Decreasing Trans and Saturated Fatty Acid Content in Food Oils
The edible oil industry has various processing and reformulation strategies available to decrease the trans and saturated fatty acid contents of food oils to increase their health value.
On July 11, 2003, the Food and Drug Administration published final rules mandating trans acid content to be included on food labels by January 1, 2006, in accordance with the Nutrition Labeling Act of 2003. This ruling will have a profound effect on the food and edible oil industries. This article provides some background and describes some strategies that will permit the food industry to comply with the new regulations.

Trans
Fats
Fats and oils are triglycerides containing both saturated and unsaturated fatty acids. Typically, common vegetable oils, including soybean, corn, cottonseed, sunflower, peanut, and olive, are relatively low in saturated acids, and the double bonds within unsaturated acids are in the cis configuration. To improve their oxidative stability and to increase their melting points, vegetable oils sometimes require hydrogenation. This is particularly true for margarines, spreads, shortenings, and frying fats. During catalytic hydrogenation, some of the double bonds are isomerized from the naturally occurring cis form to the trans form. Trans acids may also be formed in the rumen of animals. Butter, milk, beef tallow, and lard contain small amounts (2–5%) of trans acids.
The roles of saturated and trans acids in human health and nutrition are highly controversial issues. Nevertheless, in 1990, Congress passed the Nutrition Labeling and Education Act, and by 1994, labeling guidelines had been published. Of particular interest are statements regarding fat. Listing of “Total fat” must be in grams and in bold print. Listing of “Saturated fat” must be in grams, but is not required if the food contains less than 0.5% of total fat per serving and if no claims are made about fat and cholesterol content. If not required and not declared, the statement, “Not a significant source of saturated fat” must be included at the bottom of the nutrition label. Listing of “Polyunsaturated” in grams is voluntary, unless “Monounsaturated fat” or “Monounsaturated” is declared or a cholesterol or saturated fat claim is made and the total fat declared is greater than zero. Listing of “Cholesterol” must be in milligrams and in bold print, but is not required if the product contains less than 2 mg of cholesterol per serving and makes no claim about fat, saturated fat, or cholesterol; and, if not declared, the statement, “Not a significant source of cholesterol” must be included at the bottom of the nutrition label.
Several groups petitioned the Food and Drug Administration to include trans acids as part of the nutrition label, and proposed rules issued in 1999 mandated both trans labeling and statements to the effect that trans acid consumption should be kept to a minimum. After opening the rules for public comment several times, final regulations were issued on July 11, 2003 (FDA, 2003). By January 1, 2006, trans content will be required as a separate line on labels, but the aforementioned statement regarding consumption was eliminated.
Soybean and Palm Oils
An edible oil quality triangle has been described as having oxidative stability, functionality, and nutrition as the three legs (Lui, 1999). The ideal fat or oil should have excellent oxidative stability at high and ambient temperatures, contain enough solid fat for use as margarines, shortenings, and frying fats, and be both low in saturated fatty acid, and high in polyunsaturated acids, thus satisfying the requirements of the triangle. However, no single fat or oil fully satisfies these requirements.
Worldwide consumption of edible fats and oils in 2001–02 amounted to just over 92 million metric tons, of which 59% can be accounted for by soybean oil (29 million metric tons) and palm oil (25.4 million metric tons). While soybean oil is relatively low in saturated acids (15%), it represents a major source of trans fatty acids in our food supply because it must be hydrogenated to achieve functional properties and oxidative stability for use in salads/cooking, shortenings, and margarines/spreads. On the other hand, palm oil, while containing no trans acids, contains about 50% saturated acids, making it attractive for use in the these products, particularly when modified by interesterification and/or fractionation.
--- PAGE BREAK ---
Thus, just two fats and oils dominate and dictate oil processing worldwide, and any discussion of strategies to reduce trans and saturated acids in the food supply must focus on soybean and palm oils.
To improve the quality triangle for functionality and oxidative stability, various fat-modification techniques—hydrogenation, interesterification, fractionation, and combinations—are employed by the industry. A decade ago, it was estimated that about one-third of the edible oils produced worldwide were hydrogenated, while 10% were processed by interesterification/fractionation (Haumann, 1994). While more recent data are lacking, it is anticipated that the latter techniques will see increased usage in the future.
Over the past several decades, a number of oilseeds have been introduced with modified fatty acid composition. These include canola and soybean oils with low linolenic acid content; corn, soybean, and sunflower oils with high oleic acid content; and soybean oils with high saturated acids and low saturates (Lui, 1999; Gunstone, 2001; Gupta, 1998, 2001). Many of these oils show promise in reducing trans and/or saturated acids in food oils because the high-oleic oils are much more oxidatively stable and do not require hydrogenation, while the high-saturated oils are trans free.
