Functional Foods from Probiotics and Prebiotics
Combining prebiotics with probiotics to modify gastrointestinal flora has the potential to provide maximum health benefits, but proper strain selection is necessary.
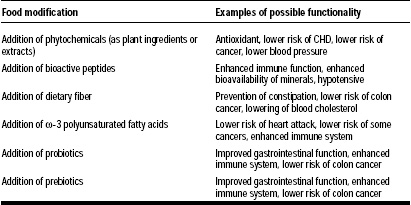
The functional food market is growing at a rate of 15–20% per year, and the industry is claimed to be worth $33 billion (Hilliam, 2000). Functional foods—also known as nutraceuticals, designer foods, medicinal foods, therapeutic foods, superfoods, foodiceuticals, and medifoods—are defined as “foods that contain some health-promoting component(s) beyond traditional nutrients” (Berner and O’Donnell, 1998). Foods can be modified by addition of phytochemicals, bioactive peptides, ω-3 polyunsaturated fatty acids, and probiotics and/or prebiotics to become “functional” (Berner and O’Donnell, 1998). Some examples are listed in Table 1.
 Since lactic acid bacteria used for the manufacture of various fermented dairy foods such as yogurt do not survive in the gastrointestinal tract, a trend today is to incorporate probiotic bacteria such as Lactobacillus acidophilus and bifidobacteria into fermented foods. Probiotic foods are defined as “foods containing live microorganisms, which actively enhance health of consumers by improving the balance of microflora in the gut when ingested live in sufficient numbers” (Fuller, 1992).
Since lactic acid bacteria used for the manufacture of various fermented dairy foods such as yogurt do not survive in the gastrointestinal tract, a trend today is to incorporate probiotic bacteria such as Lactobacillus acidophilus and bifidobacteria into fermented foods. Probiotic foods are defined as “foods containing live microorganisms, which actively enhance health of consumers by improving the balance of microflora in the gut when ingested live in sufficient numbers” (Fuller, 1992).
Traditionally, probiotics have been added to yogurt and other fermented foods, but they have also recently been incorporated into drinks, as well as marketed as supplements in the form of tablets, capsules, and freeze-dried preparations. Today there are more than 70 bifidus- and acidophilus-containing products produced worldwide, including sour cream, buttermilk, yogurt, powdered milk, and frozen desserts. More than 53 different types of milk products that contain probiotic organisms are marketed in Japan alone. Probiotics are very popular in Europe, but their use is largely restricted to yogurt (Hilliam, 2000).
Probiotic Cultures
Traditionally, yogurt is manufactured using Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus. These yogurt bacteria are claimed to provide some health benefits, but they are not natural inhabitants of the intestine and do not survive under the acidic conditions and bile concentrations usually encountered in the gastrointestinal tract. Therefore, for yogurt to be considered as a probiotic product, L. acidophilus and bifidobacteria are incorporated as a dietary adjunct. Yogurt containing these two probiotic bacteria is referred to as “AB” yogurt. A recent trend has been to incorporate Lactobacillus casei in addition to L. acidophilus and bifidobacteria; such products are known as “ABC” yogurt.
Fermented milk with only L. acidophilus and/or bifidobacteria could be manufactured, but the incubation period is long and the product quality is affected, since bifidobacteria produce appreciable amounts of acetic acid. Thus the normal practice is to make product with both yogurt and probiotic bacteria.
There are 56 species of lactobacilli and 29 species of bifidobacteria. The main probiotic organisms used worldwide are listed in Table 2. The main species believed to have probiotic characteristics are L. casei, bifidobacteria, and L. acidophilus (Krishnakumar and Gordon, 2001).
A probiotic yogurt may contain L. acidophilus only or L. acidophilus and bifidobacteria or L. acidophilus, bifidobacteria, and L. casei as probiotic organisms in addition to the two yogurt bacteria. Thus probiotic yogurts may contain up to five different groups of bacteria. Unlike yogurt bacteria, probiotic bacteria grow very slowly.
A number of issues need to be considered in developing a probiotic product:
--- PAGE BREAK ---
• Viability of Probiotic Organisms.
To realize health benefits, probiotic bacteria must be viable and available at a high concentration, typically106 cfu/g of product. Despite the importance of viability, surveys conducted to validate viability claims have shown low populations of probiotic bacteria in probiotic foods (Anonymous, 1992; Shah et al., 1995, 2000). It is questionable whether such products can provide the claimed benefits if the populations of probiotic bacteria are low.
