Peanuts: Bioactive Food in a Shell
Research has identified numerous functional compounds in peanuts that promote health and may reduce the risk of cardiovascular disease, cancer, and diabetes.

Disease Prevention
Several epidemiological studies have shown that frequent peanut consumption reduced the risk of type 2 diabetes, cardiovascular disease (CVD), cancer, and weight gain. A daily intake of two tablespoons equivalent to 1 oz or 32 g of peanut butter or a handful (1 oz or 28 g) of peanuts and other nuts reduced the risk of type 2 diabetes in women by 21% (Nurses’ Health Study; Jiang et al., 2002), while as little as 0.4–3.1 g daily intake of peanuts by Chinese women reduced the risk of type 2 diabetes by 20% (Shanghai Women’s Health Study; Villegas et al., 2008).
Women with type 2 diabetes had a 44% risk reduction of CVD after daily consumption of at least 1 tablespoon (16 g) of peanut butter or 1 oz (28 g) of peanuts or other nuts (Nurses’ Health Study; Li et al., 2009). Healthy adults who regularly ate peanuts improved their indices of CVD risk, including lowered serum triacylglycerol (TAG) and increased serum magnesium, folate, alpha-tocopherol, copper, and arginine concentrations (Alper and Mattes, 2003). Similarly in Ghana, healthy adults who consumed 500 kcal of peanuts daily reduced their total cholesterol and TAG levels by 7.2% and 20%, respectively (Lokko et al., 2007).
An inverse relation between frequent peanut consumption and risk of colorectal cancer in Taiwanese women was observed with a remarkable 58% risk reduction (Yeh et al., 2006). Regular peanut intake improved the diet quality of consumers as evidenced by higher intake of vitamins A, E, and folate, calcium, magnesium, zinc, iron, and dietary fiber; and by lower intake of saturated fat and cholesterol, without increasing their body mass index despite higher energy intake over a two-day period (Griel et al., 2004). Furthermore, during a 30-wk study with daily peanut consumption, participants maintained their ratings for pleasantness or hunger for peanuts and did not prefer other snack foods with different tastes and fat contents (Alper and Mattes, 2002).
Phenolics and Antioxidants
A comparison of total phenolics and antioxidant capacities (AOC) of food, nutraceuticals, and dietary supplements is problematic because they are analyzed using different analytical methods. Among these methods, three assays were proposed for standardization at the First International Congress on Antioxidant Methods held in June 2004: (1) Folin-Ciocalteau, (2) oxygen radical absorbance capacity (ORAC), and (3) Trolox equivalent antioxidant capacity (TEAC) (Prior et al., 2005). All three methods are necessary for routine assessment of AOC, and together they measure the multiple reaction mechanisms of antioxidants toward various radical or oxidant sources in a mixed or complex system as food (Prior et al., 2005).
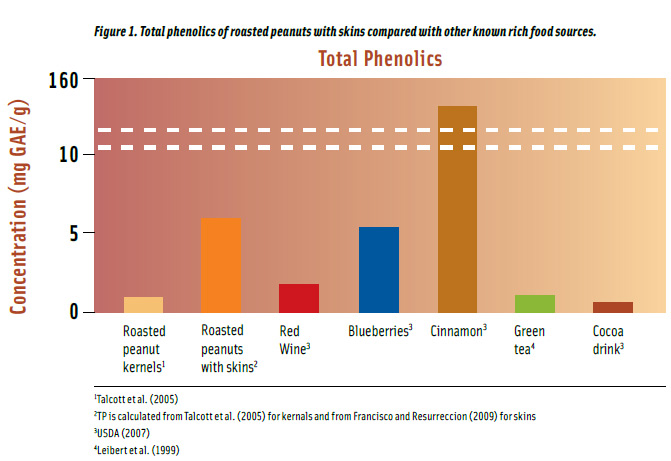
The Folin-Ciocalteau method uses an electron transfer mechanism to measure reducing capacity of samples and results are normally expressed as total phenolic contents (Prior et al., 2005). Roasted peanut kernels have about 1 mg/g total phenolics but the concentration increases six-fold when they are consumed with skins.
--- PAGE BREAK ---
Roasted peanuts have higher total phenolics compared to well-known food sources such as green tea, red wine, blueberries, and cocoa drink (Figure 1). Roasted peanuts with skins have 10% more phenolics, on a per gram basis, than blueberries and more than twice the phenolics in red wine. They also contain approximately 6 and 8 times more total phenolics than in green tea and cocoa drink, respectively.