Improvements in oil processing technology also provide options for trans and saturated acid reduction. Traditional random interesterification, in which fatty acids are redistributed across the glycerol backbone, is usually accomplished with a chemical catalyst and is thus nonspecific. Numerous advances have been made by use of enzymes which are stereo-specific, allowing production of tailor-made fats (MaCrae, 1983). Indeed, shortening and confectionery fats are now produced commercially by this technology. The time-honored hydrogenation process offers potential for reducing trans acids in food oils (Hasman, 1995; King et al., 2001; Warner et al., 2000).
Trans
and Saturated Acid Contents
Nearly 18 billion lb of soybean oil are consumed domestically per year, as follows: shortening 8.57 billion lb; margarine/spreads 1.24 billion lb; salad/cooking oil 7.9 billion lb; and other edible products 125 million lb (Soyatech, 2003). The shortening and margarine categories contain hydrogenated components. Based on the assumption that soft and stick margarines/spreads contain an average of 15% trans acids and shortenings contain an average of 20% trans acids, about 1.9 billion lb of trans acids are produced each year by hydrogenation.
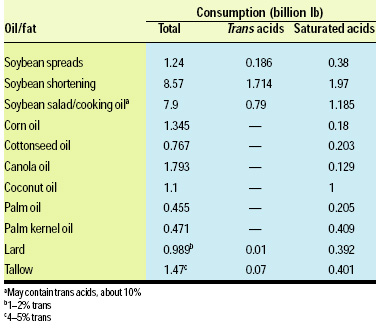
During 2000–01, corn oil consumption amounted to 1.41 billion lb, canola oil 1.75 billion lb, and cottonseed oil 767 million lb. No data are available on the amounts of these oils going into shortenings, margarines, and salad cooking oils.
Significant amounts of edible food and oils high in saturated acids are consumed per year in the U.S., including coconut oil 1.1 billion lb, lard 989 million lb, palm and palm kernel oils 826 million lb, and edible tallow 1.47 billion lb—a total consumption of 2.48 billion lb of saturated acids.
Thus, the U.S. consumption of trans and saturated acids is about equal—1.9 vs 2.48 billion lb, respectively. The value for trans consumption is low because of lack of data for the amounts of corn, cottonseed, and canola oil processed by hydrogenation (Table 1).
--- PAGE BREAK ---
A survey of the trans content of soft tub margarine/spreads in the U.S. was reported by List et al. (2000). The samples were premium products that were taken from grocery store shelves and, according to their label, were formulated from hydrogenated and liquid soybean oil. From 1992 to 1999, the overall trans reduction ranged from about 25% to 82%, with an average reduction of nearly 56%. The 1992 data indicate an average of nearly 20% trans in soft tub products, whereas the 1999 data show less than 9% trans. Stick/spreadable products have shown decreased trans acid contents over the past decade—trans acids averaged 26.8% in 1989, but by 1999 the average had dropped to 16.9%, a 37% overall reduction (Postmus et al., 1989; List et al., 2000).
Thus, the domestic edible oil industry has made concerted efforts to reduce trans acids in edible products. This has been accomplished through reformulation methods in which a multiple basestock system employing three hydrogenated oils has been replaced with a single hydrogenated component. Typically, soybean oil, hydrogenated to an iodine value (IV)—a measure of degree of unsaturation—of about 65 (40% trans), is blended with 25–50% liquid soybean oil to yield components suitable for a wide variety of spreads, including soft tub, spreadable stick, and stick products.
The trans acid content of margarines and spreads taken from the literature over a seven-year period (1995–2002) show some interesting trends in oil processing (Alonzo et al., 2000; Oveson et al., 1998; Ratnayke et al., 1998; Tsanev et al., 1998; Tekin et al., 2002; Bayard and Wolff, 1995). Low-trans products are arbitrarily defined as those having 5% or less trans acids, while zero-trans products may have smaller amounts (1–2%). Of the 228 samples reported, 87(38.2%) may be considered zero- or low-trans. The largest number of samples reported include those from Canada and the U.S., where 10 of the 126 were zero- or low-trans.
These results indicate that margarines/spreads formulated in North America are formulated primarily from hydrogenated components rather than by interesterification. The products produced in Denmark are formulated from interesterified oil components and represent the only country surveyed where hydrogenation has been replaced entirely. However, if the 59 samples from Denmark are excluded, 28 of 169 (16.6%) were low- or zero-trans. Thus, it would appear that hydrogenation remains the technology of choice to formulate margarine/spread products throughout most of the world.