Several factors have been claimed to be responsible for the loss of viability of probiotic organisms: acidity of products, acid produced during refrigerated storage (also known as post-acidification), level of oxygen in products, oxygen permeation through the package, sensitivity to antimicrobial substances produced by yogurt bacteria, and lack of nutrients in milk (Dave and Shah, 1997; Shah and Lankaputhra, 1997). Strategies to improve viability of probiotic organisms are discussed by Shah (2000).
• Acid and Bile Tolerance. One of the most important criteria for selection of probiotic organisms is their ability to survive in the acidic environment of the product and in the stomach, where the pH can reach as low as 1.5. Similarly, the organisms must be able to survive in the bile concentrations usually encountered in the intestine. Lankaputhra and Shah (1995) showed that, among several strains of L. acidophilus and Bifidobacterium spp. studied, only a few strains survived under the acidic conditions and bile concentrations normally encountered in fermented products and in the gastrointestinal tract. Thus it cannot be generalized that all probiotic strains are acid and bile tolerant. Clark et al. (1993) and Lankaputhra and Shah (1995) showed that Bifidobacterium longum survives better in acidic conditions and is able to tolerate a bile concentration as high as 4%. Acid and bile tolerance is strain dependent, and care should be taken to select strains on the basis of these attributes. Reports suggest that Bifidobacterium animalis (which has recently been reclassified as Bifidobacterium lactis) is commonly used in probiotic yogurt in Europe and elsewhere (Shah et al., 1995). It is an animal strain and is reported to with-stand harsh conditions, compared to other strains.
• Antagonism Among Bacteria. L. acidophilus and L. casei produce lactic acid as the main end product of fermentation. Bifidobacteria produce acetic acid and lactic acid in a molar ratio of 3:1. In addition to lactic and acetic acids, probiotic organisms produce other acids, such as citric acid and hippuric acid. Lactic acid bacteria also produce hydrogen peroxide, diacetyl, and bacteriocin as antimicrobial substances. These inhibitory substances create hostile environments for foodborne pathogens and spoilage organisms. Yogurt bacteria are reported to produce bacteriocin against probiotic bacteria and vice versa (Dave and Shah, 1997). L. acidophilus is shown to produce bacteriocin against several strains of L. delbrueckii ssp. bulgaricus, Lactobacillus helveticus, Lactobacillus jugurti, and L. casei. Yogurt may contain one or more groups of these organisms, and production of bacteriocins by L. acidophilus may therefore affect the viability of L. delbrueckii ssp. bulgaricus and L. casei. Thus, before any combination of probiotic bacteria and yogurt bacteria is used in a product, their antagonism should be checked.
• Adherence Properties. Adherence is one of the most important selection criteria for probiotic bacteria. The desired effects of probiotic microorganisms are produced only if they are able to adhere, colonize, and multiply in the intestine. The ability of probiotic bacteria to adhere to the intestine will improve their chances of winning the competition against “unfriendly bacteria” to occupy the intestinal “niches.”
Adherence to the intestinal cell wall is an important prerequisite for colonization in the gastrointestinal tract (Bernet et al., 1993; Coconnier, et al., 1992). However, thus far only a few Lactobacillus species such as Lactobacillus gasseri ADH, L. acidophilus BG2FO4 and L. casei GG have been studied for their colonization property.
--- PAGE BREAK ---
Among bifidobacteria, Bifidobacterium breve, B. longum, Bifidobacterium bifidum, and Bifidobacterium infantis have been studied (Bernet et al., 1993). However, not all probiotic bacteria adhere to the intestinal cells adequately. Only a few strains of bifidobacteria are able to adhere to intestinal cells and colonize. Most of the studies have reported adherence properties of bifidobacteria based on their adherence to Caco2 and Ht-29 cell lines. L. acidophilus strains inhabit the upper parts of the intestine, whereas bifidobacteria are found in the lower part of the intestine, particularly in the colon. In a study by Lankaputhra and Shah (1998a), only one out of 6 strains of L. acidophilus adhered properly, whereas 2 out of 9 strains of bifidobacteria showed good adherence properties. In general, Bifidobacterium spp. adhered better than L. acidophilus. B. infantis and B. longum showed the best adherence properties.
