Evaluating the Safety and Nutritional Value of Mycoprotein
Here’s how a panel of experts evaluated the suitability of mycoprotein for food use in the United States.
The survival of human civilizations has depended on the ability of society to continuously discover and develop new sources of food. No animal, other than the rat, consumes as broad a variety of foods as the human. Humans surpass the rat because they have the ability to add further to their food resources by manipulation of the environment and modification of potential food sources.
The last half of the 20th century saw an almost logarithmic acceleration in the pace of modern biology and its application to the production of food. Genetic modification, increased knowledge of the role of foods in the etiology of disease, and the identification of new food sources has added spectacularly to the capability of society to meet the nutritional needs of a burgeoning population.
The application of new science to food production and processing has also resulted in new problems in the evaluation of their safety and wholesomeness. Traditional food safety protocols were designed for food chemicals, not foods, and are based on the assumption that feeding the test substance at multiples of the estimated exposure will increase the sensitivity of the tests and permit a conservative estimate of the risk. These approaches are not suitable for food, since feeding exaggerated levels of the food is virtually impossible. Several protocols and decision trees have been proposed, including a proposed approach in the Food and Drug Administration’s Toxicological Principles for Evaluating the Safety of Food Ingredients (FDA, 2000). While differing in detail, they share several points in common. All emphasize the need for chemical characterization of the product, and the need for in-vitro testing where appropriate, animal studies, and human exposure studies prior to general release.
Recently, a new food, mycoprotein, was introduced in products available in the United Kingdom, Belgium, Holland, Ireland, Sweden, and Switzerland. A petition for its use in the United States is under review by FDA. An expert panel reviewed the data and, using the paradigms described above, evaluated the suitability of mycoprotein for food use.
Properties and Composition
Mycoprotein is derived from the RNA-reduced cells of Fusarium venenatum (PTA 2684) (Yoder and Christianson, 1998), a mushroom like plant originally discovered growing in a field in Buckinghamshire in the U.K. The organism chosen for production was selected from more than 3,000 samples from all over the world.
In the production process, F. venenatum is grown axenically in a continuous fermentation system using food-grade carbohydrate substrates and other safe and suitable cofactors. After harvest, the mycelium is heat processed to reduce RNA content to safe levels (UN Protein Advisory Group, 1972).
The structure and composition of mycoprotein are typical of the class Fungi-Imperfecti (Table1). On the basis of dry cell weight, mycoprotein supplies about 50% protein, 13% lipid, and 25% fiber. Significant quantities of ergosterol are present, but mycoprotein does not contain cholesterol. The cell wall constitutes approximately one-third of the dry cell weight and is composed of chitin (poly N-acetyl glucosamine) and β glucans (β-1,3 and β-1,6 glucosidic linkages).
Safety Evaluation
A wide variety of safety tests have been conducted on mycoprotein (Fig. 1).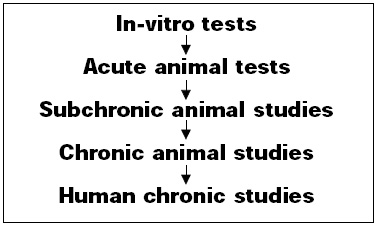
• In-Vitro Tests. The application of mutagenicity tests to a food poses special problems in the choice of test procedures. The most commonly used procedure, the Salmonella reverse mutation test, relies on the histidine dependence for growth of the test organism. The inclusion of high-protein test material would be expected to generate false positive results. As a partial solution, a modification of the protocol was developed in which the test is performed in liquid suspension. In these tests, mycoprotein was compared to chicken meat. Both mycoprotein and chicken meat tested with and without the presence of the S9 mix metabolic activation system were nonmutagenic in this test.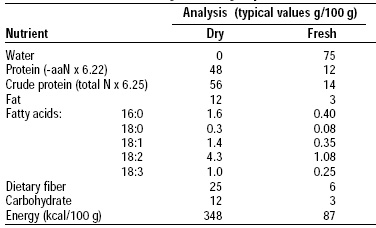
--- PAGE BREAK ---
• Acute Tests. Mycoprotein provoked no reaction when applied to the intact or abraded skin of the rabbit as a 5 or 10% aqueous suspension twice daily for 14 days. Guinea pigs showed mild erythema after similar treatment with a 10% suspension. Intradermal injections of mycoprotein induced a granulomatous response in rabbits and guinea pigs associated with the insoluble component of mycoprotein. The response was no different from that provoked by mushrooms or other food-grade microorganisms. The estrogenic potential of mycoprotein was tested in the mouse and in the Landrace pig. Neither showed any response.