Matsuzaki et al. (2002) analyzed margarines marketed in 11 countries, including Europe, Scandinavia, and the U.S., for their trans fatty acid and other compositional data. Their data confirm that any decrease in trans fatty acids is achieved at the expense of increased saturated acids content. Margarines produced in the U.S. had the lowest trans/saturated acid content (41.4%) and the highest ratio of polyunsaturated fatty acids/trans fatty acids plus saturated acids compared to the rest of the world. The same trend was observed for soft tub margarines. 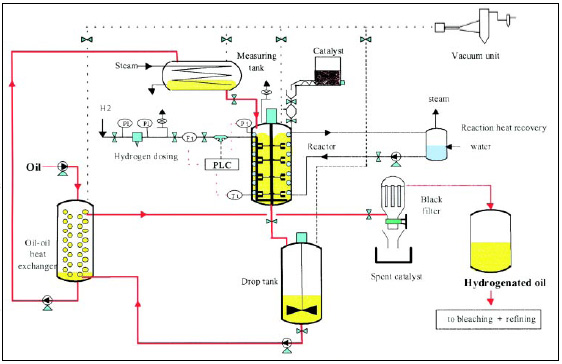
Hydrogenation of Soy-Based Oils
Fig. 1 is a flow diagram of the hydrogenation process. Temperature, pressure, agitation, and catalyst concentration are the most important factors governing the course and speed of hydrogenation. However, with other factors being equal, temperature has the largest effect on trans acid formation. Industrially, hydrogenation is carried out under selective conditions favoring reduction of polyunsaturated groups over that of monoenic acids.
--- PAGE BREAK ---
Typically, selective conditions involve high temperature (160–220°C) and low hydrogen pressure (10–40 psi) in the presence of 0.02–0.04% nickel metal catalyst supplied as 25% nickel on a support. Although selective conditions promote trans fatty acid formation, it is imperative that the amount of stearic acid formed at lower IVs be kept at a minimum, since any tristearin formed in the reaction will unduly raise the melting point of the finished oil such that the sharply melting “trans” functionality is compromised.
Partially hydrogenated winterized soybean salad/cooking oil (PHWSBO) has been produced commercially in the U.S. since the early 1960s. It is typically produced by hydrogenation under selective conditions (160–170°C, low hydrogen pressure, and 0.02% nickel) where the IV is reduced from approximately 130–132 to 110–115. The feedstock is then chilled slowly, allowing the higher-melting triglycerides to crystallize. The liquid oil is then recovered by filtration. The higher-melting fraction, stearine, may be incorporated into margarine/spread or shortening products.
The composition and properties of PHWSBO are given in Table 2, along with hydrogenation conditions used in its manufacture. The product with an IV of 110–112 will remain clear at refrigerator temperatures and is superior to unhydrogenated oil for light frying in the home.
Further discussion of the hydrogenation process can be found in reviews by Hastert (1996), Patterson (1994), and Erickson and Erickson (1995).
Margarine Basestock and Formulation of Spreads
Margarine oil basestocks—hydrogenated components that, when blended with liquid oil, produce desired functional properties—require “selective conditions” in which stearic acid formation needs to be controlled very carefully. Typically, low pressure and high temperature are used. The preparation of multi-purpose margarine basestocks involves reducing the IV of soybean oil from about 130 to 65–70 in the presence of a nickel catalyst at about 220°C and low hydrogen pressure.
Margarine oils typically show the following solid fat content (SFC): tub products 8–14 at 10°C, 4–8 at 21.1°C, and 1–2 at 33.3°C; stick products 20–25 at 10°C, 10–12 at 21.1°C, and 2–4 at 33.3°C. The hydrogenated soybean oil, when blended with 25–50% liquid oil, results in SFCs suitable for tub and stick products, respectively. The melting point of the blended oils will usually be 32–35°C. The trans isomer content of the basestock is about 40%, which, after blending, yields final trans values for tub and stick products of 10 and 20%, respectively. Trans-containing triglycerides are very sharply melting materials at low temperatures. They provide functionality for spreadability and resistance to oil-off at room temperatures, yet they melt very quickly at body temperature to yield a pleasant, cooling sensation in the mouth.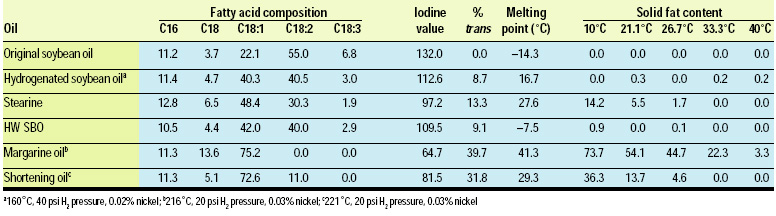
Shortening Basestock and Formulation of Shortening
Shortening basestocks are typically prepared by hydrogenation of soybean oil to an IV of about 80. Lower temperatures and higher pressures are used than for margarine basestocks.
