Probiotics
This Scientific Status Summary addresses the scientific basis of the hypothesis that consumption of probiotics can positively influence human health. Product and regulatory issues are also briefly addressed.
A PUBLICATION OF THE INSTITUTE OF FOOD TECHNOLOGISTS ’ EXPERT PANELON FOOD SAFETY AND NUTRITION
Probiotics are defined as live microbial food ingredients that have a beneficial effect on human health (Salminen et al., 1998). The concept of probiotics evolved at the turn of the 20th century from a hypothesis first proposed by Nobel Prize winning Russian scientist Elie Metchnikoff (Bibel, 1988), who suggested that the long, healthy life of Bulgarian peasants resulted from their consumption of fermented milk products. He believed that when consumed, the fermenting bacillus (Lactobacillus) positively influenced the microflora of the colon, decreasing toxic microbial activities.
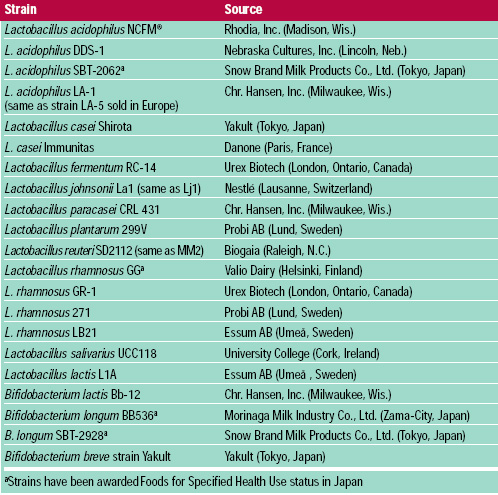 The historical association of probiotics with fermented dairy products, still true today, stems from these early observations. Investigations in the probiotic field during the past several decades, however, have expanded beyond bacteria isolated from fermented dairy products to those of intestinal origin. The probiotic bacteria most commonly studied include members of the genera Lactobacillus and Bifidobacterium. Saccharomyces boulardii, (McFarland et al., 1994), Escherichia coli (Kruis et al., 1997) and Enterococcus strains are used as probiotics in non-food formats. Many probiotic strains have been identified, studied, and commercialized (Table 1).
The historical association of probiotics with fermented dairy products, still true today, stems from these early observations. Investigations in the probiotic field during the past several decades, however, have expanded beyond bacteria isolated from fermented dairy products to those of intestinal origin. The probiotic bacteria most commonly studied include members of the genera Lactobacillus and Bifidobacterium. Saccharomyces boulardii, (McFarland et al., 1994), Escherichia coli (Kruis et al., 1997) and Enterococcus strains are used as probiotics in non-food formats. Many probiotic strains have been identified, studied, and commercialized (Table 1).
Probiotic-containing products are common in Japan and Europe (Lee et al., 1999; Sanders and Huis in’t Veld, 1999). In the United States, probiotics are just now receiving attention by the food industry as healthful ingredients for an increasingly health-conscious consumer. The passage in 1994 of the Dietary Supplement Health and Education Act invigorated the sale of probiotic products as dietary supplements.
This Scientific Status Summary provides an overview of the current status of the proposed mechanisms of activity of probiotics, their efficacy in humans, and some marketing and regulatory considerations. Extensive reviews of these topics may be consulted for additional information.
These include Bengmark (1998), Elmer et al. (1999), Fonden et al. (1999), Holzapfel et al. (1998), Lee et al. (1999), Naidu et al. (1999), Salminen et al. (1996), Sanders (1998a), Sanders and Huis in’t Veld (1999), and Tannock (1999a).
Scientific Basis of Functionality
Hundreds of microbial species live in association with humans—on skin and in oral, intestinal and vaginal tracts. Bacterial populations have been estimated to reach 100,000,000,000,000 cells at all sites of the human body (Tannock, 1994), a number that is more amazing when considered in the context of exceeding by 10-fold the number of human cells associated with the human body. Studies with germ-free (gnotobiotic) animals prove that microbial colonization is not required for survival. In fact, microbial colonization can have negative effects as a result of the toxic, genotoxic, mutagenic, or carcinogenic potential of microbial metabolites (Hill et al., 1971). Germ-free animals, however, are more susceptible to infection than conventional counterparts (Hentges, 1992). The increased susceptibility to infection is attributed, at least in part, to poor immune function and the absence of “colonization resistance” (competition of normal microflora with invading microorganisms; Vollaard and Clasener, 1994). Differences between conventional and germ-free animals provide a basis for the belief that microbial colonization has important health implications for host organisms.
It is a big jump, however, from the assertion that colonizing microflora have a profound effect on normal human health to the probiotic hypothesis that the addition of certain exogenous microorganisms to the intestinal ecosystem will have a positive effect. The intestinal tract is a fairly stable microbial ecosystem in the adult (Tannock, 1990). Acute perturbations as might result from antibiotic use, disease, or certain dietary changes seem to be self-correcting (Tannock, 1983). Probiotic bacteria consumed even in high numbers do not become permanent colonizers and are rarely detectable in fecal or intestinal samples beyond a couple of weeks after ingestion. This residence time likely coincides with washout kinetics, extended perhaps by some in situ replication of probiotics suited to the environment. Therefore, it is necessary to consider that probiotic effects may, in fact, be mediated by associations and mechanisms less intimate and more transient than those of native microflora.
--- PAGE BREAK ---
Whether or not a specific probiotic bacterium will have beneficial, detrimental, or no effect on health cannot be presumed strictly through determination of its genus or species. The tempting speculation that the members of one genus or species will consistently mediate specific effects is not supported by research. Strain-specific effects are frequently reported in a diversity of assays. Conversely, for targets including immune function, anti-cancer effects, and anti-diarrheal effects, similar positive effects have been demonstrated for different strains of different genera, e.g., lactobacilli, bifidobacteria and enterococci. Although direct comparisons of different strains are rarely done, it appears that generalizations about the probiotic performance of genera and species are difficult to make. Until mechanisms are better understood and controlled studies comparing isogenic strains differing in a well-defined manner are completed, it is prudent to assume that probiotic effects are strain-specific. In addition, physiological conditions of the host are likely to be as important to probiotic efficacy as the microbial strain.
Justification of Use
Are efforts to understand the role that probiotic bacteria may play in human health justifiable, apart from interest in yet another functional ingredient to lure purchasing dollars from an increasingly health-conscious consumer? Are benefits from these bacteria going to make a substantive difference in the health of the average consumer? At this point, the answers to these questions are speculative. The research characterizing the health effects of probiotic bacteria is not sufficient, although hundreds of publications address the topic. Mechanisms of action are not thoroughly established; results for some endpoints are equivocal; results are spread among many different targets, population groups, endpoints, strains and doses; and properly controlled human intervention trials are limited. Yet the emergence of new public health risks, even in industrialized nations, suggests ways in which effective probiotic bacteria may play an important role in maintaining human health.
Epidemiological and research evidence suggests that intestinal mucosal immunity diminishes with age, leaving the elderly especially susceptible to infectious disease. This situation will continue to expand with the aging of the population. Furthermore, some infections, once thought benign and self-limiting or readily treatable with antibiotics, are now recognized as more serious health threats. For example, a diarrheal disease associated with antibiotic therapy is caused by an opportunistic pathogen, Clostridium difficile, carried asymptomatically by many. This infection is generally treated successfully with a second antibiotic. Some infections, however, recur in spite of antibiotic therapy. The association of this disease with disruption of normal intestinal microflora during antibiotic treatment suggests the importance of finding alternative approaches to treatment.
Campylobacter jejuni, believed to be the leading cause of bacterial gastroenteritis (Altekruse et al., 1999), can cause Guillain-Barré syndrome (leading to acute neuromuscular paralysis) in 0.1% of cases of infection. Other potentially life threatening foodborne pathogens, e.g., enterohemorrhagic Escherichia coli strains (Buchanan and Doyle, 1997), have emerged. Multiple antibiotic resistance is a continual threat in the battle against once-treatable infections, with vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus serious concerns, especially in hospital environments. And in non-industrialized nations, infections such as rotavirus claim the lives of hundreds of thousands of infants yearly (Parashar et al., 1998). For these reasons, a safe, cost-effective, “natural” barrier to microbial infection or to the negative effects of indigenous microorganisms may be significant to human health.