• Subchronic Studies. Four subchronic studies were performed. In the first, rats were fed mycoprotein at dietary levels ranging from 26 to 52% for 22 weeks. With exception of caecal enlargement attributed to the high fiber content of the food, no significant findings were observed.
In a second study, mycoprotein in the form of a dried cooked food product was fed to rats for 13 weeks. The mycoprotein was fed at 13 and 35% of the diets and compared to a casein control. Again with the exception of caecal enlargement, no significant differences were found in growth, blood parameters, organ weights, and histopathology.
A similar study was performed with an undried form of mycoprotein. No significant differences were found in all parameters studied, including availability and balance of calcium, phosphorus, magnesium, iron, copper, and zinc.
A fourth study was performed using a similar protocol in which mycoprotein was fed at 20% and 40% of the diets for 90 days. In addition, the study included in-utero exposure and further 90-day studies in the offspring. Ophthalmoscopic examinations were included in this study.
All diets demonstrated normal growth and development. At weaning, however, both male and female pups fed the high-mycoprotein diet had lower body weights at weaning than the casein controls. While the proportional differences diminished during the 90-day feeding period, statistical differences remained. In addition, plasma cholesterol and triglycerides were lower in the rats fed mycoprotein. Liver weights were also lower. All of these effects could be attributed to the reduction in food intake observed in these animals. To test this hypothesis, the physical form of the diet was changed to improve its palatability. The differences in food intake and weight gain were abolished, confirming the hypothesis.
• Chronic Studies. Three chronic studies were used to evaluate the safety of mycoprotein. These included a two-year carcinogenicity study in rats with an in-utero phase, a one-year study in dogs, and a two-generation study in the rat with a teratology phase.
The two-year feeding study used a freeze-dried mycoprotein that was fully representative of the commercial product. The mycoprotein was fed at 41 and 21% of the diet. At 41%, all of the dietary protein was derived from mycoprotein. All other nutritional components were balanced to be nutritionally similar to a commercial diet. A casein control diet was also used. All diets supported satisfactory growth and reproduction in the F-0 generation. Sufficient litters were produced to permit the selection of the rats for the 2-year study. Although minor intergroup variations were found in some parameters, no significant adverse effects were noted in both phases of the study on growth, survival, incidence or onset of tumors or in hematological, urinary or histopathological examination (Edwards et al., 2001; Milburn et al., 2001). For example, pigmentation of various organs was more prominent in rats fed mycoprotein. This observation was considered to reflect the high levels of polyunsaturated lipids found in these diets.
A one-year study in beagle dogs fed diets containing 40 and 20% mycoprotein examined growth, clinical condition, urine, hematology, and histopathology. All diets supported good growth. No clinical signs that were diet related were reported. As in the rat, dogs fed diets containing mycoprotein showed lower plasma cholesterol and triglycerides. Females (but not males) fed mycoprotein, showed marginally greater thyroid weight. No gross or microscopic pathological findings were made that were diet related (Hodge et al., 2001).
--- PAGE BREAK ---
A multigenerational rat study was designed to determine the ability of mycoprotein to sustain normal reproduction and development. Four diets were used: mycoprotein as 50, 25, and 12.5% of the protein in the diet, and casein as 50% of the protein in the diet. A conventional commercial rat diet was used as a laboratory control.
All diets supported good growth and maturation in both parental generations and offspring. Fertility was within normal limits. No changes in reproductive function attributable to mycoprotein were observed. No microscopic or pathological findings were reported.
A series of special tests were performed to study teratogenesis and embryo toxicity in rats and rabbits. No evidence of teratogenic or embryo-toxic effects associated with mycoprotein-containing diets was observed.
• Human Chronic Studies. Four studies to assess the tolerance of humans to mycoprotein were performed. Levels of mycoprotein, ranging from 10 to 40 g/day were fed to human volunteers for periods ranging from 1 to 30 days. Subjects recording adverse responses were rechallenged under controlled conditions. In each case, the reported events did not reoccur, or the subject was shown to be atopic to another fungus. The results indicated that mycoprotein was well tolerated by humans and has an extremely low allergenic potential.