• Proteolytic Activity. Yogurt has been traditionally made using Streptococcus thermophilus and L. delbrueckii ssp. bulgaricus as a starter culture. L. delbrueckii ssp. bulgaricus produces essential amino acids owing to its proteolytic nature, and the symbiotic relationship of L. delbrueckii ssp. bulgaricus and S. thermophilus is well established, the former producing amino nitrogen for the latter. These two bacteria grow rapidly.
On the other hand, L. acidophilus and bifidobacteria grow slowly in milk because of a lack of proteolytic activity, so the usual practice is to add yogurt bacteria to probiotic products to reduce the fermentation time. With probiotic bacteria only, the fermentation time could be as long as 24 hr, compared to approximately 4 hr with yogurt bacteria (Dave and Shah, 1997). As a result, yogurt bacteria are used as the main starter culture and probiotic bacteria are added as an adjunct starter culture.
Because of the slow-growing nature of probiotic organisms, L. delbrueckii ssp. bulgaricus and S. thermophilus out-compete L. acidophilus and bifidobacteria, causing loss of viability of the latter organisms (Dave and Shah, 1998). Shihata and Shah (2000) studied the proteolytic profiles of several strains of S. thermophilus, L. delbrueckii ssp. bulgaricus, L. acidophilus and bifidobacteria and reported that S. thermophilus and L. delbrueckii spp. bulgaricus showed higher proteolytic activity than probiotic bacteria. One out of 7 strains of L. acidophilus and one out of 14 strains of bifidobacteria showed some proteolytic activity. Thus it is important to select strains based on their proteolytic activity. Proteolytic enzymes are required to degrade milk casein to oligopeptides, which are degraded by peptidases to peptides and amino acids. Milk does not contain sufficient free amino acids and peptides to allow growth of lactic acid bacteria, particularly fastidious organisms such as L. acidophilus and bifi-dobacteria.
• β-D-Galactosidase Activity. Yogurt bacteria produce higher β-galactosidase activity than probiotic bacteria (Shah and Jelen, 1990; Shah, 1994).
Proteolytic activity and β-D-galactosidase activity explain why yogurt bacteria grow faster than probiotic bacteria and why yogurt bacteria are used as the main starter bacteria and probiotic bacteria as an adjunct starter.
Beneficial Effects of Probiotics
There is sufficient evidence to support the view that oral administration of lactobacilli and bifidobacteria is able to restore the normal balance of microbial populations in the intestine. In addition to their established role in gastrointestinal therapy, the probiotic organisms are claimed to offer several nutritional and therapeutic benefits. Probiotics have been successfully employed to treat antibiotic-associated diarrhea. They also have various other functional properties:
• Antimicrobial Properties. With the emergence of antibiotic-resistant bacteria and natural ways of suppressing pathogens, the concept of probiotics has attracted much attention. The belief in the beneficial effects of probiotics is based on the knowledge that the intestinal microflora provides protection against various diseases. Probiotic bacteria produce organic acids such as lactic and acetic acids, hydrogen peroxide, and bacteriocins. Lactic and acetic acids account for more than 90% of the acids produced. Other acids produced in small quantities include citric, hippuric, orotic, and uric (Lankaputhra and Shah, 1998a). Lowering of pH due to lactic acid or acetic acid produced by these bacteria in the gut has a bactericidal or bacteriostatic effect.
--- PAGE BREAK ---
The antimicrobial substances suppress the multiplication of pathogenic and putrefying bacteria. Because of these virtues, probiotic bacteria show strong antimicrobial properties against Gram-positive bacteria such as Staphylococcus aureus and Clostridium perfringens rather than against Gram-negative bacteria such as Salmonella typhimurium and Escherichia coli. Hydrogen peroxide in the presence of organic acids such as lactic acid is more inhibitory to bacteria than hydrogen peroxide or lactic acid alone (Lankaputhra and Shah, 1998b).
Two types of lactic acid, L(+) and D(-), are produced during fermentation by lactic acid bacteria. Some species of bacteria such as L. delbrueckii ssp. bulgaricus and Lactococcus lactis produce only D(-) lactic acid, whereas some lactic streptococci and L. casei produce L(+) lactic acid. L. helveticus and L. acidophilus produce a racemic mixture of L(+) and D(-) lactic acid. D(-) lactic acid is not metabolized to pyruvic acid in the body because of a lack of D2-hydroxy acid dehydrogenase, and this results in acidosis in neonatal infants. L(+) isomer is completely harmless. Bifidobacteria and L. casei produce L(+) lactic acid. Thus the lactic acid produced by bifidobacteria and L. casei is easily metabolized, while providing antimicrobial properties (Shah, 1999).