Spices are the highest sources of total phenolics (USDA, 2007). For example, cinnamon has 26 times more phenolics than roasted peanuts with skins (Figure 1). However, the spice is consumed in such minute quantities to have a considerable dietary impact. On a per serving basis, a serving (24.8 g or 1 oz) of roasted peanuts with skins will provide 40% more total phenolics than a serving (0.76 g or 1/3 tsp) of cinnamon, and as much as a serving of cocoa drink (236 mL), about half a glass of red wine (147 mL), half a cup of green tea (236 mL), and about one fifth of a cup of blueberries (148 g).
Several chronic and degenerative diseases such as cancer, heart, Alzheimer’s and Parkinson’s disease, and aging are theorized to be caused by oxidative stress and reactive oxygen species, damaging biomolecules such as proteins, lipids, and DNA (Prior and Cao, 1999). While the human body has developed a number of systems to eliminate free radicals from the body, it is far from 100% efficient.
The two recommended methods for measuring AOC (Prior et al., 2005) are based on differing mechanisms for terminating free radical reactions. ORAC is a hydrogen atom transfer-based assay that measures quenching of peroxylfree radicals, whereas TEAC is a single electron transfer-based assay that quantifies the scavenging ability of antioxidants to the radical cation ABTS.+. Both methods measure hydrophilic and lipophilic antioxidants (Prior et al., 2005). AOC in ORAC and TEAC assays is expressed in micromoles of Trolox, a water-soluble vitamin E analog, or Trolox equivalents (TE).
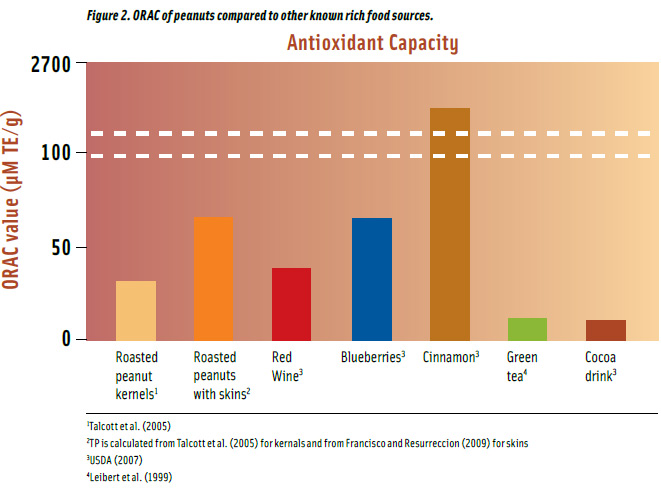
Peanuts have higher AOC compared to well-known foods such as green tea and red wine (Figure 2). Roasted peanuts have 32 μM TE/g ORAC on a per gram basis, and when consumed with skins, they have more than double the amount. Roasted peanuts with skins have the equivalent ORAC as blueberries and almost twice as much as in red wine. They also contain approximately 5 and 6 times more than in green tea and cocoa drink, respectively. Spices are the highest food sources of antioxidants with cinnamon having 40 times greater ORAC than peanuts with skins. A 1-oz serving of roasted peanuts with skins provides as much ORAC as a serving of cinnamon (1/3 tsp) and a cup of green tea (236 mL) but less than red wine (147 mL), blueberries (148 g,) and cacao drink (236 mL).
Bioactive Compounds
Since 1973, studies have found many phenolic bioactive compounds in peanuts which are classified into four major classes— stilbenes, flavonoids, phenolic acids, and phytosterols (Francisco and Resurreccion, 2008). Many scientists believe it is a combination of these plant polyphenols and not just a single component that provides the most stable source of health benefits.
Stilbenes are a class of phytoalexins or low molecular mass secondary metabolites produced by plants (Boue et al., 2009) in response to infection, injury, or stress (Soleas et al., 1997). The major stilbene found in peanuts is resveratrol, the most widely known bioactive compound, discovered in 1990 in red wine and associated with the so called “French paradox”—a phenomenon seen in France where mortality from coronary heart disease is relatively low (33% less) despite relatively high levels of dietary saturated fat, as compared to Western diets (Stanley and Mazier, 1999). This observation led to the conclusion that resveratrol present in red wine might provide some protection from coronary heart disease.
