Parasites and the Food Supply
This Scientific Status Summary, prepared for the Institute of Food Technologists’ Expert Panel on Food Safety and Nutrition, discusses the sources and incidence of human infection by foodborne parasites and the new technologies that are being developed for their prevention, detection, and inactivation.
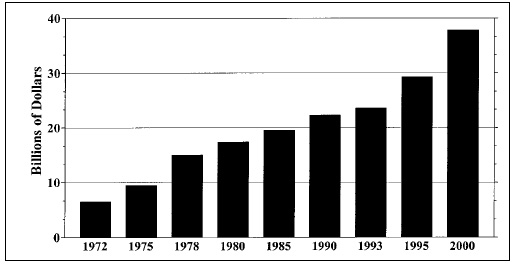 Patterns of travel, trade in foods and food consumption have
changed, exposing consumers to pathogens—including
parasitic animals— not previously encountered. The globalization
of America’s food supply increased substantially during the 1990s
(Fig. 1). Correspondingly, so did the risk to American consumers of
acquiring a foodborne parasite. In 1990, about 13 species of parasitic
animals were of concern to food scientists in the United States (Jackson,
1990). Today, that figure has multiplied by more than a factor of 8.
Patterns of travel, trade in foods and food consumption have
changed, exposing consumers to pathogens—including
parasitic animals— not previously encountered. The globalization
of America’s food supply increased substantially during the 1990s
(Fig. 1). Correspondingly, so did the risk to American consumers of
acquiring a foodborne parasite. In 1990, about 13 species of parasitic
animals were of concern to food scientists in the United States (Jackson,
1990). Today, that figure has multiplied by more than a factor of 8.
In the past, the risk of human infection with parasites was considered to be limited to distinct geographic regions because of parasites’ adaptations to specific definitive hosts, select intermediate hosts and particular environmental conditions. These barriers are slowly being breeched—first by international travel developing into a major industry, and then by rapid, refrigerated food transport which became available to an unprecedented degree at the end of the 20th century.
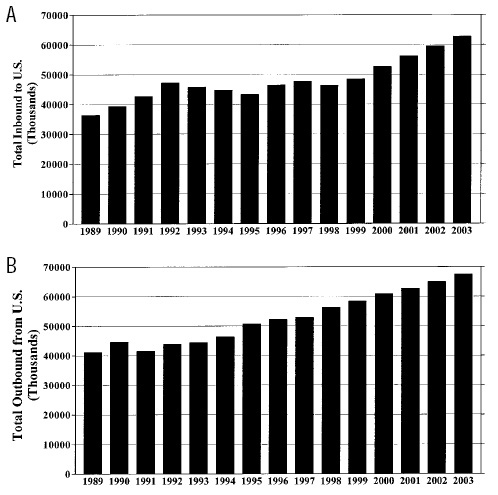 Figs. 2A and B trace the numbers of international travelers to and from the U.S. over the past decade and provide further projections for international travel through 2003. International travel and population migration are the primary mechanisms by which immunologically naive (previously unexposed) consumers may come into contact with emerging parasites.
Figs. 2A and B trace the numbers of international travelers to and from the U.S. over the past decade and provide further projections for international travel through 2003. International travel and population migration are the primary mechanisms by which immunologically naive (previously unexposed) consumers may come into contact with emerging parasites.
Another factor to consider is the rapid transport of foreign food products to the U.S., which has further enhanced the chances that parasites come into contact with consumers. Now, produce picked and seafood harvested or caught early in the week, thousands of miles from our borders, can be consumed fresh in America’s heartlands before the weekend. Moreover, cultural habits have shifted toward the consumption of fresh, i.e., raw and undercooked, foods that bypass important preparatory measures intended to reduce or prevent infections by pathogens—especially the long-surviving encysted forms of foodborne parasites.
Food parasitology is an emerging discipline. Although its beginnings coincided with the beginnings of microscopy more than 300 years ago (Dobell, 1920), few of its testing methods are as standardized as those of food bacteriology. Reasons for the underdeveloped state of this science include our inability to readily culture most parasites (Smyth, 1990; Taylor and Baker, 1978) and, for forms encysted on or within plant or animal tissues, our inability to design seeding experiments that are equivalent to working with natural samples.
Frequently, parasitology in its entirety is relegated to the status of a sub-specialty in between microbiology and zoology. Moreover, pathogenic parasites of humans are sometimes considered only in the context of tropical medicine, despite mounting evidence of their prevalence in temperate and arctic climates.