• Antimutagenic Properties. Studies have shown a negative correlation between the incidence of certain cancers and consumption of fermented milk products (Orrhage et al., 1994). Antimutagenic activity of fermented milk has been demonstrated in vitro against a large spectrum of mutagens, including 4-nitroquinoline-N'-oxide, 2-nitrofluorene, and benzopyrene, and against a range of mutagens and promutagens in various test systems based on microbial and mammalian cells (Ayebo et al., 1982). The mechanism of antimutagenicity of probiotic bacteria has not been identified so far and remains speculative. It has been suggested that microbial binding of mutagens could be the possible mechanism (Orrhage et al., 1994).
Lankaputhra and Shah (1998b) studied the antimutagenic activity of acetic, lactic, pyruvic, and butyric acids against eight mutagens and promutagens using the Ames Salmonella test. While acetic acid showed higher antimutagenic activity than lactic or pyruvic acids, butyric acid showed a broadspectrum antimutagenic activity against all mutagens or promutagens studied. Butyric acid is claimed to prevent carcinogenic effects at the molecular (DNA) level (Smith, 1995).
Lankaputhra and Shah (1998b) also reported that live bacterial cells showed higher antimutagenicity than killed cells against the mutagens studied. This suggests that live bacterial cells may metabolize or bind the mutagens. Inhibition of mutagens and promutagens by probiotic bacteria appeared to be permanent for live cells and temporary for killed cells. Killed cells released mutagens and promutagens when extracted with di-methyl sulfoxide. The results emphasized the importance of consuming live probiotic bacteria and of maintaining their viability in the intestine to provide efficient inhibition of mutagens.
• Anticarcinogenic Properties. Lactic acid bacteria and fermented products made from them have potential anticarcinogenic activity (Mitsuoka, 1989). B. longum and B. infantis are effective antitumor agents. Their mode of action may be by suppression of bacterial enzymes, activation of the host immune system, and reduction of intestinal pH. The antitumor effect of L. acidophilus was reported by Goldin and Gorbach (1977, 1984). Oral dietary supplements containing viable cells of L. acidophilus decreased β-glucuronidase, azoreductase, and nitroreductase, bacterial enzymes which catalyze conversion of procarcinogens to carcinogens. Anticarcinogenic effects of L. acidophilus or bifidobacteria may be due to direct removal of procarcinogens and activation of the body’s immune system
Direct removal of procarcinogens by probiotic bacteria might involve a reduction in the rate at which nitrosamines are produced. Probiotic bacteria may remove the sources of procarcinogens or the enzymes that lead to the formation of carcinogens. It has been shown that probiotic bacteria can greatly reduce the mutagenicity of nitrosamines (Mitsuoka, 1989). This may be due to the fact that certain species of probiotic bacteria, such as B. breve, have high absorbing properties for carcinogens, such as those produced upon charring of meat products.
--- PAGE BREAK ---
• Improvement in Lactose Metabolism. Lactose malabsorption is a condition in which lactose, the principal carbohydrate of milk, is not completely digested into its component monosaccharides, glucose and galactose. Since lactose is cleaved into its constituent monosaccharides by β-D-galactosidase, lactose malabsorption results from a deficiency of this enzyme. Lactose malabsorbers often complain of “gastric distress” after consuming fresh, unfermented milk or milk products (Onwulata et al., 1989).
The traditional cultures used in making yogurt—L. delbrueckii ssp. bulgaricus and S. thermophilus—contain substantial quantities of β-D-galactosidase, and it has been suggested that consumption of yogurt may assist in alleviating the symptoms of lactose malabsorption. Several studies have reported that yogurt or probiotic yogurt is tolerated well by lactose malabsorbers; however, the mechanism is not very clear. Some lactose is hydrolyzed by yogurt bacteria during fermentation.