--- PAGE BREAK ---
Resveratrol is associated with reduced CVD, including atherosclerosis protection and inhibition of platelet aggregation (Kris-Etherton et al., 2002). Reseveratrol also has cancer-preventive (Jang et al., 1997), anti-estrogenic (Kris-Etherton et al., 2002), strong antioxidant, and anti-inflammatory (Chang et al., 2006) activities. The role of resveratrol in anti-aging or longevity (Baur et al., 2006) and therapeutic potential in Alzheimer’s disease was recently discovered (Marambaud et al., 2005).
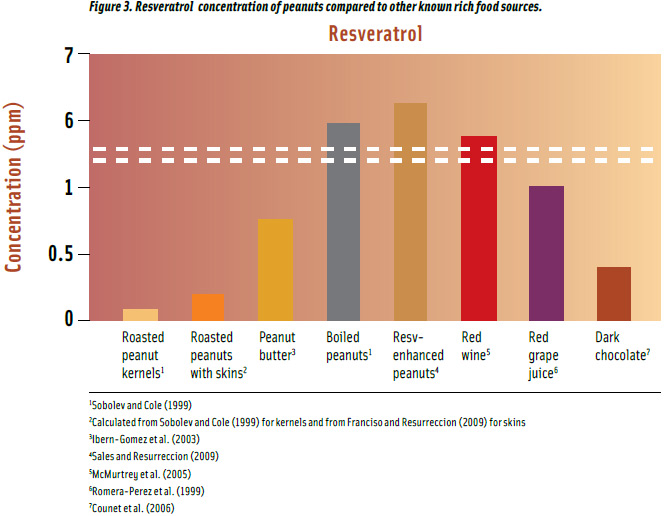
Roasted peanuts are one of the important food sources of resveratrol after red wine, red grape juice, and dark chocolate (Figure 3) with a maximum concentration of 0.08 ppm (Sobolev and Cole, 1999), and when consumed with skins, provide about 3 times more resveratrol. Peanut butter has 3.4 times more resveratrol than roasted peanuts with skins, and boiled peanuts may contain up to 23 times more. A patented method developed at the University of Georgia enhanced resveratrol even further to 29 times more than roasted peanuts with skins, up to 6.39 ppm using ultrasound (Sales and Resurreccion, 2009). Likewise, UV treatment increased resveratrol 11 times up to 2.36 ppm, similar to that of red wine, but not as effective as ultrasound (Potrebko and Resurreccion, 2009). Resveratrol in red wine, red grape juice, and dark chocolate falls within concentrations found in peanut products.
Flavonoids are secondary plant phenolics widely distributed in leaves, seeds, bark, and flowers. They play many different roles in the ecology of plants, such as natural pesticide, catalyst, visual attractor, and light screen. Some flavonoids are extremely potent odorants and have bitter and astringent flavors. Over 4,000 flavonoids have been identified and categorized into several classes, based on the differences of their structure, including flavonols, flavones, flavanols, flavanones, anthocyanidins, and isoflavonoids (Liu, 2004).
A growing number of studies suggest that a high flavonoid intake may be correlated with decreased risk of cancer (Pignatelli et al., 2000). Men with higher intakes of flavonoids, myricetin or quercetin, had lower prostate or lung cancer incidences, respectively (Knekt et al., 2002). Flavonoids decreased atherosclerosis by uppressing LDL oxidation (Yen and Hsieh, 2002). Flavonols, flavones, and catechin reduced mortality risk of coronary artery disease by 65% (Arts and Hollman, 2005). Quercetin and catechin inhibit platelet aggregation (Pignatelli et al., 2000) whereas procyanidin dimers and trimers are antioxidants that protect cell membrane disruption (Verstraeten et al., 2005). In addition, coumarin derivatives and flavonoid glucosides have therapeutic potential for weight control (Moreno et al., 2006).
Isoflavones (e.g., daidzein, genistein, glycitein, formononetin) are found in peanuts and legumes (Francisco and Resurreccion, 2008). Soybeans have the highest concentration of isoflavones and chick peas have the lowest. Peanuts have 211 ppm of total isoflavones, placing them between soybeans and chick peas.