Nutrition Studies
Protein quality was evaluated using both the FDA Protein Digestibility-Corrected Amino Acid Scoring (PDCAAS) method and a human volunteer study. The amino acid pattern for mycoprotein is shown in Table 2 and its PDCAAS in comparison to other food proteins is shown in Table 3. Mycoprotein has an excellent pattern of amino acids, and its PDCAAS is 0.91, based on an estimate of 78% digestibility.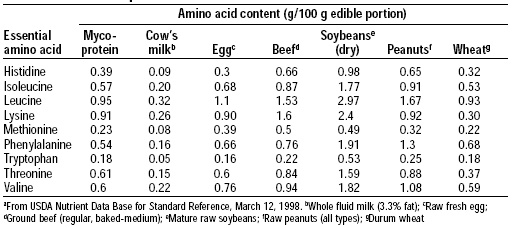
The protein quality of mycoprotein is similar to that of soy protein. When incorporated into fabricated products, the small amount of egg albumin and milk proteins used in fabrication of the product enhance the protein quality. Udall et al. (1984) confirmed the results of the animal bioassays in humans. 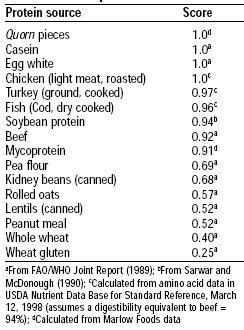
The fat content of mycoprotein is typically 13 g/100 g dry basis. As consumed, the fat content is close to 34 g/100 g. The fatty acid pattern is more like that of vegetable fat than animal fat, with a low proportion of saturated fat and a high proportion of mono- and polyunsaturated fatty acids (Table 4).
As indicated earlier, mycoprotein contains 6 g of dietary fiber/100 g. Studies performed at the Dunn Laboratories, Cambridge, UK, showed that mycoprotein does not contain any phytic acid or phytic salts and, as a result, had no significant effect on the absorption of calcium, magnesium, phosphorus, zinc, or iron.
Human Experience
Mycoprotein products have been widely consumed in the U.K. and Europe since 1985. More than 15 million people have consumed these products, more than 10 million in the UK alone. Table 5 shows the number of reported adverse reactions to mycoprotein in 1994–2000. The incidence of intolerance to mycoprotein appears to be below that reported for other foods in common use.
To estimate the probable risk associated with the consumption of a new food product, it is necessary to estimate the daily intake of the product. Based on British market data for homemade recipes and ready-to-eat meals and Pennington et al. (1983) and NLSMB (1994), the estimated daily intakes for mycoprotein are 0.01–0.18 g dry weight/kg/day for the general U.S. population and 0.24–0.46 g dry weight/kg/day for the meat avoider/vegetarian U.S. population.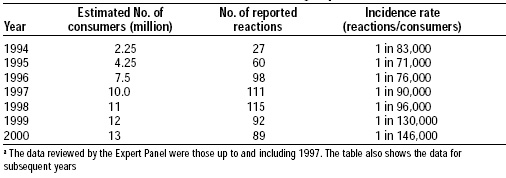
--- PAGE BREAK ---
Safe and Suitable
As a novel food, the safety evaluation of mycoprotein presents a different set of issues from those of the conventional additives for which current safety protocols were developed. Whole foods present a complex chemical matrix, in contrast to food additives that are often purified single substances. Foods also represent a significant portion of the diet and contribute to its nutritional pattern. The fact that mycoprotein may form a significant part of the diet limits the potential to apply substantial multiples of estimated exposure (typically 100-fold) to the animal models, as required in the traditional safety assessment models. As a result, the safety assessment of such foods as mycoprotein must follow a different protocol in which sources of information other than traditional feeding studies are used to expand the database and provide increased certainty to the conclusions. These include chemistry, in-vitro studies, and controlled human tests.
In designing such studies for whole foods, several issues need to be particularly considered. For example, the similarity in composition to conventional foods means that toxicity related to major nutrients or toxic substances is unlikely. Nevertheless, it is possible that some minor component or unusual interaction of nutrients may exert an adverse effect. Therefore, animal feeding studies are still required but must be carefully designed to incorporate the special needs for evaluating whole foods.
Even when all of these considerations are taken into account, minor intergroup variations in parameters such as growth or body weight can still be expected. These, in turn, may be more associated with properties of the food such as palatability rather than metabolic dysfunction.
The need for studies in human volunteers becomes more important under conditions where the traditional increased sensitivity obtained in feeding large multiples of human exposure to animals cannot be attained. Moreover, human studies are needed to estimate the potential allergenicity that may be associated with a new protein.