Another reason for better tolerance could be that the bacterial enzyme autodigests lactose intracellularly before reaching the intestine, and a third reason could be slower oral–caecal transit time. Gastric emptying and intestinal transit time play an important role in improved lactose tolerance. Viscous foods such as yogurt or foods with higher solids content may delay gastric emptying and thus may be effective in alleviating lactose-intolerance symptoms. As a result, fermented acidophilus milk may be better tolerated than sweet acidophilus milk, since coagulated milk, because of its viscous nature, may pass more slowly through the gut than unfermented milk (Shah et al., 1992).
Although there are limited studies on the efficacy of bifidus products in management of lactose malabsorption, the effects of acidophilus milk in alleviation of lactose malabsorption have been thoroughly researched (Onwulata et al., 1989).
• Reduction in Serum Cholesterol. Studies have shown that consuming certain cultured dairy products can help reduce serum cholesterol level. Feeding of fermented milks containing very large numbers of probiotic bacteria (~109/g) to hypercholesterolemic human subjects has lowered cholesterol levels from 3.0 g/L to 1.5 (Homma, 1988). The role of bifidobacteria in reducing the serum cholesterol is not completely understood.
Mann and Spoerry (1974) observed a decrease in serum cholesterol levels in men fed large quantities of milk fermented with Lactobacillus. This may have been due to the production of hydroxymethyl glutarate by lactic acid bacteria, which inhibit hydroxymethylglutaryl-CoA reductases required for the synthesis of cholesterol. Rao et al. (1981) reported that metabolites from orotic acid formed during fermentation of dairy products may help lower cholesterol level. According to Jaspers et al. (1984), uric acid inhibits cholesterol synthesis and orotic acid and hydroxymethyl glutamic acid reduce serum cholesterol.
However, the role of bifidobacteria in lowering cholesterol is still debated. Klaver and Meer (1993) reported that removal of cholesterol from the culture medium by L. acidophilus and other species is due not to bacterial uptake of cholesterol but to the deconjugation of bile acid by L. acidophilus. The deconjugated bile acid does not absorb lipid as readily as the conjugated counterpart, leading to a reduction in cholesterol level.
L. acidophilus and bifidobacteria actively assimilate cholesterol and other organic acids. Gilliland et al. (1985) showed that L. acidophilus itself may take up cholesterol during growth in the small intestine and make it unavailable for absorption into the bloodstream. The effects of lactic acid bacteria on cholesterol levels are therefore inconsistent and range from a significant reduction to no reduction. The exact mechanism remains unknown.
• Immune System Stimulation. Immunomodulation by L. acidophilus and bifidobacteria has been observed (Schiffrin et al., 1995), but the mechanism is not clearly understood. Translocation of a small number of ingested bacteria via M cells to the Payer’s patches of the gut-associated lymphoid tissue in the small intestine is claimed to be responsible for enhancing immunity. Ingestion of probiotic yogurt has been reported to stimulate cytokine production in blood cells (Solis and Lemonnier, 1996) and enhance the activity of macrophages (Marteau et al., 1997).
--- PAGE BREAK ---
Prebiotics and Synbiotics
The beneficial effects of the presence of bifidobacteria in the gastrointestinal tract are dependent on their viability and metabolic activity. Their growth is dependent on the presence of complex carbohydrates known as oligosaccharides. Certain oligosaccharides are considered prebiotics, which are defined as “non-digestible food that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon” (Gibson and Roberfroid, 1995). To maximize effectiveness of bifidus products, prebiotics are used in probiotic foods.
Some oligosaccharides, because of their chemical structure, are resistant to digestive enzymes and therefore pass into the large intestine, where they become available for fermentation by saccharolytic bacteria. Compounds which are either partially degraded or not degraded by the host and are preferentially utilized by bifidobacteria as a carbon and energy source are referred to as “bifidogenic factors.” Some bifidogenic factors of commercial significance include fructooligosaccharides, lactose derivatives such as lactulose, lactitol, galactooligosaccharides, and soybean oligosaccharides (O’Sullivan, 1996). Resistant starch and non-starch oligosaccharides are classified as colonic foods, but not as prebiotics because they are not metabolized by certain beneficial bacteria (O’Sullivan, 1996).
Djouzi and Andrieux (1997) studied the effects of three oligosaccharides on metabolism of intestinal microflora in germ-free rats inoculated with human faecal flora. Fructooligosaccharides and galactooligosaccharides increased the bifidobacteria number by 2 log cycles.