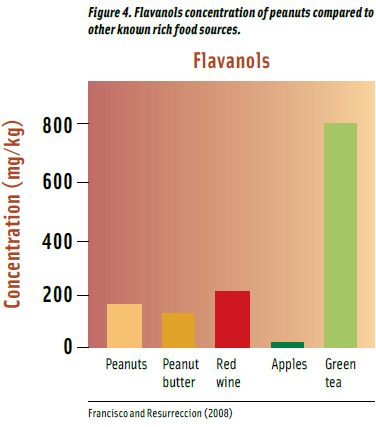
Flavanols (e.g., catechin, gallocatechin, proanthocyanidins) are found in fruits, green tea, red wine, and peanuts (Francisco and Resurreccion, 2008; Figure 4). Peanuts contribute 160 ppm of total flavanols content on a per gram basis, slightly higher than peanut butter and less than red wine. Green tea provides 5 times more flavanols than peanuts, depending upon the brewing conditions.
Phenolic acids are derivatives of benzoic and cinnamic acids (Liu, 2004). They are abundant in food and account for about one-third of the phenolic compounds in the diet (Liu, 2004). Phenolic acids can affect the color, sensory, nutritional, and antioxidant properties of foods.
--- PAGE BREAK ---
Phenolic acids are associated with reduced risk of CVD. Protocatechuic acid inhibits LDL oxidation (Yen and Hsieh, 2002), whereas ferulic acid has a hypotensive effect and decreases blood glucose, cholesterol, and LDL levels (Ardiansyah et al., 2008; Balasubahini et al., 2003). Ferulic acid has therapeutic potential in Alzheimer’s disease (Ono et al., 2005).
One class of phenolic acids is hydroxybenzoic acid, including gallic, protocatechuic, and α-hydroxybenzoic acids. Their major food sources are berries and peanuts. Peanuts contribute 40–60% more hydroxybenzoic acids, on a per gram basis, than strawberries and raspberries, but have half the amount found in blackberries (Francisco and Resurreccion, 2009).
The most frequently occurring class of phenolic acids is hydroxycinnamic acid, such as caffeic, ferulic, sinapic, chlorogenic, and coumaric acids. Their major food sources are fruits, coffee, and peanuts (Francisco and Resurreccion, 2009). Roasted peanuts contain about 300 ppm of hydroxycinnamic acid. Apples contain twice the amount of roasted peanuts, on a per gram basis; plums and coffee contain 4 to 6 times more. Blueberry has the highest concentration, which is about 8 times more than in roasted peanuts.
Phytosterols are found chiefly in vegetable oils and have chemical structures similar to cholesterol. Beta-sitosterol is the main phytosterol (Awad et al., 1998) and is abundant in Western diets (Kris-Etherton et al., 2002).
As a source of phytosterols, peanuts receive much attention due to their cancer-preventive activities such as inhibiting colon, prostate, and breast cancers (Awad et al., 2000; Awad et al., 2007) and protection from atherosclerosis (Awad et al., 2004). β-sitosterol inhibits colon and prostate cancers (Awad et al., 1998; von Holtz et al., 1998) and reduces cholesterol levels (Kris-Etherton et al., 2002). The U.S. Food and Drug Administration in 2000 approved the use of a labeling health claim for certain foods about the relationship between plant sterols and the reduced risk of coronary heart disease. Plant sterols and stanols work by blocking the absorption of cholesterol from the diet (Ohr, 2003).
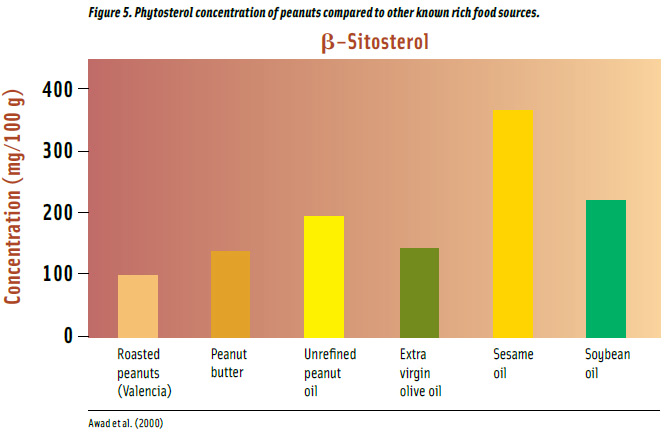
Vegetable oils are the major source of β-sitosterol with unrefined oils having greater amounts than refined (Awad et al., 2000; Figure 5). Unrefined peanut oil has 33% more β-sitosterol than extra virgin olive oil and 15% less than soybean oil. Roasted peanuts have 94 mg per 100 g, which is 43% less than peanut butter and 52% less than extra virgin olive oil. Sesame oil has the highest concentration of the compound and contains 2 times more than peanut oil.