 Effects on Human Health
Effects on Human Health
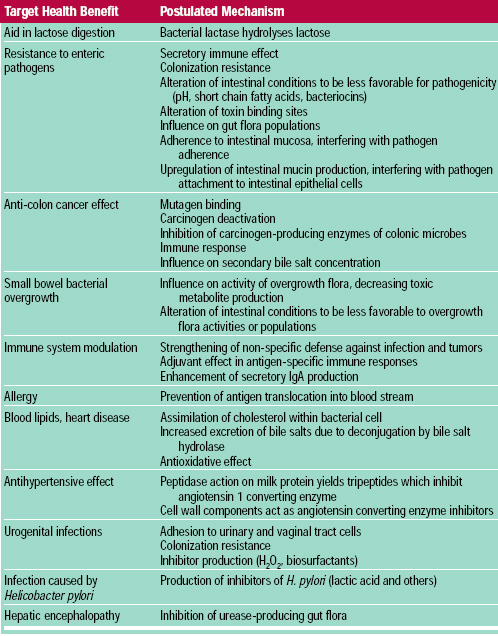
Research suggests that probiotic bacteria may mediate a variety of health effects through numerous proposed mechanisms (Table 2). Some blinded, randomized, placebo-controlled studies conducted with a meaningful number of human subjects have yielded sufficiently positive results (Aso and Akazan, 1992; Belloma et al., 1980; Gade and Thorn, 1989; McFarland et al., 1994; Saavedra et al., 1994) to justify further investigation of the probiotic hypothesis. Alleviation of lactose intolerance symptoms and anti-diarrheal effects are the best substantiated effects. Anti-cancer and immune modulation effects are encouraging, but need more thorough substantiation in humans. Modulation of the gut microflora (populations and activities) and influence on mucosal immunity are mechanisms of probiotic function with potential to broadly influence human physiology. Probiotics have ameliorated acute toxic effects of gut flora metabolism in clinical systems, such as small bowel bacterial overgrowth (Simenhoff et al., 1996) and liver disease (Nanji et al., 1994; Read et al., 1966). A brief assessment of probiotic effects targeted toward several endpoints, with emphasis on results from human studies, where possible, follows.
--- PAGE BREAK ---
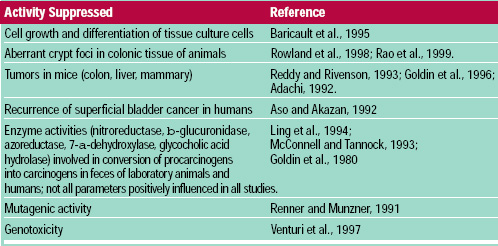 • Cancer. Research has demonstrated that dietary components may increase or decrease cancer incidence (Williams and Wynder, 1996). Evidence that probiotic bacteria may be a dietary constituent that reduces cancer risk (Table 3) is derived from several lines of evidence (Hirayama and Rafter, 1999; Mital and Garg, 1995; Rafter, 1995). One study demonstrated that a powder preparation of L. casei (1010 CFU three times/day for one year) increased the recurrence-free period among human subjects with superficial bladder cancer (Aso and Akazan, 1992). Additional population studies and human intervention trials are needed to confirm this type of effect. Such studies can be justified by the positive results seen to date from studies using a variety of biomarkers and the plausibility of proposed mechanisms.
• Cancer. Research has demonstrated that dietary components may increase or decrease cancer incidence (Williams and Wynder, 1996). Evidence that probiotic bacteria may be a dietary constituent that reduces cancer risk (Table 3) is derived from several lines of evidence (Hirayama and Rafter, 1999; Mital and Garg, 1995; Rafter, 1995). One study demonstrated that a powder preparation of L. casei (1010 CFU three times/day for one year) increased the recurrence-free period among human subjects with superficial bladder cancer (Aso and Akazan, 1992). Additional population studies and human intervention trials are needed to confirm this type of effect. Such studies can be justified by the positive results seen to date from studies using a variety of biomarkers and the plausibility of proposed mechanisms.
Results suggest that probiotic bacteria appear able to counteract mutagenic and genotoxic effects in the colon and other organ sites. Additionally, mechanistic studies suggest that probiotic bacteria or their byproducts influence epithelial cell kinetics in the colon, decreasing cancer cell proliferation. What cannot be determined from scientific evidence to date is to what extent regular probiotic consumption might influence cancer in humans. In addition, the cumulative evidence involves many different probiotic strains, feeding levels, exposure times, target cancer sites, and study methods. Therefore, precise translation of these results into specific recommendations is difficult.
• Intestinal Tract Function. Gastrointestinal disturbances can range from annoying to life-threatening. Diarrheal illnesses have a host of microbiological, immunological and physiological causes, some related to the disruption of normal microecology. Improper function of the immune system that is related to decreased tolerance to indigenous microflora can lead to immunopathogenesis in chronic inflammatory bowel disease (Merger and Croitoru, 1998), which can be refractory to conventional treatment. Underlying pathology (e.g., chronic kidney failure) and achlorhydria (brought on by aging) can result in bacterial overgrowth of the small bowel and lead to abnormal and harmful microbial activities (Nanji et al., 1994; Simenhoff et al., 1996). Disruptions in intestinal permeability barriers can lead to translocation of bacteria into the blood stream (Wells et al., 1988). Consumption of non-digestible foodstuffs (e.g., lactose for lactose intolerant people, soluble fibers) can provide fermentable substrates for growth of intestinal microbes, sometimes with deleterious effects.
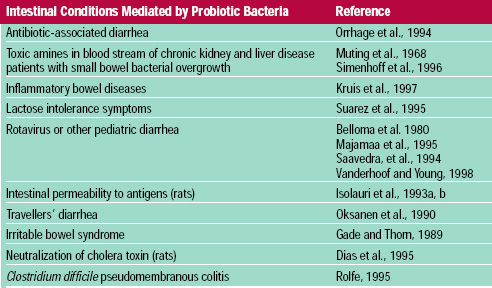 Probiotic bacteria have been shown to improve the clinical outcome in many intestinal disease targets (Table 4; Elmer et al., 1996; Salminen et al., 1998a). Most convincing are the blinded, placebo-controlled trials with human subjects (Bello ma et al., 1980; Gade and Thorn, 1989; McFarland et al., 1994; Saavedra et al., 1994). Although the clinical protocols frequently fall short of the double-blinded, placebo-controlled ideal, the scientific evidence suggests that probiotic bacteria, consumed at high levels (109-1011/day), can decrease the incidence, duration, and severity of some intestinal illnesses.
Probiotic bacteria have been shown to improve the clinical outcome in many intestinal disease targets (Table 4; Elmer et al., 1996; Salminen et al., 1998a). Most convincing are the blinded, placebo-controlled trials with human subjects (Bello ma et al., 1980; Gade and Thorn, 1989; McFarland et al., 1994; Saavedra et al., 1994). Although the clinical protocols frequently fall short of the double-blinded, placebo-controlled ideal, the scientific evidence suggests that probiotic bacteria, consumed at high levels (109-1011/day), can decrease the incidence, duration, and severity of some intestinal illnesses.
• Immune Function. Exposure to foreign antigens elicits a complex cascade of responses from the human body, including launching protective reactions against pathogens and suppressing activity against food antigens and colonizing microflora. Improperly directed or balanced immunologic activity can lead to serious health problems; the suffering brought on by allergic and inflammatory reactions is a testimony to these occurrences. Immune function can be compromised in the elderly and in those on immuno-suppressing medications and suffering from certain diseases. The role of functional foods, directed for the most part toward a healthy population with competent immune function, is not clear. However, it is an area of much active research, consumer interest, and product positioning.
--- PAGE BREAK ---
The oro-gastro-intestinal system is a prime means of interface between foreign antigens, (either pathogens, harmless bacteria, or food antigens) and mucosal surfaces. The total mucosal surface area of the adult human gastrointestinal tract is up to 300 m2, the largest body area interacting with the environment. The gut-associated lymphoid tissue (GALT) transforms the gastrointestinal tract into the largest immune organ in the human body. Approximately 1010 immunoglobulin-producing cells per meter of small bowel are estimated to be present, accounting for approximately 80% of all immunoglobulin-producing cells in the body (Targan and Shanahan, 1994). Subsequently, the intestinal tract can evoke a variety of immunological responses from many different local immune cells (McCracken and Gaskins, 1999). It is through this mechanism that probiotic bacteria are postulated to influence the immune response, although genera in addition to Lactobacillus and Bifidobacterium have been evaluated as immune stimulators or “biological response modifiers” with varying degrees of success. The influence of probiotic bacteria on the immune response has been thoroughly reviewed by Marteau and Rambaud (1993), Mc-Cracken and Gaskins (1999), Perdigón and Alvarez (1992), and Perdigón et al. (1995).