The majority of yogurts marketed in Australia, the United States, and Europe in recent years contain probiotic bacteria and some form of prebiotics. Such combination products are referred to as “synbiotics.” Japan is the world leader in probiotic and prebiotic products.
In conclusion, there is mounting evidence of health benefits for probiotics, and the market trend is to develop product formulations that combine these health benefits with product appeal and versatility. Probiotics will be seen in many different markets beyond what is known today.
by Nagendra P. Shah
The author, a Professional Member of IFT, is Associate Professor of Food Science, School of Life Sciences and Technology, Victoria University of Technology, P.O. Box 14428, Melbourne City Mail Center, Victoria 8001, Australia.
Edited by Neil H. Mermelstein,
Editor
References
Anonymous. 1992. Yogurt and probiotics. Choice 11:
Ayebo, A.D., Shahani, K.M., Dam, R., and Friend, B.A. 1982. Ion exchange separation of the antitumor components in yogurt dialyzate. J. Dairy Sci. 65: 2388-2390.
Berner, L. and O’Donnell, J. 1998. Functional foods and health claims legislation: Applications to dairy foods. Intl. Dairy J. 8: 355-362.
Bernet, F.M., Brassart, D., Neeser, J.R., and Servin, A. 1993. Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interaction. Appl. Environ. Microbiol. 59: 4121-4128.
Clark, P.A., Cotton, L.N., and Martin, J.H. 1993. Selection of bifidobacteria for use as dietary adjuncts in cultured dairy foods: II. Tolerance to simulated pH of human stomachs. Cult. Dairy Prod. J. 28: 11-14.
Coconnier, M.H., Klaenhammer, T.R., Kerneis, S., Bernet, M.F., and Servin, A.L. 1992. Protein mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus secreting cell lines in culture. Appl. Environ. Microbiol. 58: 2034-2039.
Dave, R.I. and Shah, N.P. 1997. Viability of yogurt and probiotic bacteria in yogurts made from commercial starter culture. Intl. Dairy J. 7: 31-41.
Dave, R.I. and Shah, N.P. 1998. Ingredients supplementation effects on viability of probiotic bacteria in yogurt. J. Dairy Sci. 81: 2804-2816.
Djouzi, Z., and Andrieux, C. 1997. Compared effects of three oligosaccharides on metabolism of intestinal microflora in rats inoculated with human faecal flora. Brit. J. Nutr. 78: 313-324.
Fuller, R. 1992. “Probiotics—The Scientific Basis.” Chapman and Hall, London.
Gibson, G.R. and Roberfroid, M. 1995. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 125: 1401-1412.
Gilliland, S.E., Nelson, C.R., and Maxwell, C. 1985. Assimilation of cholesterol by Lactobacillus acidophilus. Appl. Environ. Microbiol. 49: 377-381.
Goldin, B.R. and Gorbach, S.L. 1977. Alterations in faecal microflora enzymes related to diet, age, lactobacillus supplement and dimethyl hydrazine. Cancer 40: 2421-2426.
Goldin, B.R. and Gorbach, S.L. 1984. The effect of milk and lactobacillus feeding on human intestinal bacterial enzyme activity. Am. J. Clin. Nutr. 39: 756-761.
Hilliam, M. 2000. Functional food: How big is the market? World of Food Ingredients 12: 50-53.
Homma, N. 1988. Bifidobacteria as a resistance factor in human beings. Bifidobacteria Microflora 7: 35-43.
Jaspers, D.A., Massey, L.K., and Leudecke, L.O. 1984. Effect of consuming yogurts prepared with three culture strains on human serum lipoproteins. J. Food Sci. 49: 1178-1181.
Klaver, F.A.M. and Meer, R.V.D. 1993. The assumed assimilation of cholesterol by lactobacilli and Bifidobacterium bifidum is due to their bile salt deconjugating activity. Appl. Environ. Microbiol. 59: 1120-1124.
Krishnakumar, V. and Gordon, I.R. 2001. Probiotics: Challenges and opportunities. Dairy Ind. Intl. 66(2): 38-40.
Lankaputhra, W.E.V. and Shah, N.P. 1995. Survival of L. acidophilus and Bifidobacterium spp. in the presence of acid and bile salts. Cult. Dairy Prod. J. 30: 2-7.
