Understanding and Combating Food Fraud
The goal of food fraud is economic gain. It differs from intentional contamination but could pose a public health risk. The behavioral sciences and criminology offer tools to deter food fraud.

Food fraud is a collective term used to encompass the deliberate and intentional substitution, addition, tampering, or misrepresentation of food, food ingredients, or food packaging; or false or misleading statements made about a product for economic gain (Spink and Moyer, 2011a). The types of fraud include adulteration, tampering, overrun, theft, diversion, simulation, and counterfeiting (Spink and Moyer, 2011b).
Food-related fraud is not new. Most early food laws and codes had a major focus on adulterants for economic gain. Concerns were reported by philosophers as far back as the fourth century B.C. and Roman law punishment for food adulteration included servitude or exile (Fortin, 2009). The U.S. Pure Food and Drug Act of 1906 was partially intended to address “fraud jokesers.” In 1938, the U.S. Food Drug and Cosmetics Act (FD&C) was ratified after deadly product adulteration with diethylene glycol, a sweet-tasting chemical used to make antifreeze that can be lethal for humans and pets. Even as food protection laws continue to evolve, such as the U.S. Food Safety Modernization Act passed in 2011, the risk from this specific adulterant persists.
Several international food standards and regulations address the risk of fraud-related food adulteration. These include Codex Alimentarius, Food Chemicals Codex (U.S. Pharmacopeia), and the European Union (EU) Directives. For example, EU Food Labeling Directive 2000/13, Article 2 requires that consumers not be misled regarding “as to the characteristics of the foodstuff and, in particular, as to its nature, identity, properties, composition, quantity, durability, origin or provenance, method of manufacture or production.” Also, the Global Food Safety Initiative (GFSI) has continued work on a Food Fraud Think Tank to consider if, or how, food fraud fits into their benchmarking. More generally, the International Standards Organization (ISO) has a technical committee on Fraud Countermeasures and Controls that includes food in “material goods.” The international focus on fraud complements the U.S. focus on adulteration.
The overarching concept of food protection includes food quality, food safety, food fraud, and food defense (Figure 1) (Spink and Moyer, 2011a; Moore et al., 2012; FDA, 2009). While all types of intentional contamination or intentional adulteration from attacks or breaches (including food fraud) could theoretically be categorized under food defense, in practice food defense is usually defined more specifically as counterterrorism (DHS, 2003).
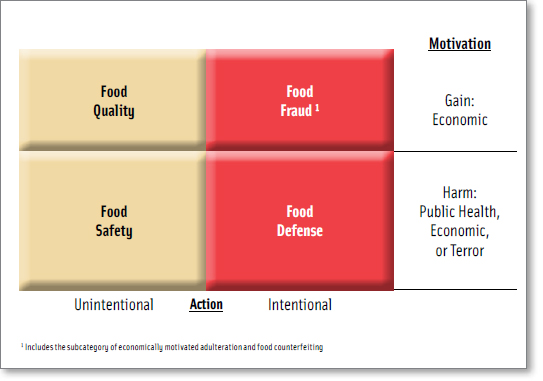 Preventing a food fraud incident is very different than preventing a terrorist who is intent on harming citizens or undermining the economy (Spink, 2011). This is because the fraudster is economically motivated to cheat the consumer and may inadvertently create a public health threat (e.g., melamine in infant formula, known Salmonella contamination in peanuts, over-treatment or glazing of more ice than allowed in seafood, etc.) (Everstine et al., under review). Only if a fraudulent food is identified as a public health threat does the traditional food safety intervention and response become engaged. While the cause of a food fraud incident is economic gain, if a public health threat is identified, the effect is an adulterated product (Spink and Moyer, 2011a).
Preventing a food fraud incident is very different than preventing a terrorist who is intent on harming citizens or undermining the economy (Spink, 2011). This is because the fraudster is economically motivated to cheat the consumer and may inadvertently create a public health threat (e.g., melamine in infant formula, known Salmonella contamination in peanuts, over-treatment or glazing of more ice than allowed in seafood, etc.) (Everstine et al., under review). Only if a fraudulent food is identified as a public health threat does the traditional food safety intervention and response become engaged. While the cause of a food fraud incident is economic gain, if a public health threat is identified, the effect is an adulterated product (Spink and Moyer, 2011a).
Economically Motivated Adulteration
Food fraud is the act while adulteration is often the regulatory violation in the U.S. Fraud is an act of deceit or trickery. The goal of fraud is economic gain. One who perpetrates fraud is a fraudster. With that in mind, food fraud is a human behavior with the intent to deceive utilizing food. Economically motivated adulteration (EMA) is a mode with which they often commit fraud. Adulteration of food is usually the regulatory violation as defined by the U.S Food and Drug Administration (FDA). This holds true for countries with similar food adulteration laws. More specifically, EMA is defined in the Federal Register and uses the adulteration term as defined in the FD&C.