To formulate shortenings, liquid oil is blended to decrease the SFC, and addition of completely hydrogenated hardstock (10–12%) raises the 33.3°C and 40°C SFC values.
The baking industry employs a number of shortenings. Their trans acid content varies from about 12% to nearly 25%. Reduction in the trans acid content of shortenings is possible by alternative processing techniques such as interesterification. However, no- and low-trans shortenings, while equivalent in performance to hydrogenated products, may be more expensive and will have elevated saturated acid contents.
--- PAGE BREAK ---
High-Stability Oils
High-stability oils (HSOs) were developed more than 30 years ago (Gooding, 1972). Compared to commodity oils (soybean, corn, cottonseed, canola, peanut), they are expensive, but they are extremely stable and fill definite needs for the food industry. They are liquids at ambient temperature and perform well as spray oils and in products with large surface areas or where long shelf life is required.
HSOs are at least four times more resistant to oxidation and hydrolysis than commodity salad oils; this translates into slower development of off-flavors and marked improvement in color stability. Typical applications include use as carriers of flavors, moisture barriers, viscosity modifiers, gloss enhancers, lubricating/releasing agents, and anti-dusting agents, as well as use in roasting of nuts and frying operations.
Use in 2000 was estimated to be 45,000–57,000 metric tons—70% low-end HSO, 10% mid-range, and 20% high-end. These designations refer to their relative stability under active oxygen method conditions (100°C) or hours to reach a peroxide value of 100. Low, mid-range, and high-end oils have peroxide values of 50–100, 100–300, and >300, respectively (Lampert, 2000).
HSOs can be processed from both commodity oils and oils that have been modified genetically or by plant breeding. High-oleic corn, soybean, and sunflower oils meet requirements for low-end HSO without processing beyond refining, bleaching, and deodorizing, whereas soybean oil requires partial hydrogenation and dry fractionation as well. Both medium- and high-end HSOs can be prepared from both commodity oils and oils modified by either genetic engineering or plant breeding. However, light, partial or heavy hydrogenation and dry fractionation must be employed along with the usual refining, bleaching, and deodorization steps.
Low-end HSOs prepared from hydrogenated, dry-fractionated soybean oil contain 15% saturates and 32% trans, whereas high-oleic sunflower oil contains 9.5% saturates and 1% trans. HSOs based on canola and high-oleic canola oils offer opportunity for trans reduction in mid-range applications. Canola HSO shows 18–29% trans, compared to 51% for soy-based oil. Similarly, high-oleic-based, high-end oils have 33% trans compared to 48% for cottonseed- or soybean-based oils (Lampert, 2000).
HSOs are liquids at ambient temperature and are highly functional and convenient to use. They are used at low levels, often 0.2 and 1%, and are most commonly sprayed onto the surface of the food or ingredient. Although more expensive than commodity oils, processing costs are reduced, making final pricing comparable.
It is expected that increased use of HSOs will occur in the future to reduce the fat content in foods, improve shelf life, and improve health and nutrition.
Canola Oil
Hydrogenation of canola oil has been studied extensively in the laboratory (see review by Koseoglu and Lusas, 1990). Although canola oil is widely used in margarines/spreads and shortenings, the trans isomer content of these products tends to be somewhat higher than soybean-based products (Postmus et al., 1989).
Compared to soybean oil, hydrogenation of canola oil results in more trans acids at a given IV level. For example, canola oil (IV 113), when hydrogenated to IV 80 for shortening stock, contains about 45% trans acids, compared to 31–32% for soybean oil at the same IV. Canola margarine stock (IV 75) contains 50% trans, compared to 40% for soybean oil at IV 65. Lightly hydrogenated canola (IV 90) used as a frying fat contains 20% trans, whereas soybean oil at IV 110–116 contains about 10–12% trans (Eskin et al., 1996).
--- PAGE BREAK ---
These phenomena can be accounted for by the differences in fatty acid composition of the two oils. Canola oil contains 60% oleic acid and about 32% polyunsaturated acids (22% linoleic acid and 10% linolenic acid), whereas soybean oil contains about 22% oleic acid and 63% polyunsaturated acids. Thus, the high content of oleic acid renders canola oil more amenable to isomerization/trans fatty acid formation during hydrogenation; also sulfur-containing glucosinulates found in canola oil tend to poison the catalyst, thus promoting formation of trans acids. Canola oil tends to crystallize in the undesirable beta (highest-melting) form because of the low levels of palmitic acid found in the oil. To improve the crystal habit, the addition of palm oil prior to hydrogenation reportedly stabilizes the oil in the desirable beta-prime form desired for margarines and shortenings (Eskin et al., 1996).