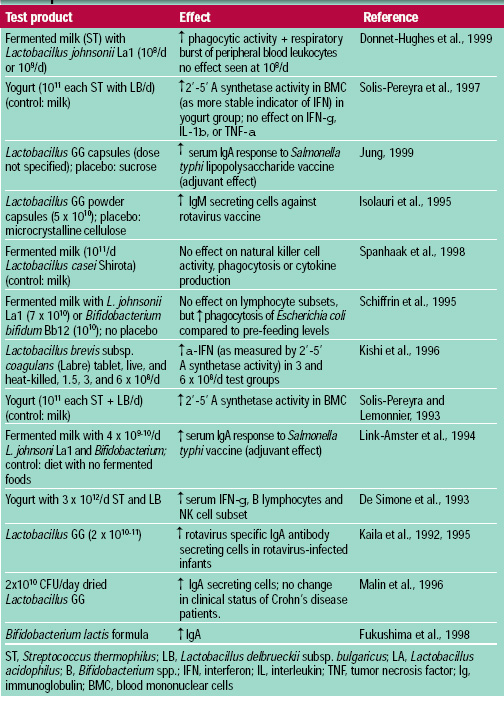 Many studies using in vitro assessments of immune response or non-oral routes of probiotic introduction have questionable relevance. Animal models and human studies (Table 5) provide a baseline understanding of the degree and type of probiotic-induced immune response. From these studies, it appears that probiotic bacteria are able to enhance both non-specific and specific immune responses by activating macrophages; increasing levels of cytokines; increasing natural killer cell activity; and increasing levels of immunoglobulins, especially secretory IgA. Viable probiotic cells, dead cells, or fermentation products have been shown to mediate immune activity. Important in this capability is the positive influence of probiotics on immune activity without eliciting a harmful inflammatory response.
Many studies using in vitro assessments of immune response or non-oral routes of probiotic introduction have questionable relevance. Animal models and human studies (Table 5) provide a baseline understanding of the degree and type of probiotic-induced immune response. From these studies, it appears that probiotic bacteria are able to enhance both non-specific and specific immune responses by activating macrophages; increasing levels of cytokines; increasing natural killer cell activity; and increasing levels of immunoglobulins, especially secretory IgA. Viable probiotic cells, dead cells, or fermentation products have been shown to mediate immune activity. Important in this capability is the positive influence of probiotics on immune activity without eliciting a harmful inflammatory response.
Biological effects correlated with enhanced immune function, such as protection against viral and bacterial pathogens and tumor inhibition, have also been measured (Marteau and Rambaud, 1993). This effort is important because it provides a physiologically relevant measure of functionality. It is difficult to interpret statistically significant results in immune function studies in terms of what degree of influence these changes would be expected to have on human health. Importantly, long-term studies have not established whether the immune response to exogenous probiotic strains is a temporary response upon exposure to a foreign strain or one that would continue to be expressed after extended feeding (Tannock, 1999b).
• Allergy. Preliminary research on probiotic-mediated modulation of certain allergic reactions has been reported. A breakdown of the intestine’s mucosal barrier function, allowing extensive antigen challenge, may be a factor in some allergic reactions. Since probiotic bacteria have been shown to improve mucosal barrier function, the hypothesis that they may play a role in moderating allergic response was tested.
In one study, changes in receptors mediating phagocytosis was measured in eight milk-hypersensitive (not lactose intolerant) adults consuming milk and milk with Lactobacillus GG (Pelto et al., 1996). Consumption of the probiotic-containing milk did not result in a significant increase in receptor expression whereas consumption of regular milk did, suggesting that this strain may suppress a milk-induced immune inflammatory response. Unfortunately, symptoms were not evaluated.
Symptoms were evaluated, however, in a partially blinded 12-month study in which yogurt and pasteurized yogurt were fed to college-age and elderly individuals (Trapp et al., 1993). A significant decrease in allergy symptoms was observed in individuals consuming yogurt containing live, active bacteria compared to individuals consuming pasteurized yogurt and those who did not consume yogurt. No microbiological characterization of the yogurt was reported. Another report, however, did not show clinical improvement in 15 asthmatic subjects fed yogurt and yogurt with L. acidophilus in a crossover design study (Wheeler et al., 1997). Further study of the role of probiotics in allergic response is necessary.
• Stomach Health. The ability of probiotic bacteria to influence colonization and activity of Helicobacter pylori, which is associated with chronic gastritis, peptic ulcers, and risk for gastric cancer (Marshall, 1994), has been evaluated. Conventionally colonized (stomach microflora is dominated by lactobacilli) mice resisted infection by H. pylori, whereas germ-free mice did not (Kabir et al., 1997). Results from animal (Aiba et al., 1998; Kabir et al., 1997) and human (Michetti et al., 1999) studies suggest that some probiotic bacteria or their end-products may inhibit H. pylori infection.
--- PAGE BREAK ---
The ability of Lactobacillus salivarius WB1004 to inhibit H. pylori colonization of human or murine gastric epithelial cells in vitro was extended in vivo in gnotobiotic mice (Kabir et al., 1997). Interestingly, complementary studies indicated that neither S. aureus nor Enterococcus faecalis afforded the same protection from H. pylori infection. Colonization by H. pylori was also reduced by feeding L. salivarius after infection with H. pylori. Aiba et al. (1998) found that L. salivarius, but not L. casei or L. acidophilus, inhibited H. pylori colonization in a mouse model. Lactic acid production was thought to correlate with inhibition. Lactic acid was shown to be more inhibitory to H. pylori in vitro than acetic or hydrochloric acids (Midolo et al., 1995).
Michetti et al. (1999) administered to humans in a double-blind, controlled clinical study a whey protein-based fermentation product supernatant fluid prepared by growth of L. johnsonii La1. They assessed effects using a carbon-13-urea breath test, endoscopy, and stomach biopsy. Reductions in 13CO2 in the 13C-urea breath test were observed in subjects consuming the probiotic product, suggesting an inhibition of infection; the inhibition extended four weeks after cessation of treatment. Gastric biopsies, however, indicated that H. pylori colonization persisted. In vitro studies suggested that inhibition resulted from more than lactic acid, and was strain-specific. These results suggest that probiotic bacteria and their metabolic end-products can inhibit H. pylori and its physiological effects. Results regarding the influence of probiotics on H. pylori infection must be extended and confirmed.
• Urogenital Health. Similar to the intestinal tract, the urogenital tract in women is highly colonized and susceptible to infection upon colonization disruption (Reid et al., 1998). Both urinary and genital tract infections have been linked to bacteria originating from the colon. This has led to the hypothesis that modulation of gut microflora through consumption of bacteria can have an effect on urogenital ecosystems (Sobel, 1999). Populating the colon with lacto-bacilli enables the colon to serve as a source of beneficial, not just harmful, bacteria.
Several studies have correlated vaginal health (absence of infections) with the presence of lactobacilli, and specifically hydrogen peroxide-producing lactobacilli (Hillier et al., 1992). Some clinical substantiation of the ability of probiotics to decrease recurrence of urogenital infections in women exits (Bruce and Reid, 1988; Hallen et al., 1992; Hilton et al., 1992; Reid et al., 1995; Shalev et al., 1996). Most of these studies were conducted with intravaginal instillations of probiotic bacteria. Oral consumption of certain probiotic-containing products, however, was found to mediate decreased recurrence of Candida infections and bacterial vaginosis (Hilton et al., 1992; Shalev et al., 1996). To confirm a role of dietary sources of probiotic bacteria in the prevention of urogenital infections, placebo-controlled, blinded studies using oral routes of introduction with sufficiently large study groups are needed.
• Cholesterol. Elevated levels of certain blood lipids are a risk factor for cardiovascular disease. The observation that conventional animals excrete higher levels of cholesterol in feces than germ-free animals suggests that colonizing microbes may influence cholesterol excretion, with potential implications for serum cholesterol levels (Eyssen, 1973). Studies of the effects of culture-containing dairy products or probiotic bacteria on cholesterol levels has yielded equivocal results (Taylor and Williams, 1998).Since 1974, 15 published studies have evaluated blood lipids in human subjects consuming fermented milk products, with a total of 534 subjects. Statistically significant lowering of cholesterol ranged from 2.4 – 23.2% for total cholesterol and 9 – 9.8% for low-density lipoprotein cholesterol. The studies have been criticized for failure to stabilize baselines prior to onset of the feeding protocol, small sample size, short study duration, unreasonably large fermented milk intake requirements, small differences, and failure to control for diet and physical activity of subjects. In the studies showing statistically significant results on the lowering of either total cholesterol or low-density lipids, the treatment duration was six weeks or less. Furthermore, one report showed increases in both total cholesterol and low-density lipoprotein (Rossouw et al., 1981) and another showed decreases in high-density lipoprotein (Anderson and Gilliland, 1999).
The effect of probiotic bacteria on reduction of serum cholesterol and mechanisms of any effects are unknown. One hypothesis suggests that some strains of L. acidophilus can assimilate the cholesterol molecule (Gilliland et al., 1985); this activity has been reported in in vitro assays (Gilliland et al., 1985; Rasic et al., 1992). However, some have questioned the physiological relevance of binding or assimilation observed in an in vitro, aqueous assay conducted at pH 6.0 or lower. It has been suggested that pH-dependent, transient cholesterol precipitation in laboratory media were misinterpreted as assimilation (Klaver and Meer, 1993; Tahri et al., 1996).