Living Long, Living Well
Research has clearly demonstrated that the bioactive components in peanuts may reduce disease risks. Peanuts, peanut products, and their by-products are a source of a wide array of functional compounds, phenolics, and antioxidants that provide positive healthful benefits. To date, we have only scratched the surface of this area of research and scientists are discovering more bioactive compounds with beneficial effects. A quote from the book, “Longevity Factor” (Maroon, 2009) appropriately sums up the exciting prospects provided by bioactive compounds, “Plants and the amazing small molecules that they produce under stress have provided humans an edge in our struggle for survival, and now they may offer us the key to achieving a more ambitious goal: Living long and living well.”
Anna V.A. Resurreccion, Ph.D., a Professional Member of IFT and IFT Fellow, is Professor, Dept. of Food Science and Technology, University of Georgia, 1109 Experiment St., Griffin, Georgia 30223-1731 ([email protected]). Jocelyn M. Sales, a Student Member of IFT, is a Ph.D. in Food Science Candidate, Dept. of Food Science and Technology, University of Georgia ([email protected]). Inna Potrebko, a Student Member of IFT, is Research Coordinator, Dept. of Food Science and Technology, University of Georgia ([email protected]). Maria Leonora Lotis dL. Francisco, Ph.D., is Associate Professor, Department of Food Science and Nutrition, University of the Philippines, Diliman, Quezon City, Philippines ([email protected]). Henry L. Hitchcock is Research Professional II, Dept. of Food Science and Technology, University of Georgia ([email protected]).
This article was adapted from a conference presentation by author Resurreccion in Peanuts: High Value Super Food, The Peanut Institute, Napa, CA, May 8–11, 2009.
The authors wish to thank the U.S. Dept. of Agriculture through National Research Initiative Grant. No. 20063550316989, the USDA Cooperative State Research, Education, and Extension Service NRA 71.1 Improving Quality and Value, and the USAID Peanut Collaborative Research Program Grant No. LAG-G-00-96-00013-00 for partial funding of this research.
References
Alper, C.M. and Mattes, R.D. 2002. Effects of chronic peanut consumption on energy balance and hedonics. Int. J. Obesity 26: 1129-1137.
Alper, C.M. and Mattes, R.D. 2003. Peanut consumption improves indices of cardiovascular disease risk in healthy adults. J. Am. Col. Nutr. 22(2): 133-141.
Ardiansyah, Ohsaki, Y., Shirakawa, H., Koseki, T., and Komai, M. 2008. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J. Agric. Food Chem. 56: 2825-2830.
Arts, I.C.W. and Hollman, P.C.H. 2005. Polyphenols and disease risk in epidemiological studies. Am. J. Clin. Nutr. 81(suppl): 317S-325S.
Awad, A.B., Hartati, M.S., and Fink, C.S. 1998. Phytosterol feeding induces alteration in testosterone metabolism in rat tissues. J. Nutr. Biochem. 9: 712-717.
Awad, A.B., Chan, K.C., Downie, A.C., and Fink, C.S. 2000. Peanuts as a source of b-sitosterol, a sterol with anticancer properties. Nutr. Cancer 36: 238-241.
Awad, A.B., Toczek, J., and Fink, C.S. 2004. Phytosterols decrease prostaglandin release in cultured P388D1/MAB macrophages. Prostaglandins, eukotrienes and Essential Fatty Acids 70: 511-520.
Awad, A.B., Chinnam, M., Fink, C.S., and Bradford, P.G. 2007. b-Sitosterol activated Fas signaling in human breast cancer cells. Phytomed. 14:747-754.
Balasubahini, S.M., Rukkumani, R., and Menon, V.P. 2003. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 40: 18-122.