Cottonseed Oil
Cottonseed oil has an IV of about 110 and contains about 25% saturated acids. A basestock system for formulation of shortenings, margarines, and spreads consists of five hydrogenated oils along with a hardstock or completely hydrogenated component (IV>5). Oils hydrogenated to an IV of 75–80 under non-selective conditions (350°F, 30–45 psi, 0.02% nickel catalyst) possess flat solid fat index (SFI) curves suitable for shortenings, whereas the IV 58, 65, and 70 oils hydrogenated under selective conditions (440°F, 11–15 psi, 0.04–0.08% nickel catalyst) possess steep SFI curves needed for margarine/spreads. The IV 58 oils have SFIs and melting properties very similar to those of IV 65 soybean oil and are similar in trans acid content. In 1997, only 8 million lb of cottonseed oil was used in margarines/spreads in the U.S.
O’Brien and Wan (2001) provide an excellent review of food uses and processing of cottonseed oil.
Corn Oil
During 1955–85, corn oil usage in margarines/spreads increased from less than 500,000 lb to 210 million lb, but dropped to about 61 million lb by 1997. Corn oil contains about 13% saturated acids and has an IV of approximately 127. Soft and stick margarines formulated from blends of hydrogenated soybean oil and liquid corn oil are available commercially.
Soybean oil basestocks are produced by hydrogenation of the oil to IVs of 65–67 under selective conditions and contain about 48% trans acids. The IV 65 oil blended with 25–30% liquid corn oil is suitable for soft products, while the IV 67 oil blended with 45–47% liquid corn oil is suitable for stick products. The trans acid content of soft and stick products is 12–14% and 20–24%, respectively.
Strategies to Reduce trans Acids
There are a number of strategies to reduce the trans fatty acid content of oils:
• Interesterification. The patent literature is replete with information on interesterification as a route to low-trans fats and oils. Gillies (1974) covered the patent literature from 1960 to 1974. A further search of the patent literature using margarine/shortening and interesterification as key words yields more than 46,000 references since 1976 alone. Thus, a comprehensive review would not be practical. Instead, several examples will be given from the author’s laboratory.
Random interesterification of 80% liquid soybean oil with 20% completely hydrogenated soybean oil provides a route to soft margarine oil having suitable solid fat and crystal structures (List et al., 1977). Further work in which the interesterified oils were formulated into soft margarines in the pilot plant demonstrated that the 80:20 blend produced margarine that was harder and more difficult to spread than hydrogenated controls. Adding 20% liquid oil resulted in a product with suitable spreadability, sensory properties, and resistance to oil/water loss (List et al., 1995a).
--- PAGE BREAK ---
Random interesterification of other liquid oils, including corn, peanut, cottonseed, canola, and palm, with completely hydrogenated soybean or cottonseed flakes yields basestocks suitable for formulation of zero-trans margarines and shortenings (List et al., 1995b).
Shortening oils require higher, flatter SFI curves and higher melting points than margarine oils. To obtain these properties, it is necessary to incorporate more stearine into the interesterified blend. The desired SFIs and melting points are achieved by blending the interesterified basestock with additional liquid oil.
Typically, all-purpose shortening prepared from hydrogenated components show SFI values of 18–23, 14–19, 13–14, and 7–11 at 50, 70, 92, and 104°F, respectively. A 50:50 blend of interesterified and liquid soybean oil closely matches these values. Fluid shortenings can be prepared by blending 35% of the basestock with 65% soybean, corn, peanut, cottonseed, or canola oil.
• Fractionation. Rapid growth of the palm industry, beginning in the mid-1970s, prompted development of improved fractionation technology. Historically, solvent and detergent processes had been used, but today physical or dry fractionation is the standard industry method.
Palm oil (IV 51–53) is fractionated into olein (IV 56–59) and stearine (IV 32–36) fractions. The olein fraction is further fractionated into mid-fractions, super oleins, and top oleins. The palm mid-fractions (IV 42–48) are further processed into harder fractions (IV 32–36). Fractionation of the stearine (IV 32–36) yields soft and super stearines (IV 40–42 and 17–21, respectively) (Tirtiaux, 1996). These fractions can be incorporated into margarines/shortenings and confectionery fats. However, replacing trans fat with highly saturated palm fractions will increase saturated acid content.
Further discussion of the fractionation process can be found in reviews by Krishnamurthy and Kellens (1996), Timms (1997), and Deffense (1985).
Palm Oil
Zero-trans margarine fats can be prepared via random interesterification of soybean oil with palm stearine or fully hydrogenated soybean oil in ratios of 80:20. Petrauskaite et al. (1998) concluded that increasing the amount of fully hydrogenated soybean oil to 30 parts produced zero-trans oils suitable for shortening; 40 parts palm stearine randomized with 60 parts soybean oil also produced shortening oil very similar to commercial products.