There is utility in differentiating between the act of a fraudster and the legal violation itself. This differentiation helps focus on deterrence or detection. In a broader sense, the act is consumer fraud.
EMA became the focus of greater discussion at the FDA’s May 2009 Public Meeting on Economically Motivated Adulteration. An FDA working definition of EMA from that meeting is now being used by the FDA’s Workgroup on Economically Motivated Adulteration (WEMA) and the U.S. President’s Food Safety Working Group. The meeting notice defined EMA as “The fraudulent, intentional substitution or addition of a substance in a product for the purpose of increasing the apparent value of the product or reducing the cost of its production, i.e., for economic gain. EMA includes dilution of products with increased quantities of an already-present substance (e.g., increasing inactive ingredients of a drug with a resulting reduction in strength of the finished product, or watering down of juice) to the extent that such dilution poses a known or possible health risk to consumers, as well as the addition or substitution of substances in order to mask dilution.” An important and not thoroughly clear phrase is regarding “a known or possible health risk to consumers.”
--- PAGE BREAK ---
Later in 2009, the National Center for Food Protection and Defense (NCFPD) funded a Michigan State University (MSU) Anti-Counterfeiting and Product Protection Program grant, which culminated in the Journal of Food Science article “Defining the Public Health Threat of Food Fraud” (Spink and Moyer, 2011a). The NCFPD is a U.S. Dept. of Homeland Security Center of Excellence funded by the Dept. of Homeland Security (DHS), FDA, and U.S. Dept. of Agriculture (USDA). More recently in 2012, MSU and the Michigan Dept. of Agriculture and Rural Development were awarded a grant through the FDA’s Innovative Food Defense Program to create a Food Fraud Tabletop Exercise.
Public Health Threat
While food fraud may be economically motivated, the public’s health is at risk. These threats are potentially more risky than other types of food risks because there are a near infinite number of unconventional adulterants and contaminants. To prevent incidents, there must be a focus on why the fraudsters perceive an opportunity in the first place. In contrast to a food defense attack where perpetrators typically want to be found out, food fraudsters are clandestine, stealthy, and actively seek to avoid detection (Spink, 2011).
An adulterant is something that is intentionally added while a contaminant is something that occurs by accident or in association with another act. Predicting adulterants is somewhat more straightforward since we assume rational choices by the fraudsters (i.e., Rational Choice Theory). Trying to predict contaminants is much more difficult because these can be a function of fraudsters’ substandard manufacturing or procurement practices. The FDA defines the end result of both events as adulteration. Both can lead to potential public health risks, product recall, and manufacturers’ liability.
While detection focuses on the adulterants and contaminants, deterrence encompasses understanding the fraudsters and their motivations. Any harm from food fraud is through negligence on the part of the fraudsters and not due to intent. This is another reason why food fraud prevention extends beyond classic product adulteration. This is philosophically a very important shift, which is why the food fraud definition includes all types of fraud. Research (Spink and Moyer, 2011a) has identified seven types of food fraud. They are adulteration, tampering, overrun, theft, diversion, simulation, and counterfeiting (Figure 2). It is important to note that some types of fraud include adulteration, some are misbranding, and some don’t exactly fit into either category.
 We need to consider the fraudsters are criminals not concerned with laws, sociopaths not concerned with others, and they are not aware of the potential risks of their actions.
We need to consider the fraudsters are criminals not concerned with laws, sociopaths not concerned with others, and they are not aware of the potential risks of their actions.
Applying traditional risk analysis, food fraud risks can be categorized as direct, indirect, and technical. Direct food fraud includes immediate or imminent risk such as from an acutely toxic adulterant or contaminant. In these cases, a single exposure has an immediate adverse effect. Indirect food fraud includes adverse effects through longer term and repeated exposures. This could include a chronically toxic adulterant or contaminant, or the long-term absence of a beneficial ingredient. Technical food fraud risks are nonmaterial in nature, such as stolen, diverted, tampered, repackaged, incorrect weight or quantity, and/or mislabeled products including trademark counterfeiting. In a plausible scenario, reused genuine packaging could lead to recall of the wrong batch of product while the dangerous product remains in the marketplace.
Combating Food Fraud
Fortunately, the behavioral sciences and criminology can be applied to deter food fraud and its perpetrators. While these may be new concepts for food scientists or food defense practitioners, they are well established and based on the scientific method. Once the base criminology concepts are understood, applications such as deterrence are straightforward.