Baur, J.A., Pearson, K.J., Price, N.L., Jamieson, H.A., Lerin, C., Kaira, A., Prabhu, V.V., Allard, J.S., Lopez-Lluch, G., Lewis, K., Pistell, P.J., Poosala, S., Becker, K.G., Boss, O., Gwinn, D., Wang, M., Ramaswany, S., Fishbein, K.W., Spencer, R.G., Lakatta, E.G., Le Couteur, D., Shaw, R.J., Navas, P., uigserver, P., Ingram, D.K., de Cabo, R., and Sinclair, D.A. 2006. Resveratrol improves health and survival of mice on a high calorie diet. Nature 444(7117): 337-342.
Boue, S.M., Cleveland, T.E., Carter-Wientjes, C., Shih, B.Y., Bhatnagar, D., McLanchlan, J.M., and Burow, M.E. 2009. Phytoalexin-enriched functional food. J. Agric. Food. Chem. 57: 2614-2622.
Chang, J.C., Lai, Y.H., Djoko, B., Wu, P.L., Liu, C.D., Liu, Y.W., Chiou, R.Y.Y. 2006. Biosynthesis enhancement and antioxidant and anti-inflammatory activities of peanut (Arachis hypogaea L.) arachidin-1, arachidin-3, and isopentadienylresveratrol. J. Agric. Food Chem. 54: 10281-10287.
Counet, C., Callemien, D., Collin, S. 2006. Chocolate and cocoa: New sources of trans-resveratrol and trans-piceid. J. Food Chem. 98: 649–657.
Francisco, M.L.L.D. and Resurreccion, A.V.A. 2009. Total phenolics and antioxidant capacity of heat-treated peanut skins. J. Food Comp. Anal. 22: 16-24.
Francisco, M.L.L. D.L. and Resurreccion, A.V.A. 2008. Functional Components in Peanuts. Crit. Rev. in Food Sci. Nutr. 48: 715-746.
Griel, A.E., Eissenstat, B., Jututru, V., Hsieh, G., and Kris-Etherton, P.M. 2004. Improved diet quality with peanut consumption. J. Am. Coll. Nutr.23(6): 660-668.
Ibern-Gomez, M., Roig-Perez, S., Lamuela-Raventos, R.M., and de la Torre-Boronat, M.C.2000. Resveratrol and piceid levels in natural and blended peanuts. J. Agr. Food Chem. 48: 6352-6354.
Jang, M., Cai, L., Udeani, G.O., Slowing, K.V., Thomas, C.F., Beecher, C.W.W., Fong, H.S.S, Farnsworth, N.R., Kinghorn, A.D., Mehta, R.G., Moon, R.C., and Pezzuto, J.M. 1997. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275: 218-220
Jiang, R., Manson, J.E., Stampfer, M.J., Liu, S., Willet, W.C., and Hu, F.B. 2002. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 288(20): 2554-2560.
Knekt, P., Kumpulainen, J., Jarvinen, R., Rissanen, H., Heliovaara, M., Reunanen, A., Hakulinen, T., and Aromaa, A. 2002. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 76: 560-568.
Kris-Etherton, P.M., Hecker, K.D., Bonanome, A., Coval, S.M., Binkoski, A.E., Hilpert, K.F., Griel, A.E., and Etherton, T.D. 2002. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 113(9B): 71S-88S.
Li, T.Y., Brennan, A.M., Wedick, N.M., Mantzoros, C., and Rifai, N. 2009. Regular consumption of nuts is associated with lower risk of cardiovascular disease in women with type 2 diabetes. J. Nutr. 1333-1338.
Liebert, M., Licht, U., and Bohm, V. 1999. Antioxidant properties and total phenolics content of green and black tea under different brewing conditions. Z. Lebensm Unters Forsch A. 208: 217-220.
Liu, R.H. 2004. Potential synergy of phytochemicals in cancer prevention: mechanism and action. J Nutr. Supplement: 3479S-3485S.
Lokko, P., Latey, A., Armar-Klemesu, M., and Mattes, R.D. 2007. Regular peanut consumption improves plasma lipid levels in healthy Ghanaians. Int. J. Food Sci. Nutr. 58(3): 190-200.
McMurtrey, K.D., Minn, J., Pobanz, K., and Schultz, T.P. 1994. Analysis of wines for resveratrol using direct injection high pressure liquid chromatography with electrochemical detection. J. Agric. Food Chem. 42(10): 2078-2080.