Ozay et al. (1998) described the formulation of trans-free margarines from sunflower and cottonseed oils interesterified with palm oil, palm kernel oil, palm stearine, and palm kernel olein and compared them to products made by simply blending the components in various ratios. Palm oil crystallizes slowly compared to other fats and oils, leading to a phenomenon known as post hardening in which products become harder during storage. The authors observed minimal post hardening in their study and reported that the use of skim milk in preparing the emulsions prior to crystallization was effective in retarding post hardening in blends high in palm oil and palm kernel oil.
Yusoff et al. (1998) reported formulation of trans-free tub, pastry, and bakery margarines from palm oil, palm olein, palm stearine, and palm kernel olein interesterified with soybean and rapeseed oil. The normal level of palm oil in commercial soft tub products was reported to be 25%, but palm olein can be used up to a level of 40%.
--- PAGE BREAK ---
Berger (1998) reported studies on palm oil usage in a variety of food products, including margarine, baking shortenings, frying fats, and dairy products such as whipped toppings, ice cream, cheese, and vanaspati, a butter-like product with a melting point of 37–38°C used extensively in India and Pakistan. Vanaspati is manufactured with a granular structure and a minimum of free oil at room temperatures. In the early 1980s, the product (1 million tons/year), produced in Pakistan from hydrogenated components, contained about 30% trans acids. After it was reformulated with 80% palm oil and 20% liquid vegetable oil, trans acids were reduced to less than 4%. However, the saturated acid content would be on the order of 40%.
Oils With Modified Composition
Over the past several decades, a number of oilseeds with modified fatty acid compositions have been developed and commercialized. Most have resulted from traditional plant breeding techniques (Lui, 1999; Gunstone, 2001; Loh, 2000; Wilson, 1999). These include high- and mid-level-oleic sunflower oil; high-oleic corn, soybean, and safflower oils; low-linolenic canola and soybeans oils; and high-oleic/low-linolenic canola oil.
A number of laboratory frying studies have demonstrated superiority of modified oils over commodity oils (Warner and Gupta, 2003; Warner and Knowlton, 1997; Warner et al., 1997; Mounts et al., 1994; Warner and Mounts, 1993, 1994; Su et al., 2003).
Although low-linolenic canola and soybeans oils have reached commercialization, costs and production problems have impeded their success in the marketplace. However, the new labeling regulations may provide new markets and demands for modified-composition oils.
Fluid Shortenings
Fluid shortenings are stable suspensions of 2–20% hard fat in liquid vegetable oil which may or not be hydrogenated (Herzing, 1996; Andre and Going, 1957; Holman and Quimby, 1950; Mitchell, 1950). Fluid shortenings have been used for many years in baked goods where high solids contents are not required, such as fillings, cakes, and breads. They serve the same function as solid shortenings by imparting tenderness and lubricity, as well as serving as carriers for emulsifiers needed for aerating cake batter or giving crumb strength to bread. Other advantages include the fact that, since they are liquid and pumpable, they can be easily metered into batch and continuous processes. Some reduction of trans acids might be achieved by substituting liquid or unhydrogenated oil in formulations where hydrogenated oils have been traditionally used.
The author, a Professional Member of IFT, is Lead Scientist, Food and Industrial Oil Research, National Center for Agricultural Utilization Research, Agricultural Research Service, U.S. Dept. of Agriculture, 1815 N. University St., Peoria, IL 61604.
References
Alonzo, L., Fraga, M.J., and Juarez, M. 2000. Determination of trans fatty acids in margarines marketed in Spain. J. Am. Oil Chem. Soc. 77: 131-136.
Andre, J. R. and Going, L.H. 1957. Liquid shortening. U.S. patent 2,815,286.
Bayard, C. and Wolff, R.L. 1995. trans 18:1 acids in French tub margarines and shortenings. J. Am. Oil Chem. Soc. 72: 1465-1489.
Berger, K. 1998. Recent results on palm oil uses in food products. In “Proceedings of World Conference on Oilseed and Edible Oil Processing,” Vol. 1, ed. S. Koseoglu, K. Rhee, and R. F. Wilste, pp. 151-155. AOCS Press, Am. Oil Chem. Soc., Champaign, Ill.
Deffense, E. 1985. Fractionation of palm oils. J. Am. Oil. Chem. Soc. 62: 376-385.
Erickson, D.R. and Erickson, M.D. 1995. Hydrogenation and base stock formulation. In “Practical Handbook of Soybean Processing and Utilization,” ed. D.R. Erickson, pp. 218-238. AOCS Press, Champaign, Ill.