Taking an interdisciplinary approach to complex problems is not new for the food industry. For example, a food scientist charged with doubling the shelf life of a packaged product would not simply double the thickness of the package. The task would apply an interdisciplinary approach and ask various questions to determine an efficient solution. What are the characteristics of the product’s current shelf life (food science, quality management, sensory)? How and why does the product spoil (food chemistry, microbiology)? What is the current packaging and what are the commercially available options (packaging science, polymer science, mass transfer, purchasing)? Handling and storage have to be evaluated (supply chain management, logistics, marketing). The economics of potential solutions would be explored (sales, finance). Only after data are collected and analyzed can an efficient shelf-life decision be made. This type of interdisciplinary approach now adds criminology to the food science equation.
Food fraud is a crime of opportunity. Criminology provides a frame for assessing food fraud incidents and formulating strategies to reduce the fraud opportunity. The specific incident may have never occurred before and may never occur again, in part, because there are a near infinite number of fraudsters and a near infinite number of types of fraud. Based on this insight, there is a need to focus on reducing vulnerabilities and not detecting every food fraud incident—we’re not just attempting to control one type of food fraud since the fraudsters are adapting to the evolving fraud opportunities.
--- PAGE BREAK ---
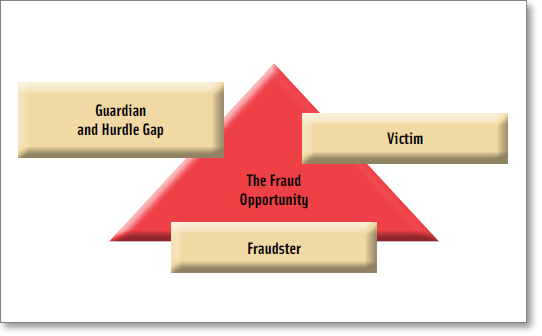
One major criminology theory that evaluates how to reduce the crime opportunity is Situational Crime Prevention. This prevention theory is applicable to food fraud due to its focus on deterrence. One helpful tool of Situational Crime Prevention is the Crime Triangle.
The Crime Triangle is a tool to deconstruct the fraud opportunity into the core characteristics of victims, fraudsters, and guardians or hurdles (Figure 3). Each characteristic plays a role in contributing to, or reducing, a fraud opportunity. Food fraud victims are consumers, retailers, manufacturers, or governments getting cheated. As brands grow (victims), the fraud opportunity grows. The fraudster is anyone cheating. Unfortunately, there are a near infinite number of fraudsters and simply arresting some will not significantly reduce the overall fraud opportunity—there are not just 10 and we can arrest eight of them. Guardians are the professionals who are trying to protect products through study, inspection, testing, or investigation. Hurdles are the countermeasures the guardians use to increase the risk of the fraudster getting caught or to increase the cost of perpetrating the fraud. It is important to note that the countermeasures do not need to be perfect or invincible. In many cases, simply changing standard operating procedures can reduce vulnerabilities. Even a minor countermeasure hurdle can cause potential fraudsters to move on to another target (i.e., crime displacement).
 Incident Database Research
Incident Database Research
To review food fraud incidents and detection test methods, a research project was conducted by the U.S. Pharmacopeia’s (USP) Food Ingredient Intentional Adulteration Expert Panel. This study leverages a range of USP projects related to adulteration and counterfeiting, including food protein products, pharmaceutical active ingredients or excipients, and dietary supplements. The results were published in a Journal of Food Science article and are published in the Food Chemicals Codex, 8th Edition (www.FoodFraud.org) (Moore et al., 2012).
The project collected 1,305 records between 1980 and 2010 primarily from scholarly journals that identified food ingredient adulteration. The scholarly journals were an important resource since the test equipment and adulterants were clearly identified, and overall research methods were evaluated by expert editors. While the exact adulterated products and their adulterants are of interest, more significant were the characteristics of the products that are targeted. Common product characteristics include liquid and ground products because they allow homogenous dilution with an adulterant. Additionally, the types and methods of the frauds provide insight to the fraud opportunity. Seven food ingredients represented more than half of the recorded frauds: olive oil, milk, honey, saffron, orange juice, coffee, and apple juice.
Another important aspect of the study was to identify the test equipment and methods. The most common analytical detection methods were high-performance liquid chromatography and infrared spectroscopy. Chemometrics data analysis was used in many reports and shows great promise to address a wide range of emerging fraud incidents.