--- PAGE BREAK ---
The ability of certain probiotic lactobacilli and bifidobacteria to enzymatically deconjugate bile acids has also been suggested to have a role in regulating cholesterol levels in humans. Deconjugated bile acids are more efficiently excreted (De Smet et al., 1994). Because cholesterol is a precursor of bile acids, this could lead to reduction of serum cholesterol as cholesterol molecules are converted to bile acids to replace those lost through excretion. The desirability of bile salt hydrolysis as a probiotic attribute, however, is questionable. Deconjugated bile acids escaping re-entry into the hepatic circulation enter the colon where they can be converted into secondary bile acids by colonic microbes. Secondary bile acids are known cancer promoters; hence, this activity could result in a health risk. A potential increased risk of colon cancer may outweigh any benefit of reduction of serum cholesterol levels. These hypotheses have not been confirmed in animal or human studies, although Gilliland et al. (1985) established a cholesterol-lowering effect of a cholesterol-assimilating (but not a non-assimilating) strain in boars. Further research on any mechanisms should be preceded by evidence for clinical effect in at least one thorough, long-term human study.
• Hypertension. Dietary recommendations accompany more aggressive strategies to control hypertension, and some preliminary evidence suggests that food products derived from probiotic bacteria could possibly contribute to blood pressure control (Takano, 1998). This antihypertensive effect has been documented with studies in spontaneous hypertensive rats (Nakamura et al., 1995; 1996) and one human clinical study (Hata et al., 1996). Two tripeptides, valine-proline-proline and isoleucine-proline-proline, isolated from fermentation of a milk-based medium by Saccharomyces cereviseae and Lactobacillus helveticus have been identified as the active components. These tripeptides function as angiotensin-I-converting enzyme inhibitors and reduce blood pressure. Based on this technology, Calpis (Kanagawa, Japan) developed a pasteurized product (Ameal-S) having Foods for Specified Health Use (FOSHU) status. Unlike many other probiotic-induced effects, it is important to note that this effect is mediated by a fermentation end-product, not viable probiotic cells.
Another antihypertensive activity was associated with cell wall fragments of Lactobacillus casei YIT9018 (Sawada et al., 1990). Tested in a placebo-controlled trial with 28 human hypertensive subjects, powdered cell extracts (not viable cells) were administered orally and effects on systolic pressure, diastolic pressure, and heart rate were determined. Small, but statistically significant, decreases in all three measurements were noted. These results suggest that probiotic bacteria or their fermentation end-products may be effective in mediating a mild antihypertensive effect. Confirmation in long-term, placebo-controlled human subjects is needed.
• Other Health Effects. Probiotic influence on a variety of other clinical targets has been evaluated. Probiotic-mediated reduction in the severity of reaction to exposure to radioactive isotopes has been shown in mice (Dong et al., 1987) and humans (Henriksson et al., 1995; Korschunov et al., 1996; Salminen et al., 1988). The effects of endotoxemia associated with alcoholic liver disease were reduced by probiotics (Nanji et al., 1994). Probiotics have been used in enteral feeding to provide nutritional support of surgery patients (Bengmark and Gianotti, 1996).
In addition to proposed direct effects on humans, probiotics may also have implications for human health through their use in animal agriculture. Probiotics have been tested for prevention of food animal colonization with pathogens. Probiotics may also benefit animal agriculture through greater resistance of farm animals to infectious diseases, increased growth rate, improved feed conversion, and increased yield of milk and eggs (Fuller, 1998). Commercial probiotic products for use in animal agriculture are available.
U.S. Regulatory Issues and Safety
Several of the bacteria used in the United States as probiotics are listed by the Food and Drug Administration (FDA) on its “Partial List of Microorganisms and Microbial-Derived Ingredients that are Used in Foods” (http://vm.cfsan.fda.gov/~dms/). The usual approach for safety assessment for marketing probiotic bacteria in the United States is presumption of safety, reasoned by a long history of safe use in fermented dairy products.
--- PAGE BREAK ---
The safety of lactobacilli and bifidobacteria has been recently reviewed (Salminen and von Wright, 1998). The general conclusion is that the pathogenic potential of lactobacilli and bifidobacteria is quite low. This conclusion is based on the widespread consumption of these microorganisms in fermented foods, their presence as normal colonizers in the human body, failure to isolate these bacteria as primary pathogens, and lack of negative side effects of the bacteria when fed in high levels to immunocompromised humans (premature infants, elderly, AIDS patients, Chron’s disease patients, and people with enteric infections).
However, isolated reports of association of lactobacilli (Aguirre and Collins, 1993; Saxelin et al., 1996) and bifidobacteria (Gasser, 1994) with human infection (commonly endocarditis) in patients with compromised health suggest that these microbes may have some opportunistic capability. Safety concerns were expressed regarding the use of enterococci as probiotic microbes (Adams and Marteau,1995). The association of Enterococcus faecium and Enterococcus faecalis with bacteremia and the increased incidence of antibiotic resistance in these strains provide rationale for excluding them from food formulations (Lai, 1996). Enterococci, however, are associated with some traditional food fermentations (Giraffa et al., 1997) and are currently used in some dietary supplement formulations.
In general, health-related statements have not been used in the United States on the labels of probiotic-containing food products (Sanders, 1998b). Inconspicuous mention of genus and probiotic species contained in the food is common, although this taxonomic information is not always accurate (Yeung et al., 1999). Although structure/function statements about gastrointestinal tract health, immune function, or improved digestion are used in the labeling of probiotic dietary supplements, most food manufacturers avoid them. Manufacturers avoid them although structure/function claims appear to be allowable on foods (with no requirement for the FDA disclaimer statement —that the claim has not been evaluated by FDA—or 30-day postmarket notification of FDA as required for supplements; McNamara, 1998). This caution may stem from food industry concern with the body of scientific substantiation of probiotic effects and whether it meets the “truthful and not misleading” requirement of the FDA for structure/function statements.
Product Issues
The current market in the United States for probiotics is difficult to assess (Sanders, 1998b). It is an undeveloped market, if trends in Japan and Europe are any indication. Yogurt manufacturers in the U.S. have added L. acidophilus, and in some cases Bifidobacterium species, to their products for years, and unfermented fluid milk containing probiotic bacteria comprises a small niche market. Some recent moves by U.S. food companies toward a warmer embrace of the probiotic concept are exhibited with the marketing of Lactobacillus GG as a dietary supplement (Culturelleâ) by ConAgra (Omaha, Neb.), the entry of Dannon (Tarrytown, N.Y.) into the dairy beverage market with Actimel (labeled as a dietary supplement, containing yogurt cultures and L. casei), and the addition by Stonyfield Farms (Londonderry, N.H.) of four probiotics (L. casei, Bifidobacterium, L. acidophilus and L. reuteri) to all of its yogurt products. The approach taken by Dannon is unique with U.S. foods because it uses (and claims on the label) very high populations of viable probiotic (1010 L. casei/serving). The outer wrap on the package of four 100-ml bottles reads “Helps fortify your body’s natural defenses.” This product does not, however, identify the specific strain of L. casei used. Because many dietary supplement products are formulated with strains with no proven clinical efficacy, strain identification is useful to identify responsibly formulated products.
Opportunity exists for food manufacturers to formulate a new generation of probiotic-containing products. Such an endeavor should consider:
• Choice of strain or strain combinations. This should be based on evaluations of health effects, mechanisms of action, and stability characteristics of the specific strain using validated biomarkers in in vitro, animal, and human subject studies.
• Definition of a physiologically relevant (dose which research suggests provides a beneficial health effect or reduced risk of disease) consumption levels.
--- PAGE BREAK ---
• Definition of the active principle of the probiotic product. Non-viable cells, fermentation end-products, and enzymes have been shown to mediate probiotic effects (Ouwehand and Salminen, 1998). In recognition of this fact, a definition of probiotics was recently proposed (Salminen et al., 1999), which extends the definition beyond only viable cells to include “components of microbial cells.” A clear understanding of the active component of a probiotic product is essential to development of appropriate quality control measures as well.
• Consumer communication program that discusses the basis of probiotic functionality and specifics of the probiotic product.
• Package labeling to communicate approximate numbers of viable cells at the end of shelf life, and the genus, species, and strain used. Use of a strain designation on package labeling communicates the importance of strain specificity in probiotic effects, clearly links the specific strain used to published studies, and provides a measure of protection against confusion if taxonomic developments alter the species to which a specific strain is assigned. A strain remains the same regardless of changeable taxonomic placements.
• Package labeling to communicate product health benefits. No approved health claim exists for probiotic bacteria and any health statements, such as structure/function statements, must comply with FDA regulations (McNamara, 1998; Sanders and Huis in’t Veld, 1999).
• Compatibility of probiotic with food format. Probiotic survival in foods is an important consideration in this regard. Factors involved in probiotic survival are complex—involving strain genetics, strain cultivation procedures, preservation method, preservation compounds, food composition, storage temperature, and storage time, among others. In general, probiotic survival benefits from storage at refrigeration temperatures, a fact sometimes ignored in the storage of dried probiotic preparations. The requirement for refrigerated storage of fermented dairy products encourages probiotic survival.