Eskin, N.A.M., McDonald, B.E., Przybyski, R., Malcomson, L.J., Scarth, R., Mag, T., Ward, K., and Adolph, D. 1996. Canola oil. In “Bailey’s Industrial Oil and Fat Products,” 5th ed., ed. Y.H. Hui, pp. 1-96. John Wiley and Sons, New York.
FDA. 2003a. Food labeling: trans fatty acids in nutrition labeling. Food and Drug Admin., Fed. Reg. 68: 41433-41506.
Gillies, M.T. 1974. “Shortenings, Margarines and Food Oils,” pp. 142-226. Noyes Data Corp., Park Ridge, New Jersey.
Gooding, C.M. 1972. Production of high stability liquid vegetable oils. U.S. patent 3,674,821.
Gunstone, F.D. 2001. Oilseed crops with modified fatty acid composition. J. Oleo. Sci. 50: 269-279.
Gupta, M. 1998. NuSun—The future generation of oils. Inform 9: 1150-1154.
Gupta, M.K. 2001. NuSun: A healthy non-transgenic sunflower oil. In “Proceedings of World Conference on Oilseed and Edible Oil Processing,” ed. R.F. Wilson, pp. 80-83. AOCS Press, Am. Oil Chem. Soc., Champaign, Ill.
Hasman, J. 1995. trans suppression in hydrogenated oils. Inform 6: 1206-1213.
Hastert, R.C. 1996. Hydrogenation. In “Bailey’s Industrial Oil and Fat Products,” 5th ed., ed.Y.H. Hui, pp. 213-300. John Wiley and Sons, New York.
Haumann, B.F. 1994. Tools: Hydrogenation, interesterification. Inform 5: 668-678.
Herzing, A.C. 1996. Fluid shortenings in bakery products. Inform 7: 165-167.
Holman, G.W. and Quimby, O.T. 1950. Process of preparing suspensions of solid triglyceride and liquid oil. U.S. patent 2,521,219.
King, J.W., Holliday, R.L., List, G.R. and Snyder, J.M. 2001. Hydrogenation of soybean oil in supercritical carbon dioxide and hydrogen. J. Am. Oil Chem. Soc. 78: 107-113.
Koseoglu, S. and Lusas, E. 1990. Hydrogenation of canola oil. In “Canola and Rapeseed,” ed. F. Shahidi., pp. 123-148. AVI, New York.
Krishnamurthy, R. and Kellens, M. 1996. Fractionation and winterization. In “Bailey’s Industrial Oil and Fat Products,” 5th ed., ed.Y.H. Hui, pp. 301-337. John Wiley and Sons, New York.
Lampert, D. 2000. High stability oils: What are they? How are they made? and Why do we need them. In “Physical Properties of Fats, Oils and Emulsifiers,” ed. N. Widlak, pp. 238-246. AOCS Press, Am. Oil Chem. Soc., Champaign, Ill.
List, G.R., Pelloso, T., Orthoefer, F., Chrysam, M., and Mounts, T.L. 1995a. Preparation and properties of zero trans soybean oil margarines. J. Am. Oil Chem. Soc. 72: 383-384.
List, G.R., Mounts, T.L., Orthoefer, F., and Neff, W.E. 1995b. Margarine and shortening oils by interesterification of liquid and trisaturated triglycerides. J. Am. Oil Chem. Soc. 72: 379-382.
List, G.R., Emken, E.A., Kwolek, W.F., Simpson, T.D., and Dutton, H.J. 1977. “Zero trans” margarines: Preparation, structure, and properties of interesterified soybean oil-soy trisaturate blends. J. Am. Oil Chem. Soc. 54: 408-413.
List, G.R., Steidley, K.R., and Neff, W.E. 2000. Commercial spreads formulation, structure and properties. Inform 11: 980-986.
Loh, W. 2000. Biotechnology and vegetable oils: First generation products in the marketplace. In “Physical Properties of Fats, Oils and Emulsifiers,” ed. N. Widlak, pp. 247-253. AOCS Press, Am. Oil Chem. Soc., Champaign, Ill.
Lui, K. 1999. Soy oil modifications: Products and applications. Inform 10: 868-877.
MaCrae, A.R. 1983. Lipase catalyzed interesterifcation of oils and fats. J. Am.Oil Chem. Soc. 60: 233a-346a.
Matsuzaki, H., Okamoto, T., Oyama, M., Mavruyama, T., Niiya, I., Yanagita, T., and Sugano, M. 2002. trans fatty acids marketed in eleven countries. J. Oleo. Sci. 51: 551-565.
Mitchell, P.J. 1950. Permanently pumpable oleaginous suspensions. U.S. patent 2,521,242.
Mounts, T.L., Warner, K., List, G.R., Neff, W.E., and Wilson, R.F. 1994. Low linolenic acid soybean oil—Alternatives to frying oils. J. Am. Oil Chem. Soc. 71: 495-499.