Characteristics of EMA Events
Another research project focused on incidents from the perspective of the fraud-related food product events. The objective was to identify and evaluate characteristics of all types of food products or ingredient events from 1980 to 2011(Everstine et al., under review). In contrast to foodborne disease outbreaks, “EMA events present a particular challenge to the food industry and regulators since they are deliberate adulteration acts that are intended to evade detection.” The study noted “EMA events reveal gaps in our quality assurance (QA) systems that could be exploited for intentional harm.” A key finding was that most quality assurance systems were not tuned to specifically detect the clandestine and stealthy EMA type of events. “Prevention and detection of EMA cannot depend on traditional food safety strategies focused on the most common foodborne pathogens.” The study identified 137 unique events in 11 food categories: fish and seafood (24), dairy products (15), fruit juices (12), oils and fats (12), grain products (11), honey and other natural sweeteners (10), spices and extracts (8), wine and other alcoholic beverages (7), infant formula (5), plant-based proteins (5), and other food products (28).
FSMA and Intentional Adulteration
Another significant milestone for understanding the concepts of food fraud and the EMA of food was the January 2011 passage of the Food Safety Modernization Act (FSMA). FSMA includes 11 mentions of intentional adulteration and over 70 mentions of prevention (FDA, 2011). Unfortunately, the FSMA does not include a definition of intentional adulteration. While the FSMA focus was on the term “adulteration,” it was frequently modified with “or misbranded.” This further distinguished adulteration and misbranding as two similar but unique types of fraud. In the absence of more clarity from a guidance document, the law insinuates, but does not explicitly define, a simultaneous focus on both. The lack of clarity perpetuates confusion between intentional adulteration and EMA. The lack of a FSMA definition was recognized in the October 2011 GAO report “Food and Drug Administration: Better Coordination Could Enhance Efforts to Address Economic Adulteration and Protect the Public Health” (GAO, 2011). The first recommendation by the GAO was to “adopt a working definition of economic adulteration.”
Next Steps
While it may seem daunting for the food protection system to expand again to include another discipline, we did successfully expand in the past to include food defense. (The original U.S. term was “food security,” which then adapted to the more global term “food defense”). Similarly, it may be difficult to remember a time before the existence of HACCP or supplier audits and quality assurance testing of incoming goods. Perhaps in a few years, after incorporating criminology into food safety and food science, it may be equally difficult to remember a food safety program that did not include a Food Fraud Manager.
Training these future practitioners and leaders will be a challenge requiring collaboration among academia, government agencies, and industry. Recent efforts include Michigan State University’s online graduate courses that culminate in a Certificate in Food Fraud Prevention or a Certificate in Counterfeiting Criminology (www.a-cappp.msu.edu). This is an important first step but more public-private partnership and teamwork with industry is required.
John Spink, Ph.D., is Assistant Professor, School of Criminal Justice, Michigan State University ([email protected]) and
Douglas C. Moyer, is Instructor, Program in Public Health, College of Human Medicine, Michigan State University ([email protected]). More information is available at www.msu.edu.
References
DHS, U.S. Dept. of Homeland Security 2003. Homeland Security Presidential Directive 7: Critical Infrastructure Identification, Prioritization, and Protection.
Everstine, K., Spink, J., and Kennedy, S. Under Review. Economically Motivated Adulteration and Food Fraud: Indicators and Countermeasures for Foodstuffs. J of Food Protection, Under Review.
FDA, U.S. Food and Drug Administration 2009. Public Meeting: Economically Motivated Adulteration. http://www.fda.gov/NewsEvents/MeetingsConferencesWorkshops/ucm163619.htm.
FDA 2011. Food Safety Modernization Act (FSMA), in 111th Congress Public Law 353.
Fortin, N.D. 2009. Food regulation: law, science, policy, and practice. Wiley. Hoboken, NJ.
GAO, U.S. Government Accountability Office 2011. Food and Drug Administration, Better Coordination Could Enhance Efforts to Address Economic Adulteration and Protect the Public Health, in Report to Congressional Requesters, October, GAO-12-46.
Moore, J.C., Spink, J., and Lipp, M. 2012. Development and Application of a Database on Food Ingredient Fraud and Economically Motivated Adulteration from 1980-2010. J of Food Sci 77(4): R118-126.
Spink, J. 2011. The Challenge of Intellectual Property Enforcement for Agriculture Technology Transfers, Additives, Raw Materials, and Finished Goods against Product Fraud and Counterfeiters. J of Intellectual Property Rights 16(2): 183-193.
Spink, J. and Moyer, D.C. 2011a. Defining the Public Health Threat of Food Fraud. J of Food Sci 76(9): R157-162.
Spink, J. and Moyer, D.C. 2011b. Backgrounder: Defining the Public Health Threat of Food Fraud, in Research Grants. National Center for Food Protection and Defense (NCFPD). http://www.ncfpd.umn.edu. Minneapolis, MN. p. 7.