 Considerations for the Future
Considerations for the Future
The increased worldwide interest in probiotics has set the stage for expanded marketing of these products, even though much research remains to be done. Some research goals are shown in Table 6. Broadened clinical evaluation in both healthy and diseased human populations will do much to increase understanding of important aspects of probiotic bioactivity, including strain specificity, dose requirements and extent of clinical efficacy. One exciting area of current research is chromosome sequencing of probiotic Lactobacillus species, including L. johnsonii La1 (Zink, 1999) and L. acidophilus (Cano and Willoughby, 1999; Klaenhammer, 1998). The information gleaned from sequence data will provide opportunity to improve probiotic functionality and expand understanding of mechanisms.
The application of gene-based technologies to track the influences of probiotic consumption on gut microecology is another exciting area of research (Kitts, 1999). Dynamic models of the stomach, small intestine, and colon to simulate the in-vivo gastrointestinal environment have been developed (Marteau et al., 1997; Minekus et al., 1995) and may provide an important link between conventional in-vitro methods and in-vivo clinical studies. Advances in the understanding of genetic transfer systems and gene regulation of the lactobacilli (Klaenhammer, 1995) have enabled the construction of isogenic strains of lactobacilli that can be applied to animal or human feeding studies to determine which strain attributes are essential for probiotic effects. The use of a Lactobacillus-free mouse colony could be quite useful for such controlled studies (Tannock et al., 1988). Increased clinical evaluation is paramount, and will be most likely to succeed if well-defined probiotic bacteria are used in established clinical systems.
--- PAGE BREAK ---
The benefit of probiotics as healthful ingredients could be enhanced if used in combination with other health-promoting dietary strategies. In the United States, the growth of the probiotics market is occurring mostly in the dietary supplement arena. Whole foods, however, represent a very attractive vehicle for delivery of probiotics, although delivery through a traditional dietary supplement is convenient for some (especially clinical) applications. Probiotic bacteria have always had a natural association with dairy foods. Dairy products containing probiotics provide not only viable bacteria, but also high-quality macronutrients and unique micronutrients (such as calcium, fermentation end-products, bioactive peptides, sphingolipids, and conjugated linoleic acids) found in fermented milk products (McBean, 1998; 1999; Van der Meer et al., 1998). At the same time, the natural buffering of stomach acid by the food carrier would enhance stability of the probiotic after consumption. Formulation of food products with additional vitamins, non-digestible carbohydrates, soluble fiber, phytochemicals, or other bioactive ingredients could further enhance the dietary value of the product.
Conclusions
Studies documenting probiotic effects in humans are limited, although results in several biological systems are intriguing. The degree of evidence required to substantiate bioactivity of food ingredients is not clearly established. This is a complex issue involving both regulatory and scientific considerations. The commercial use of probiotics, however, has proceeded because essentially no risk is associated with consumption of well-defined probiotics in foods and many benefits are possible. Perhaps the most compelling evidence for probiotic efficacy is in the areas of anti-diarrheal effects and improved digestion of lactose in lactose-intolerant people, because these findings have been substantiated in human studies and in more than one laboratory. These studies, however, have focused on populations with a disease or lactase deficiency, necessitating extrapolation of results to healthy or lactase-sufficient populations.
Human data on anti-hypertensive effects, cholesterol-lowering, urogenital health, inhibition of H. pylori, and immune function are not comprehensive enough to be considered definitive, although results generated to date are sufficiently positive to warrant further investigation. Anti-cancer activity has primarily been documented in vitro and in animal models, although several lines of research, including one human study on cancer recurrence (Aso and Akazan, 1992), suggest that probiotic bacteria may be able to mediate this effect. Epidemiological studies have not been conducted but are important for assessing the effect that regular consumption of probiotic bacteria may have in generally healthy populations. Opportunity for such investigation exists in countries, such as Japan, with a relatively high density of long-time consumers of probiotic products.
Probiotics have the potential to be exciting ingredients for foods with a “healthy” image. Probiotics could be combined with other healthful ingredients or simply used to complement the natural functional attributes of whole foods. The combination of probiotic bacteria with nutrient-dense foods, such as dairy products, will have the added benefit of enhancing consumer nutrition. Consumer awareness of probiotics in the United States is minimal. Therefore, success with probiotics in the United States will require consumer education, which could also contribute to changing the common perception that “bacteria are bad.”
The vast array of health benefits attributed to probiotics may seem implausible. However, when considering that many of the effects of probiotic bacteria stem from influence on the balance or activity of the gut microflora, it is easier to comprehend the broad-reaching postulated effects. The ability of probiotic bacteria to modulate these activities is being established.
In a climate of the trend toward reduced antibiotic use, awareness of disease resulting directly from microecosystem disruption, emergence of pathogens with enhanced virulence, clinical conditions refractory to conventional treatment, and awareness that some infections lead to serious sequelae, probiotic bacteria may add a low-cost, low risk layer of protection from infection and disease.
--- PAGE BREAK ---
INSTITUTE OF FOOD TECHNOLOGEISTS
The Society for Food Science and Technology
221 N. LaSalle St., Ste. 300, Chicago, IL 60601-1291 USA
Tel. 312-782-8424
Fax: 312-782-8348
E-mail: [email protected]
URL: http://www.ift.org
This and other Scientific Status Summaries are published by the Institute of Food Technologists’ Expert Panel on Food Safety and Nutrition in Food Technology. Scientific Status Summaries, which are not necessarily written by the Expert Panel, are rigorously peer-reviewed by the Expert Panel as well as by individuals outside the panel who have specific expertise in the subject. IFT’s Expert Panel on Food Safety and Nutrition, which studies significant food-related issues and oversees timely production of Scientific Status Summaries, comprises academicians representing expertise in one or more areas of food science/technology and nutrition.
The Scientific Status Summaries may be reprinted or photocopied without permission, provided that suitable credit is given.
Acknowledgments
The author acknowledges the following individuals for their helpful comments during manuscript preparation: D. Berry, Dairy Foods Magazine (DesPlaines, Ill.); S. Bush, Rhodia, Inc. (Madison, Wis.); J. Heimbach, Environ (Arlington, Va.); L. Kam, Cedar Sanai Med. Ctr. (Los Angeles, Calif.); T. Klaenhammer, N. Carolina State Univ. (Raleigh, N.C.); L. Morelli, Instituto di Microbiologica (Piacenza, Italy); J. O’Donnell, Calif. Dairy Res. Foundation (Davis, Calif.); L. Petersen, Chr. Hansens (Milwaukee, Wis.); G. Reid, Univ. Western Ontario (London, Ontario, Canada); W. Sandine, Temecula, Calif.; G. Tannock, Univ. Otago (Dunedin, New Zealand); and R. Zink, Nestlé (Lausanne, Switzerland). The author also acknowledges the California Dairy Research Foundation for its support of probiotic-specific research in her laboratory at Cal Poly.
by MARY ELLEN SANDERS
Author Sanders, a Professional Member of IFT, is a Consultant with her company, Dairy and Food Culture Technologies, 7119 S. Glencoe Ct., Littleton, CO 80122 and Research Professor, Dairy Products Technology Center, California Polytechnic State University, San Luis Obispo, CA 93407
References
Adachi, S. 1992. Lactic acid bacteria and the control of tumours. In “The Lactic Acid Bacteria in Health and Disease,” Vol. 1, ed. B.J.B. Wood, p. 233–261, Elsevier Applied Science, London.
Adams, M. R. and Marteau, P. 1995. On the safety of lactic acid bacteria from food. Intl. J. Food Microbiol. 27: 263–264.
Aguirre, M. and Collins, M.D. 1993. Lactic acid bacteria and human clinical infection. J. Appl. Bacteriol. 75: 95–107.
Aiba, Y., Suzuki, N., Kabir, A.M.A., Takagi, A., and Koga, Y. 1998. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Amer. J. Gastroenterol. 93: 2097–2101.
Altekruse, S.F., Stern, N.J., Fields, P.I., and Swerdlow, D.L. 1999. Campylobacter jejuni–An emerging foodborne pathogen. Emerging Infect. Dis. 5: 28–35.
Anderson, J.W. and Gilliland, S.E. 1999. Effect of fermented milk (yogurt) containing Lactobacillus acidophilus L1 on serum cholesterol in hypercholesterolemic humans. J. Amer. Coll. Nutr. 18: 43–50.
Aso, Y. and Akazan, H. 1992. Prophylactic effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. Urol. Intl. 49: 125–129.
Baricault, L., Denariaz, G., Houri, J.-J, Bouley, C., Sapin, C., and Trugnan, G. 1995. Use of HT-29, a cultured human colon cancer cell line, to study the effect of fermented milks on colon cancer cell growth and differentiation. Carcinogenesis 16: 245–252.
Bellomo, G., Mangiale, A., Nicastro, L., and Frigerio, G. 1980. A controlled double-blinded study of SF68 strain as a new biological preparation for the treatment of diarhhea in pediatrics. Curr. Therapeutic Res. 28: 927-936.