These considerations were applied to the evaluation of the safety of mycoprotein. An extensive database, consisting of thorough analytical data on identity and composition, animal studies, digestibility and nutritional evaluation, and human studies were used by the Expert Panel to evaluate the safety and wholesomeness of mycoprotein as an example of a novel whole food. Animal toxicology studies did not reveal any health concerns from acute or chronic exposure, including carcinogenicity. In-utero exposure studies and multigenerational reproduction studies demonstrated no adverse effect of mycoprotein on reproduction or developmental end points. Mycoprotein has been shown to provide support for adequate growth and development at dietary levels ranging up to 100% of the dietary protein. Mycoprotein was found to have high nutritional value and does not modify the absorption of trace elements. Controlled human trials and extensive marketing experience in Europe support the animal studies that show that mycoprotein is well tolerated in humans and has low allergenic potential.
On the basis of the extensive database available, the Expert Panel concluded that, to a reasonable certainty, mycoprotein is safe and suitable for incorporation into a wide variety of foods for humans (Expert Panel, 1998). At the estimated exposures, the risk should be very small and completely acceptable.
Expert Panel on Mycoprotein
Sanford A. Miller (chair), Senior Fellow, Center for Food and Nutrition Policy, and former director of the Food and Drug Administration’s Center for Food Safety and Applied Nutrition
Johanna T. Dwyer, Professor, Schools of Medicine and Nutrition Science and Policy, Tufts University and Director, Frances Stern Nutrition Center, New England Medical Center Hospital
Eric A. Johnson, Professor, Dept. of Food Microbiology and Toxicology, Food Research Institute, University of Wisconsin-Madison
Marcus Karel, Professor Emeritus of Food Science, Rutgers University, and of Chemical and Food Engineering, Massachusetts Institute of Technology
Vernon R. Young, Professor of Nutritional Biochemistry, Massachusetts Institute of Technology
by Sanford A. Miller and Johanna T. Dwyer
Author Miller is Senior Fellow, Center for Food and Nutrition Policy, 3240 Prospect St., N.W., Washington, DC 20001. Author Dwyer is Professor of Medicine and Community Health, Tufts University Schools of Medicine and Nutrition, and Director, Frances Stern Nutrition Center, New England Medical Center Hospital, Boston, MA 02111. Send reprint requests to author Miller.
Edited by Neil H. Mermelstein, Editor
References
Edwards, D.G., Kaye, A.E., Milburn, G.M., and Pigott, G.H. 2001. Development of diets for long term studies on mycoprotein. Unpublished manuscript. Marlow Foods, Stokesley, N. Yorks., UK.
Expert Panel. 1998. Mycoprotein: Report of the Expert Panel. Unpublished report. Marlow Foods, Stokesley, N. Yorks., UK.
FAO/WHO. 1989. Protein quality evaluation. Report of the Joint FAO/WHO Expert Consultation. Food and Nutrition Paper 51, Food and Agric. Org. and World Health Org., Rome, Italy.
FDA. 2000. “Toxicological Principles for Evaluating the Safety of Food Ingredients,” Office of Pre-Market Approval, Center for Food and Applied Nutrition, Food and Drug Administration, Washington, D.C.
Hodge, M.C.E., Edwards, D.G., and Pigott, G.H. 2001. Purified ingredients diets in the safety assessment of mycoprotein: A chronic toxicity study in the dog. Unpublished manuscript. Marlow Foods, Stokesley, N. Yorks., UK.
Milburn, G.M., Duffel, S.J., Edwards, D.G., and Pigott, G.H. 2001. Practical experience with purified ingredients diets in safety assessment of mycoprotein: A lifespan study with in utero phase in the rat. Unpublished manuscript. Marlow Foods, Stokesley, N. Yorks., UK.
NLSMB. 1994. “Eating in America Today,” 2nd ed. Natl. Live Stock and Meat Board, Chicago.
Pennington, J.A. 1983. Revision of the total diet study food list and diets. J. Am. Dietetic Assn. 82: 166-173.
Sarwar, G. and McDonough, F.E. 1990. Evaluation of protein digestibility-corrected amino acid score method for assessing protein quality of foods. J. Assn. Anal. Chem. 73: 347-356.
Udall, J.N., Lo, C.W., Young, V.R., and Scrimshaw, N.S. 1984. The tolerance and nutritional value of two microfungal foods in human subjects. Am. J. Clin. Nutr. 40: 285-292.
UN Protein Advisory Group. 1972. Production of single cell protein for human consumption. Guideline 12. FAO/WHO/UNICEF Advisory Group, United Nations, New York.
USDA. 1998. USDA nutrient data base for standard reference. U.S. Dept. of Agriculture Web site (www.usda.gov/), March 12.
Yoder W.T. and Christianson L.M. 1998. Species-specific primers resolve members of Fusarium section Fusarium; Taxonomic status of the edible “Quorn” fungus reevaluated. Fungal Genetics Biol. 23: 68-80.
