairy Sci. 85: 295-350.
Kokubo, S., Sakurai, K., Hakamata, K., Tomita, M., and Yoshida, S. 1996. The effect of manufacturing conditions on the de-emulsification of fat globules in ice cream. Milchwissenschaft 51: 262-265.
Kokubo, S., Sakurai, K., Iwaki, S., Tomita, M., and Yoshida, S. 1998. Agglomeration of fat globules during the freezing process of ice cream manufacturing. Milchwissenschaft 53: 206-209.
Krog, N. 1988. The use of emulsifiers in ice cream. In “Ice Cream,” ed. W. Buchheim. Intl. Dairy Fed., Brussels, Special Issue 9803, pp. 37-44.
Larsen, P. 2001. Getting into shape—With a good definition. Presented at Inter-Ice 2001, Solingen, Germany.
Marshall, R.T., Goff, H.D., and Hartel, R.W. 2003. “Ice Cream,” 6th ed. Kluwer Academic/Plenum Publishers, New York. (in press).
Muhr, A. H., J. M. Blanshard, and S. J. Sheard. 1986. Effect of polysaccharide stabilizers on the nucleation of ice. J. Food Technol. 21: 587-603.
Nabors, L.O. 2001. “Alternative Sweeteners,” 3rd ed., Marcel Dekker, Inc., New York. Nabors, L.O. 2002. Sweet choices: Sugar replacements for foods and beverages. Food Technol. 56(7): 28-30, 32, 34, 45.
Patmore, J.V., Goff, H.D., and Fernandes, S. 2003. Cryo-gelation of galactomannans in ice cream model systems. Food Hydrocoll. 17: 161-169.
Prakash, I., Corliss, G., Ponakala, R., and Ishikawa, G. 2002. Neotame: The next-generation sweetener. Food Technol. 56(7): 36-40, 45.
Regand, A. and Goff, H.D. 2002. Effect of biopolymers on structure and ice recrystallization in dynamically-frozen ice cream model sytems. J. Dairy Sci. 85: 2722-2732.
Regand, A. and Goff, H.D. 2003. Structure and ice recrystallization in frozen stabilized ice cream model systems. Food Hydrocoll. 17: 95-102.
Russell, A .B., Cheney, P.E., and Wantling, S.D. 1999. Influence of freezing conditions on ice crystallization in ice cream. J. Food Eng. 39: 179-191.
Sahagian, M.E. and Goff, H.D. 1995. Thermal, mechanical and molecular relaxation properties of stabilized sucrose solutions at sub-zero temperatures. Food Res. Intl. 28: 1-8.
Sakurai, K., Kokubo, S., Hakamata, K. Tomita, M., and Yoshida, S. 1996. Effect of production conditions on ice cream melting resistance and hardness. Milchwissenschaft 51: 451-454.
Schmidt, K., Lundy, A., Reynolds, J., and Yee, L.N. 1993. Carbohydrate or protein based fat mimicker effects on ice milk properties. J. Food Sci. 58: 761-763, 779.
Schorsch, C., Jones, M. G., and Norton, I. T. 2000. Phase behavior of pure micellar casein/-carrageenan systems in milk ultrafiltrate. Food Hydrocolloids. 13:89-99.
Segall, K.I. and Goff, H.D. 1999. Influence of adsorbed milk protein type and surface concentration on the quiescent and shear stability of butteroil emulsions. Intl. Dairy J. 9: 683-691.
Sutton, R.L. and Wilcox, J. 1998a. Recrystallization in model ice cream solutions as affected by stabilizer concentration. J. Food Sci. 63: 9-11.
Sutton, R.L. and Wilcox, J. 1998b. Recrystallization in ice cream as affected by stabilizers. J. Food Sci. 63: 104-197.
Turan, S., Kirkland, M., Trusty, P.A., and Campbell, I. 1999. Interaction of fat and air in ice cream. Dairy Ind. Intl. 64: 27-31.
Wildmoser, H. and Windhab, E.J. 2001. Impact of flow geometry and processing parameters in ultra low temperature ice-cream extrusion (ULTICE) on ice-cream microstructure. Eur. Dairy Mag. 13(10): 26-32.