Bengmark, S. 1998. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut 42: 2–7.
Bengmark, S. and Gianotti, L. 1996. Nutritional support to prevent and treat multiple organ failure. World J. Surg. 20: 474–481.
Bibel, D.J. 1988. Elie Metchnikoff’s bacillus of long life. ASM News 54: 661–665.
Bruce, A.W. and Reid, G. 1988. Intravaginal instillation of lactobacilli for prevention of recurrent urinary tract infections. Can. J. Microbiol. 34: 339–343.
Buchanan, R.L. and Doyle, M.P. 1997. Foodborne disease significance of Escherichia coli O157:H7 and other enterohemorrhagic E. coli. A Scientific Status Summary by the Institute of Food Technologists Expert Panel on Food Safety and Nutrition, Chicago, Ill., Food Technol. 51(10): 69–76.
Cano, R. and Willoughby, V. 1999. Sequencing the genome of Lactobacillus acidophilus. J. Dairy Sci. 82: 6, abstract #D101.
DeSimone, C., Vesely, R., Bianchi-Salvidori, B., and Jirillow. E. 1993. The role of probiotics in modulation of the immune system in man and in animals. Intl. J. Immunotherapy IX: 23–28.
DeSmet, I., Van Hoorde, L., De Saeyer, N., Van de Woestyne, M., and Verstraete, W. 1994. In vitro study of bile salt hydrolase (BSH) activity of BSH isogenic Lactobacillus plantarum 80 strains and estimation of cholesterol lowering through enhanced BSH activity. Microbial Ecol. Health Dis. 7: 315–329.
Dias, R.S., Bambirra, E.A., Silva, M.E., and Nicoli, J.R. 1995. Protective effect of Saccharomyces boulardii against the cholera toxin in rats. J. Med. Biolog. Res. 28: 323-325.
Dong, M.-Y., Chang, T.-W., and Gorbach, S.L. 1987. Effects of feeding lactobacillus GG on lethal irradiation in mice. Diagn. Microbiol. Infect. Dis. 7: 1–7.
Donnet-Hughes, A., Rochat, F., Serrant, P., Aeschlimann, J. M., and Schiffrin, E.J. 1999. Modulation of nonspecific mechanisms of defense by lactic acid bacteria: Effective dose. J. Dairy Sci. 82: 863–869.
Elmer, G.W., Surawicz, C.M., and McFarland, L.V. 1996. Biotherapeutic agents: A neglected modality for the treatment and prevention of selected intestinal and vaginal infections. J. Amer. Med. Assn. 275: 870–876.
Elmer, G.W., McFarland, L., and Surawicz, C.M. 1999. “Biotherapeutic Agents and Infectious Diseases,” Humana Press, Inc., Totawa, N.J.
Eyssen, H. 1973. Role of gut microflora in metabolism of lipids and sterols. Proc. Nutr. Soc. 32: 59–63.
Fonden, R., Mogensen, G., Tanaka, R., and Salminen, S. 1999. Effect of fermented dairy products on intestinal microflora, human nutrition and health. Intl. Dairy Federation Bulletin, In Press.
Fukushima, Y., Kawata, Y., Hara, H., Terada, A., and Mitsuoka, T. 1998. Effect of a probiotic formula on intestinal imunoglobulin A production in healthy children. Intl. J. Food Microbiol. 42: 39–44.
Fuller, R. 1998. Probiotics for farm animals. In “Probiotics: A Critical Review,” ed G.W. Tannock, pp. 15–22, Horizon Scientific Press, Wymondham, U.K.
Gade, J. and Thorn, P. 1989. Paraghurt for patients with irritable bowel syndrome. Scand. J. Prim. Health Care 7: 23–26.
Gasser, F. 1994. Safety of lactic acid bacteria and their occurrence in human clinical infections. Bull. Inst. Pasteur 92: 45–67.
Gilliland, S.E., Nelson, C.R., and Maxwell, C. 1985. Assimilation of cholesterol by Lactobacillus acidophilus. Appl. Environ. Microbiol. 49: 377–381.
Giraffa, G., Carminati, D., and Neviani, E. 1997. Enterococci isolated from dairy products: A review of risks and potential technological use. J. Food Protect. 60: 732–738.
Goldin, B.R., Gualtieri, L.J., and Moore, R.P. 1996. The effect of Lactobacillus GG on the initiation and promotion of DMH-induced intestinal tumors in the rat. Nutr. Cancer 25: 197–204.
Goldin, B.R., Swenson, L., Dwyer, J., Sexton, M., and Gorbach, S. 1980. Effect of diet and Lactobacillus acidophilus supplements on human fecal bacterial enzymes. J. Natl. Cancer Inst. 64: 255–261.
Hallen, A., Jarstrand, C., and Pahlson, C. 1992. Treatment of bacterial vaginosis with lactobacilli. Sex. Trans. Dis. 19: 146–148.
Hata, Y., Yamamoto, M., Ohni, M., Nakajima, K., Nakamura, Y., and Takano, T. 1996. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Amer. J. Clin. Nutr. 64: 767–771.
Henriksson, R., Franzen, L., Sandstrom, K., Nordin, A., Arevarn, M., and Grahn, E. 1995. Effects of active addition of bacterial cultures in fermented milk to patients with chronic bowel discomfort following irradiation. Support Care Cancer 3: 81–83.
Hentges, D.J. 1992. Gut flora and disease resistance. In “Probiotics: The Scientific Basis,” ed R. Fuller, pp. 87–109, Chapman and Hall, London.
Hill, M.H., Draser, B.S., Aries, V., Crowther, J.S., Hawksworth, G., and Williams, R.E.O. 1971. Bacteria and etiology of cancer of large bowel. Lancet January: 95–100.
Hillier, S.L., Krohn, M.A., Klebanoff, S.J., and Eschenbach, D.A. 1992. The relationship of hydrogen peroxide-producing lactobacilli to bacterial vaginosis and genital microflora in pregnant women. Obstetrics Gynecol. 79: 369–373.
Hillier, S.O., Nugent, R.P., Eschenbach, D.A., Krohn, M.A., Gibbs, R.S., Martin, D.H., Cotch, M.F., Edelman, R., Pastorek, J.G., Rao, A.V., NcNellis, D., Regan, J.A., Carey, J.C., and Klebanoff, M.A. 1995. Association be tween bacterial vaginosis and preterm delivery of a low-birth-weight infant. N. Engl. J. Med. 333: 1737–1742.
Hilton, E., Isenberg, H.D., Alperstein, P., France, K., and Borenstein, M.T. 1992. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann. Intern. Med. 116: 353–357.
Hirayama, K. and Rafter, J. 1999. The role of lactic acid bacteria in colon cancer prevention: Mechanistic considerations. Antonie van Leeuwenhoek 76: 391–394.
Holzapfel, W.H., Haberer, P., Snel, J., Schillinger, U., Huis in’t Veld, J.H.J.1998. Overview of gut flora and probiotics. Intl. J. Food Microbiol. 41: 85–101.
Isolauri, E., Joensuu, J., Suomalainen, H., Luomala, M., Vesikari, T. 1995. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine 13: 310–312.
Isolauri, E., Kaila, M., Arvola, T., Majamaa, H., Rantala, I., Virtanen, E., and Arvilommi, H. 1993a. Diet during rotavirus enteritis affects jejunal permeability to macromolecules in suckling rats. Pediatr. Res. 33: 548–553.
Isolauri, E., Majamaa, H., Arvola, T., Rantala, I., Virtanen, E., and Arvilommi, H. 1993b. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterol. 105: 1643–1650.
Jung, L.K. 1999. Lactobacillus GG augments the immune response to typhoid vaccination: A double-blinded, placebo-controlled study. Presented at Experimental Biology ‘99, Washington, D.C., Apr. 17-20, Abst. 659.8.
Kabir, A.M.A., Aiba, Y., Takagi, A., Kamiya, S., Miwa, T., and Koga, Y. 1997. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut 41: 49–55.
Kaila, M., Isolauri, E., Soppi, E., Virtanen, E., Saine, S., and Arvilommi, H. 1992. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human lactobacillus strain. Pediatr. Res. 32: 141–144.
Kaila, M., Isolauri, E., Saxelin, M., Arvilommi, H., and Vesikari, T. 1995. Viable versus inactivated lactobacillus strain GG in acute rotavirus diarrhoea. Arch. Dis. Child. 72: 51-53.
Kishi, A., Uno, K., Matsubara, Y., Okuda, C., and Kishida, T. 1996. Effect of the oral administration of Lactobacillus brevis subsp. coagulans on interferon a-producing capacity in humans. J. Amer. Coll. Nutr. 15: 408–412.
Kitts, C.L. 1999. 16S rDNA TRF patterns, a DNA-based method to describe bacterial communities: Applications to definition of probiotic function. J. Dairy Sci. 82: 6, abstract #D98.
Klaenhammer, T.R. 1995. Genetics of intestinal lactobacilli. Intl. Dairy J. 5: 1019–1058.