Lankaputhra, W.E.V. and Shah, N.P. 1998a. Adherence of probiotic bacteria to human colonic cells. Biosci. Microflora 17: 105-113.
Lankaputhra, W.E.V. and Shah, N.P. 1998b. Antimutagenic properties of probiotic bacteria and of organic acids. Mutation Res. 397: 169-182.
Mann, G.V. and Spoerry, A. 1974. Studies of a surfactant and cholesterolemia in the Messai. Am. J. Clin. Nutr. 27: 464-469.
Marteau, P., Vaerman, J.P., Bord, J.P., Brassart, D., Pochart, P., Desjeux, J.F., and Rambaud, J.C. 1997. Effects of intrajejunal perfusion and chronic ingestion of Lactobacillus johnsonii strain LA1 on serum concentrations and jejunal secretions of immunoglobulins and serum proteins in healthy humans. Gastroenterol. Clin. Biol. 21: 293-298.
Mitsuoka, T. 1989. Microbes in the intestine. Yakult Honsha Co. Ltd., Yokyo, Japan.
Onwulata, C.I., Ramkishan Rao, D., and Vankineni, P. 1989. Relative efficiency of yogurt, sweet acidophilus milk, hydrolyzed-lactose milk, and a commercial lactase tablet in alleviating lactose maldigestion. Am. J. Clin. Nutr. 49: 1233-1237.
Orrhage, K., Sillerstrom, E., Gustafsson, J.A., Nord, C.E., and. Rafter, J. 1994. Binding of mutagenic heterocyclic amines by intestinal and lactic acid bacteria. Mutation Res. 311: 239-248.
O’Sullivan, M.G. 1996. Metabolism of bifidogenic factors by gut flora—An overview. IDF Bull. 313, p. 23. Internationl Dairy Federation, Brussels, Belgium.
Rao, D.R., Chawan, C.B., and. Pulusani, S.R. 1981. Influence of milk and thermophilus milk on plasma cholesterol levels and hepatic cholesterogenesis in rats. J. Food Sci. 46: 1339-1341.
Savaiano, D.A., ElAnouar, A.A., Smith, D.J., and Levitt, M.D. 1984. Lactose malabsorption from yogurt, pasteurized yogurt, sweet acidophilus milk, and cultured milk in lactase-deficient individuals. Am. J. Clin. Nutr. 40: 1219.
Schiffrin, E.J., Rochat, F., Link-Amster, H., and Aeschlimann, J.M. 1994. REMS Immunol. Med. Microbiol. 10: 55-64.
Shah. N.P. 1994. Lactobacillus acidophilus and lactose intolerance: A review. ASEAN Food J. 9: 47-54.
Shah, N.P. 1999. Probiotic bacteria: Antimicrobial and antimutagenic properties. Probiotica 6: 1-3.
Shah, N.P. 2000. Probiotic bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci. 83: 894-907.
Shah, N.P. and Jelen, P. 1990. Survival of lactic acid bacteria and their lactases under acidic conditions. J. Food Sci. 55: 506-509.
Shah, N.P. and Lankaputhra, W.E.V. 1997. Improving viability of L. acidophilus and Bifidobacterium spp. in yogurt. Intl. Dairy J. 7: 349-356.
Shah, N.P., Fedorak, R.N., and Jelen, P. 1992. Food consistency effects of quarg in lactose absorption by lactose intolerant individuals. Intl. Dairy J. 2: 257-269.
Shah, N.P., Ali, J.F., and Ravula, R.K. 2000. Populations of L. acidophilus, Bifidobacterium spp., and Lactobacillus casei in commercial fermented milk products. Biosci. Microflora 19(1): 35-39.
Shah, N.P., Lankaputhra, W.E.V., Britz, M., and Kyle, W.S.A. 1995. Survival of L. acidophilus and Bifidobacterium bifidum in commercial yogurt during refrigerated storage. Intl. Dairy J. 5: 515-521.
Shihata, A. and Shah, N.P. 2000. Proteolytic profiles of yogurt and probiotic bacteria. Intl. Dairy J. 10: 401-408.
Solis-Pereira and Lemonnier, D. 1996. Induction of human cytokines by bacteria used in dairy foods. Nutr. Res. 13: 1127-1140.
Smith, J.G. 1995. Molecular and genetic effects of dietary derived butyric acid. Food Technol. 49(11): 87-90.