Marambaud, P., Zhao, H., and Davies, P. 2005. Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J. Biol. Chem. Mol. Biol. 280(45): 37377-37382.
Maroon, J.C. 2009. The Longevity Factor: How Resveratrol and Red Wine Activate Genes for a Longer and Healthier Life. Simon and Schuter’s Atria Books. http://www.simonandschuster.com.
Moreno, D.A., Ilic, N., Poulev, A., and Raskin, I. 2006. Effects of Arachis hypogaea nutshell extract on lipid metabolic enzymes and obesity parameters. Life Sci. 78: 2927-2803.
Ohr, L.M. 2003. Fats for healthy living. Food Technol. 57(7): 91-96.
Ono, K., Hirohata, M., and Yamada, M. 2005. Ferulic acid destabilizes preformed β-mylodi fibrils in Vitro. Biochem. Biophys. Res. Commun. 336: 444-449.
Pignatelli, P., Pulcinelli, F.M., Celestini, A., Lenti, L., Ghiselli, A., Gazzaniga, P.P., and Violi, F. 2000.
The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am. J. Clin. Nutr. 72: 1150-1155.
Potrebko, I. and Resurreccion, A.V.A. 2009. Effect of ultraviolet doses in combined ultraviolet-ultrasound treatments on trans-resveratrol and trans-piceid contents in sliced peanut kernels. J. Agric. Food Chem. 57: 7750-7756.
Prior, R.L., Wu, X., and Schaich, K. 2005. Standardized methods for the determination of antioxidant capacity and phenolics in food and dietary supplements. J. Agric. Food Chem. 53: 4290-4302.
Prior, R.L. and Cao, G. 1999. Antioxidant capacity and polyphenolic components of tea: implications for altering in vivo antioxidant status. Soc. Exp. Biol. and Med. 220: 255-261.
Romero-Perez, A.I., Ibern-Gomez, M., Lamuela-Raventos, R.M., and de la Torre-Boronat, M.C. 1999. Piceid, the major resveratrol in grape juices. J. Agric. Food Chem. 47: 1533-1536.
Sales, J.M. and Resurreccion, A.V.A. 2009. Maximising resveratrol and piceid content in UV and ultrasound-treated peanuts. Food Chem. 117:674-680.
Stanley, L.L. and Mazier, M.J. 1999. Potential explanations for the French paradox. Nutr. Res. 19(1):3-15.
Sobolev, V.S. and Cole, R.J. 1999. trans-Resveratrol content in commercial peanuts and peanut products. J. Agric. Food Chem. 47: 1435-1439.
Soleas, G.J., Diamandis, E.P., and Goldberg, D.M.1997. Resveratrol, a molecule whose time has come? and gone?. Clin. Biochem. 30(2): 91-113.
Talcott, S.T., Passeretti, S., Duncan, C.E., and Gorbet, D.W. 2005. Polyphenolic content and sensory properties of normal and high oleic acid peanuts. Food Chem. 90: 379-388.
USDA. 2007. Oxygen radical absorbance capacity (ORAC) of selected foods. Agricultural Research Service, U.S. Department of Agriculture. Retrieved May 23, 2008 from Nutrient Data Laboratory home page: http://www.ars.usda.gov/nutrientdata.
Verstraeten, S.V., Hammerstone, J.F., Keen, C.L., Fraga, C.G., and Oteiza, P.I. 2005. Antioxidant and membrane effects of procyanidins dimers and trimers isolated from peanut and cocoa. J. Agric. Food Chem. 53: 5041-5048.
Villegas, R., Gao, Y-T., Yang, G., Li, H-L., Elasy, T.A., Zheng, W., and Shu. X.O. 2008. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Study. Am. J. Clin. Nutr. 87: 162-167.
von Holtz, R.L., Fink C.S., and Awad A.B. 1998. Beta-Sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr. Cancer 32: 8-12.
Yeh, C-C., You, S-L., Chen, C-J., and Sung, F-C. 2006. Peanut consumption and reduced risk of colorectal cancer in women: A prospective study in Taiwan. Gastroenterol. 12(2): 222-227.
Yen, G.C. and Hsieh, C.L. 2002. Inhibitory effects of Du-zhong (Eucommia ulmoides Oliv.) against low-density lipoprotein oxidative modification. Food Chem. 77: 449-456.