Klaenhammer, T.R. 1998. Functional activities of Lactobacillus probiotics: Genetic mandate. Intl. Dairy J. 8: 497–505.
Klaver, F.A.M. and Meer, R.V. 1993. The assumed assimilation of cholesterol by lactobacilli and Bifidobacterium is due to their bile salt-deconjugating activity. Appl. Environ. Microbiol. 59: 1120–1124.
Korschunov, V.M., Smeyanov, V.V., Efimov, B.A., Tarabrina, N.P., Ivanov, A.A., and Baranov, A.E. 1996. Therapeutic use of an antibiotic-resistant Bifidobacterium preparation in men exposed to high-dose g-irradiation. J. Med. Microbiol. 44: 70–74.
Kruis, W., Schutz, E., Fric, P., Fixa, B., Judmaier, G., and Stolte, M. 1997. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 11: 853–858.
Lai, K.K. 1996. Antibiotic-resistant enterococci and the changing face of surgical infections. Arch. Surg. 156: 338-342.
Lee, Y.-K, Nomoto, K., Salminen, S., and Gorbach, S.L. 1999. “Handbook of Probiotics,” John Wiley & Sons, Inc., N.Y.
Ling, W.H., Korpela, R., Mykkanen, H., Salminen, S., and Hanninen, O. 1994. Lactobacillus strain GG supplementation decreases colonic hydrolytic and reductive enzyme activities in healthy female adults. J. Nutr. 124: 18–23.
Link-Amster, H., Rochat, F., Saudan, K.Y., Mignot, O., and Aeschlimann, J.M. 1994. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol. Med. Microbiol. 10: 55–64.
Majamaa, H., Isolauri, E., Saxelin, M., and Vesikari, T. 1995. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J. Ped. Gastroenterol. Nutr. 20: 333–338.
Malin, M., Suomalainen, H., Saxelin, M., and Isolauri, E. 1996. Promotion of IgA immune response in patients with Crohn’s disease by oral bacteriotherapy with Lactobacillus GG. Ann. Nutr. Metab. 40: 137–145.
Marshall, B.J. 1994. Helicobacter pylori. Amer. J. Gastroenterol. 89: S116–S128.
Marteau, P. and Rambaud, J.-C. 1993. Potential of using lactic acid bacteria for therapy and immunomodulation in man. FEMS Microbiol. Rev. 12: 207–220.
Marteau, P., Minekus, M., Havenaar, R., and Huis in‘t Veld, J.H.J. 1997. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: Validation and the effect of bile. J. Dairy Sci. 80: 1031–1037.
McBean, L.D. 1998. Dairy foods: Traditional and emerging health benefits. Dairy Council Digest 69(5): 25–30.
McBean, L.D. 1999. Emerging dietary benefits of dairy foods. Nutr. Today. 34(1): 47-53.
McConnell, M.A. and Tannock, G.W. 1993. A note on lactobacilli and ß-glucuronidase activity in the intestinal contents of mice. J. Appl. Bacteriol. 74: 649–651.
McCracken, B.J. and Gaskins, H.R. 1999. Probiotics and the immune system. In “Probiotics: A Critical Review,” ed G.W. Tannock, pp. 85–111, Horizon Scientific Press, Norfolk, England.
McFarland, L.V., Surawicz, C.M., Greenberg, R.N., Fekety, R., Elmer, G.W., Moyer, K.A., Melcher, S.A., Bowen, K.E., Cox, J.L., Noorani, Z., Harrington, G., Rubin, M., and Greenwald, D. 1994. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. J. Amer. Med. Assn. 271(24): 1913–1918.
McNamara, S.H. 1998. So you want to market a food and to make health-related claims–How far can you go? What rules of law will govern the claims you want to make? Food Drug Law J. 53: 421–436.
Merger, M. and Croitoru, K. 1998. Infections in the immunopathogenesis of chronic inflammatory bowel disease. Sem. Immunol. 10: 69–78.
Michetti, P., Dorta, G., Wiesel, P.H., Brassart, D., Verdu, E., Herranz, M., Felley, C., Porta, N., Rouvet, M., Blum, A.L., and Corthesy-Theulaz, I. 1999. Effect of wheybased culture supernatant of Lactobacillus acidophilus (johnsonii) La1 on Helicobacter pylori infection in humans. Digestion. In Press.
Midolo, P.D., Lambert, J.R., Hull, R., Luo, F., and Grayson, M.L. 1995. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J. Appl. Bacteriol. 79: 475–479.
Minekus, M., Marteau, P., Havenaar, R., and Huis in’t Veld, J.H.J. 1995. A multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. Alternative To Laboratory Animals 23: 197–209.
Mital, R.K. and Garg, S.K. 1995. Anticarcinogenic, hypocholesterolemic, and antagonistic activities of Lactobacillus acidophilus. Crit. Rev. Microbiol. 21: 175–214.
Muting, D., Eschrich, W., and Mayer, J.-B. 1968. The effect of bacterium bifidum on intestinal bacterial flora and toxic protein metabolites in chronic liver disease. Amer. J. Proct. 19(5): 336–342.
Naidu, A.S., Bidlack, W.R., and Clemens, R.A. 1999. Probiotic spectra of lactic acid bacteria (LAB). CRC Crit. Rev. Food Sci. Nutr. 39: 13–126.
Nakamura, Y., Masuda, O., and Takano, T. 1996. Decrease of tissue angiotensin-I-converting enzyme activity upon feeding sour milk in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 60: 488–489.
Nakamura, Y., Yamamoto, N., Sakai, K., and Takano, T. 1995. Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin-I-converting enzyme. J. Dairy Sci. 78: 1253–1257.
Nanji, A.A., Khettry, U., and Sadrzadeh, S.M.H. 1994. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc. Soc. Exp. Biol. Med. 205: 243–247.
Oksanen, P.J., Salminen, S., Saxelin, M., Hamalainen, P., Ihantola-Vormisto, A., Muurasniemi-Isoviita, L., Nikkari, S., Oksanen, T., Porsti, I., Salminen, E., Siitonen, S., Stuckey, H., Toppila, A., and Vapaatalo, H. 1990. Prevention of travelers diarrhea by Lactobacillus GG. Ann. Med. 22: 53–56.
Orrhage, K., Brismar, B., and Nord, C.E. 1994. Effect of supplements with Bifidobacterium longum and Lactobacillus acidophilus on the intestinal microbiota during administration of clindamycin. Microbial. Ecol. Health Dis. 7: 17–25.
Ouwehand, A.C. and Salminen, S.J. 1998. The health effects of cultured milk products with viable and non-viable bacteria. Intl. Dairy J. 8: 749–756.
Parashar, U.D., Bresee, J.S., Gentsch, J.R., and Glass, R.I. 1998. Rotavirus. Emer. Infect. Dis. 4: 561–570.
Pelto, L., Salminen, S.J., and Isolauri, E. 1996. Lactobacillus GG modulates milk-induced immune inflammatory response in milk-hypersensitive adults. Nutr. Today Suppl. 31: 45S–46S.
Perdigón, G., Alvarez, S., Rachid, M., Agüero, G., and Gobbato, N. 1995. Immune system stimulation by probiotics. J. Dairy Sci. 78: 1597–1606.
Perdigón, G. and Alvarez, S. 1992. Probiotics and the immune state. In “Probiotics: The Scientific Basis.” ed R. Fuller, pp.145–179, Chapman and Hall, London.
Rafter, J.J. 1995. The role of lactic acid bacteria in colon cancer prevention. Scand. J. Gastroenterol. 30: 497–502.
Rao, C.V., Sanders, M.E., Indranie, C., Simi, B., and Reddy, B.S. 1999. Prevention of indices of colon carcinogenesis by the probiotic Lactobacillus acidophilus NCFM™ in rats. Intl. J. Oncol. 14: 939–944.
Rasic, J.L., Vujicic, I.F., Skrinjar, M., and Vulic, M. 1992. Assimilation of cholesterol by some cultures of lactic acid bacteria and bifidobacteria. Biotechnol. Lett. 14: 39–44.
Read, A. E., McCarthy, C. F., Heaton, K.W., and Laidlaw, J. 1966. Lactobacillus acidophilus (Enpac) in treatment of hepatic encephalopathy. Brit. Med. J. 1: 1267–1269.
Reddy, B. S. and Rivenson, A. 1993. Inhibitory effect of Bifidobacterium longum on colon, mammary, and liver carcinogenesis induced by 2-amino-3 methylimidazo [4,5]quinoline, a food mutagen. Canc. Res. 53: 3914–3918.
Reid, G., Bruce, A.W., and Taylor, M. 1995. Instillation of Lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infections. Microecol. Ther. 23: 32–45.
Reid, G., Bruce, A.W., and Smeianov, V. 1998. The role of lactobacilli in preventing urogenital and intestinal infections. Intl. Dairy J. 8: 555–562.