O’Brien, R.D. and Wan, P. 2001. Cottonseed oil: Processing and utilization. In “Proceedings of World Conference on Oilseed and Edible Oil Processing,” ed. R.F. Wilson, pp. 90-140. AOCS Press, Am. Oil Chem. Soc., Champaign, Ill.
Oveson, L., Leth, T., and Hansen, K. 1998. Fatty acid composition and contents of trans monounsaturated fatty acids in frying fats and margarines and shortenings marketed in Denmark. J. Am. Oil Chem. Soc. 75: 1079-1083.
Ozay, G., Yildiz, M., Mahidin, M., Yusoff, M., Yurdagul, M., and Goken, N. 1998. Formulation of trans free fatty acid margines. In “Proceedings of World Conference on Oilseed and Edible Oil Processing,” Vol. 1, ed. S. Koseoglu, K. Rhee, and R.F. Wilste, pp. 143-146. AOCS Press, Am. Oil Chem. Soc., Champaign, Ill.
Patterson, H.B.W. 1994. “Hydrogenation of Fats and Oils; Theory and Practice.” AOCS Press, Am. Oil Chem. Soc., Champaign, Ill.
Petrauskaite, V.W., DeGreyt, A., M. Kellens, M., and Huyghaebaert, A. 1998. Physical and chemical properties of trans free fats produced by chemical interesterification of vegetable oil blends. J. Am. Oil Chem. Soc. 75: 489-493.
Postmus, E., Deman, L., and Deman, J.M. 1989. Composition and physical properties of North American stick margarines. Can. Inst. Food Sci. Technol. 22: 481-486.
Ratnayake, W.M.N., Pelletier, G., Hollywood, R., Bacler, S., and D. Leyte. 1998. trans fatty acids in Canadian margarines: Recent trends. J. Am. Oil Chem. Soc. 75: 1587-1594.
Soyatech. 2003b. Soya and oilseed bluebook, p. 357. Soyatech, Bar Harbor, Mich.
Su, C., Gupta, M., and White, P. 2003. Oxidative and flavor stabilities of soybean oils with low and ultra-low linolenic acid composition. J. Am. Oil Chem. Soc. 80: 171-176.
Tekin, A., Cizmeci, M., Karabacak, H., and Kayaban, M. 2002. trans fatty acids and solid contents of margarines marketed in Turkey. J. Am. Oil Chem. Soc. 79: 443-445.
Timms, R.E. 1997. Fractionation. In “Lipid Technologies and Applications,” ed. F. Gunstone, pp. 199-222. Marcel Dekker, New York.
Tirtiaux, A. 1998. Dry fractionation—The boost goes on. In “Proceedings of World Conference on Oilseed and Edible Oil Processing,” Vol. 1, ed. S. Koseoglu, K. Rhee, and R.F. Wilste, pp. 92-98. AOCS Press, Am. Oil Chem. Soc., Champaign, Ill.
Tsanev, R., Russeva, A., Rizov, T., and Dontcheva, I.N. 1998. Content of trans fatty acids in edible margarines. J. Am. Oil Chem. Soc. 75: 143-145.
Warner, K. and Gupta, M. 2003. Frying quality and stability of ultra low and low linolenic acid soybean oils. J. Am. Oil Chem. Soc. 80: 275-280.
Warner, K. and Knowlton, S. 1997. Frying oils and oxidative stability of high-oleic corn oils. J. Am. Oil Chem. Soc. 74: 1317-1322.
Warner, K. and Mounts, T.L. 1993. Frying stability of soybean and canola oils with modified fatty acid compositions. J. Am. Oil Chem. Soc. 70: 983-988.
Warner, K., Orr, P., and Glynn, P. 1997. Effect of fatty acid composition of oils on flavor and stability of fried foods. J. Am. Oil Chem. Soc. 74: 347-356.
Warner, K., Neff, W.E., List, G.R. and Pintauro, P. 2000. Electrochemical hydrogenation of edible oils in a solid polymer electrolyte reactor. Sensory and compositional characteristics of low trans oils. J. Am. Oil Chem. Soc. 77: 1113-1117.
Wilson, R.F. 1999. Alternatives to genetically modified soybeans—The better bean initiative. Lipid Technol. 11: 107-109.
Yusoff, M., Kifli, H., Noorlida, H., and Rozig, M.P. 1998. Formulation of trans-free margarines. In “Proceedings of World Conference on Oilseed and Edible Oil Processing,” Vol. 1, ed. S. Koseoglu, K. Rhee, and R.F. Wilste, pp. 156-158. AOCS Press, Am. Oil Chem. Soc., Champaign, Ill.