Renner, H.W. and Munzner, R. 1991. The possible role of probiotics as dietary antimutagens. Mutation Res. 262: 239–245.
Rolfe, R.D. 1995. Probiotics: Prospects for use in Clostridium difficile-associated intestinal disease. In: “Probiotics: Prospects of Use in Opportunistic Infections,” ed. R. Fuller, P.J. Heidt, V. Rusch, and D.V.D. Waaij, pp. 47–66, Institute for Microecology, Herborn, Germany.
Rossouw, J.E., Burger, E.-M., Van Der Vyver, P., and Ferreira, J.J. 1981. The effect of skim milk, yoghurt, and full cream milk on human serum lipids. Am. J. Clin. Nutr. 34: 351–356.
Rowland, I.R., Rumney, C.J., Coutts, J.T., and Lievense, L.C. 1998. Effect of Bifidobacterium longum and inulin on gut bacterial metabolism and carcinogen-induced aberrant crypt foci in rats. Carcinogenesis 19: 281–285.
Saavedra, J.M., Bauman, N.A., Oung, I., Perman, J.A., and Yolken, R.H. 1994. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 344:1046–1049.
Salminen, S. and von Wright, A. 1998. Current probiotics —safety assured? Microbial Ecol. Health Dis. 10: 68–77.
Salminen, E., Elomaa, E., Minkkinen, J., Vapaatalo, H., and Salminen, S. 1988. Preservation of intestinal integrity during radiotherapy using live Lactobacillus acidophilus cultures. Clin. Radiol. 39: 435–437.
Salminen, S., Isolauri, E., and Salminen, E. 1996. Clinical uses of probiotics for stabilizing the gut mucosal barrier: Successful strains and future challenges. Antonie von Leeuwenhoek 70: 347-358.
Salminen, S., Bouley, C., Boutron-Ruault, M.-C., Cummings, J.H., Franck, A., Gibson, G.F.R., Isolauri, E., Moreau, M.-C., Roberfroid, M., and Rowland, I. 1998. Functional food science and gastrointestinal physiology and function. Brit. J. Nutr. 80 (suppl. 1): S147–S171.
Salminen, S., Ouwehand, A., Benno, Y., and Lee, Y.K. 1999. Probiotics: How should they be defined? Trends Food Sci. Technol. 10: 107–110.
Sanders, M.E. 1998a. Overview of functional foods: Emphasis on probiotic bacteria. Intl. Dairy J. 8: 341–347.
Sanders, M.E. 1998b. Development of consumer probiotics for the U.S. market. Brit. J. Nutr. 80 (Suppl. 1): S213–S218.
Sanders, M.E. and Huis in’t Veld, J. 1999. Bringing a probiotic-containing functional food to the market: Microbiological, product, regulatory and labeling issues. Antonie van Leeuwenhoek 76: 293–315.
Sawada, H., Furushiro, M., Hirai, K., Motoike, M., Watanabe, T., and Yokokura, T. 1990. Purification and characterization of an antihypertensive compound from Lactobacillus casei. Agric. Biol. Chem. 54: 3211–3219.
Saxelin, M., Chuang, M.H., Chassy, B., Rautelin, H., Makela, P.H., Salminen, S., and Gorbach, S.L. 1996. Lactobacilli and bacteremia in Southern Finland, 1989-1992. Clin. Infect. Dis. 22: 564-566.
Schiffrin, E.J., Rochat, F., Link-Amster, H., Aeschlimann, J.M., and Donnet-Hughes, A. 1995. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J. Dairy Sci. 78: 491–497.
Shalev, E., Battino, S., Weiner, E., Colodner, R., and Keness, Y. 1996. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch. Fam. Med. 5: 593–596.
Simenhoff, M.L., Dunn, S.R., Zollner, G.P., Fitzpatrick, M.E.D., Emery, S.M., Sandine, W.E., Ayres, J.W. 1996. Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Miner. Electrolyte Metab. 22: 92–96.
Sobel, J.D. 1999. Biotherapeutic agents as therapy for vaginitis. In “Biotherapeutic Agents and Infectious Diseases Diseases,” ed. G.W. Elmer, L. McFarland, and C. Surawicz, p. 221–244, Humana Press Inc., Totowa, N.J..
Solis-Pereyra, B. and Lemonnier, D. 1993. Induction of human cytokines by bacteria used in dairy foods. Nutr. Res. 13: 1127–1140.
Solis-Pereyra, B., Aattouri, N., and Lemonnier, D. 1997. Role of food in the stimulation of cytokine production. Am. J. Clin. Nutr. 66: 521S–525S.
Spanhaak, S., Havenaar, R., and Schaafsma, G. 1998. The effect of consumption of milk fermented by Lactobacillus casei strain Shirota on the intestinal microflora and immune parameters in humans. Europ. J. Clin. Nutr. 52: 899–907.
Suarez, F.L., Savaiano, D.A., and Levitt, M.D. 1995. The treatment of lactose intolerance. Aliment. Pharmacol. Ther. 9: 589–597.
Tahri, K., Grill, J.P., and Schneider, F. 1996. Bifidobacteria strain behavior toward cholesterol: Coprecipitation with bile salts and assimilation Curr. Microbiol. 33: 187–193.
Takano, T. 1998. Milk derived peptides and hypertension reduction. Intl. Dairy J. 8: 375–381.
Tannock, G.W. 1983. Effect of dietary and environmental stress on the gastrointestinal microbiota. In “Human Intestinal Microflora in Health and Disease,” ed. D.J. Hentges, pp. 517–539, Academic Press, Inc., London.
Tannock, G.W. 1990. The microecology of lactobacilli inhabiting the gastrointestinal tract. Adv. Microb. Ecol. 11: 147–171.
Tannock, G.W. 1994. More than a smell: The complexity of the normal microflora. In “Normal Microflora. An Introduction to Microbes Inhabiting the Human Body,” ed. G.W. Tannock, pp 1–36, Chapman and Hall, London.
Tannock, G.W. 1999a. “Probiotics A Critical Review.” Horizon Scientific Press, Norfolk, England.
Tannock, G.W. 1999b. The intestinal microflora. In “Probiotics A Critical Review,” ed. G.W. Tannock, pp. 5–14, Horizon Scientific Press, Norfolk, England.
Tannock, G.W., Crichton, C., Welling, G.W., Koopman, J.P., and Midtvedt, T. 1988. Reconstitution of the gastrointestinal microflora of lactobacillus-free mice. Appl. Environ. Microbiol. 54: 2971–2975.
Targan, R. and Shanahan, F. 1994. “Inflammatory Bowel Disease from Bench to Bedside.” Williams & Wilkins, Baltimore, Md.
Taylor, G.R.J. and Williams, C.M. 1998. Effects of probiotics and prebiotics on blood lipids. Brit. J. Nutr. 80 (Suppl. 1): S225–S230.
Trapp, C.L., Chang, C.C., Halpern, G.M., Keen, C.L., and Gershwin, M.E. 1993. The influence of chronic yogurt consumption on populations of young and elderly adults. Intl. J. Immunother. IX: 53–64.
Van der Meer, R., Bovee-Oudenhoven, I.M.J., Sesink, A.L.A., and Kleibeuker, J.H. 1998. Milk products and intestinal health. Intl. Dairy J. 8: 163–170.
Vanderhoof, J.A. and Young, R.J. 1998. Use of probiotics in childhood gastrointestinal disorders. J. Pediatr. Gastroenterol. Nutr. 27: 323-332.
Venturi, M., Hambly, R.J., Glinghammar, B., Rafter, J.J., and Rowland, I.R. 1997. Genotoxic activity in human faecal water and the role of bile acids: A study using the alkaline comet assay. Carcinogenesis 18: 2353–2359.
Vollaard, E.J. and Clasener, H.A.L. 1994. Colonization resistance. Antimicrobial Agents Chemother. 38: 409–414.
Wells, C.L., Maddaus, M.A., and Simmons, R.L. 1988. Proposed mechanisms for the translocation of intestinal bacteria. Rev. Infect. Dis. 10: 958–979.
Wheeler, J.G., Shema, S.J., Bogle, M.L., Shirrell, M.A.,Burks, A.W., Pittler, A., and Helm, R. M. 1997. Immune and clinical impact of Lactobacillus acidophilus on asthma. Ann. Allergy Asthma Immunol. 19: 229–233.
Williams, G.M. and Wynder, E.L. 1996. Diet and cancer: A synopsis of causes and prevention strategies. In “Nutrition and Cancer Prevention,” ed. R.R. Watson and S.I. Mufti, pp. 1–23. CRC Press, Inc., Boca Raton, Fla.
Yeung, P.S.M., Cano, R., Tong, P.S., and Sanders, M.E. 1999. Comparison of API, 16S rDNA sequencing and fatty acid analysis as methods to speciate commercial probiotic bacteria. J. Dairy Sci. 82: 6, abstract #D22.
Zink, R. 1998. Personal communication. Nestlé, Lausanne,Switzerland.