Domestically, for a multitude of reasons, parasitic infections are often not routinely considered as a source of illness when symptoms similar to bacterial infection present themselves. Therefore,many instances of parasite-related illness go undiagnosed, which may lead to a skewed reporting of the incidences of parasitic illness. As a consequence, those parasites that are now considered emerging parasitic pathogens may in fact have been a continual source of human illness in the absence of any clinical recognition.
--- PAGE BREAK ---
In the context of foods and parasitic animals, then, there is a need for increased awareness of the impact of parasites on the food supply. This includes recognition of parasites’ potential effect on public health and health care issues as well as sensitivity to economic consequences such as worker productivity and agricultural losses. In comparison to other classes of foodborne pathogens, particularly bacteria, the impact of parasites is difficult to assess primarily due to the lack of a uniform standard for monitoring the incidence of foodborne illness directly attributed to parasitic infection. As such, statistical data relating parasitic foodborne illness and economic sequelae are either approximations or lacking.
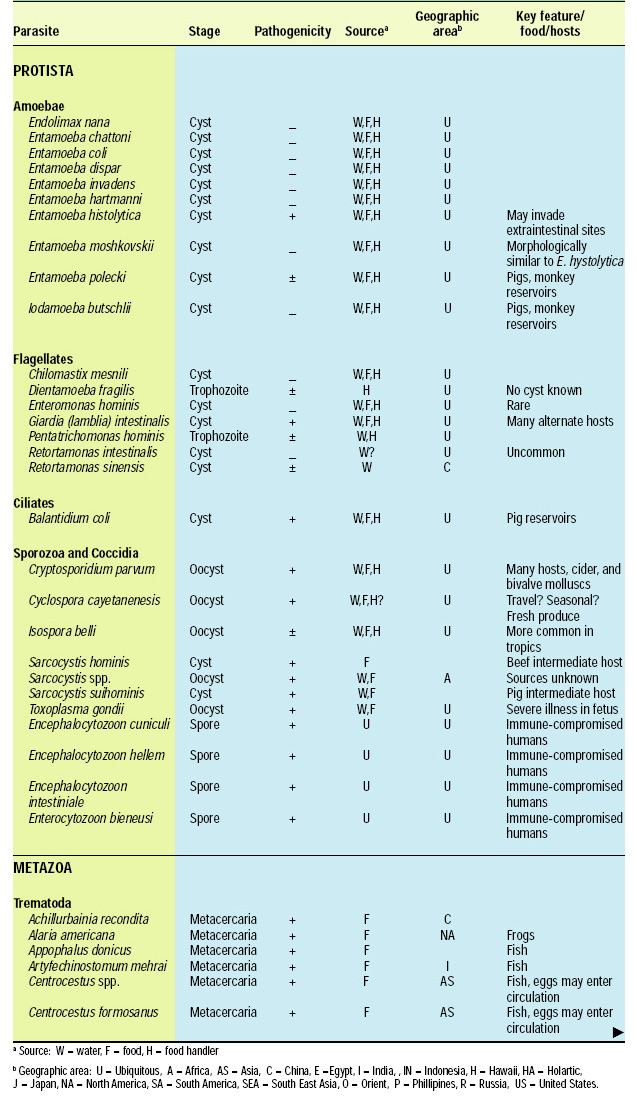
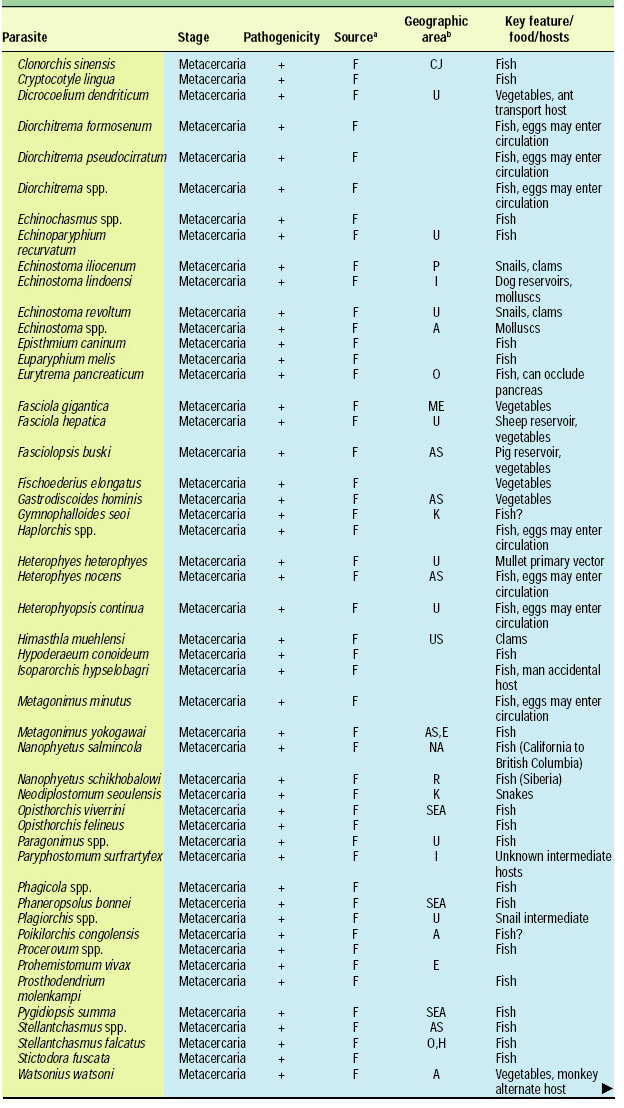
 Scope of the Problem
Scope of the Problem
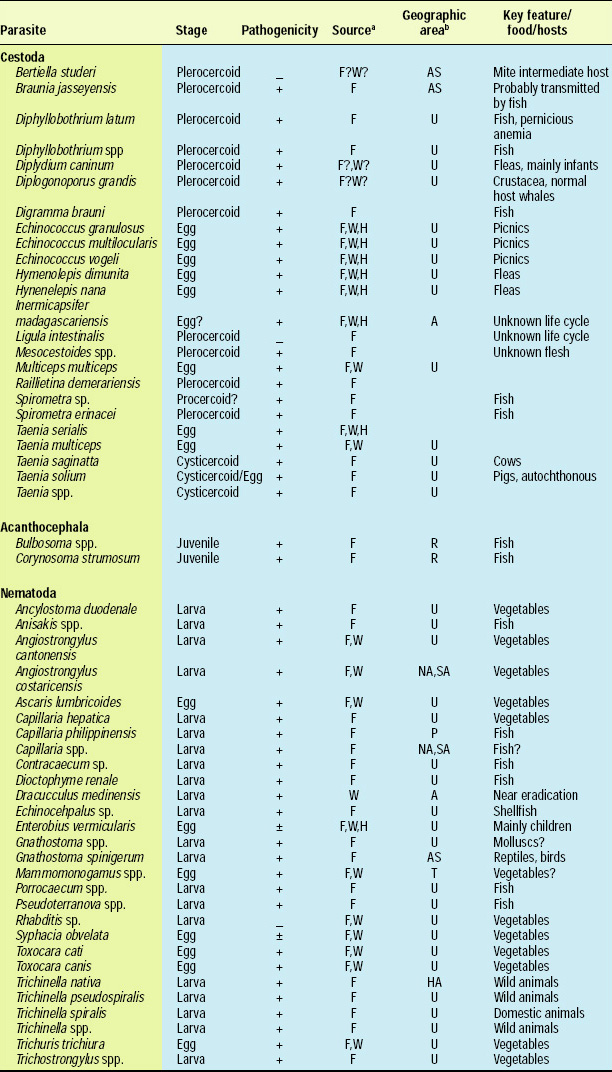
There are about 107 known species of parasitic animals that can be foodborne (Table 1). While not all species are reported to infest domestic food sources or infect consumers in the U.S. and its territories, the likelihood of this possibility has significantly increased in recent years with the emergence of a truly global market place. Planetary statistics on foodborne illnesses due to parasitic infections have been difficult to estimate. Norman R. Stoll’s classic “This Wormy World” (Stoll, 1947) estimated that in the global population of 2.2 billion people, there were 664 million Ascaris lumbricoides infections (30% prevalence) and 355 million infections with Trichuris trichiura (16%) compared to the update by Michael et al. (1997) which estimated 1273 million (24%) and 902 million (17%) infections 50 years later when the human population was 5.6 billion. What percentage of these cases is foodborne has not been determined; however, their overall impact as demonstrated by substantial incidences of foodborne trematode infections (Opistorchis, Clonorchis) in Southeast Asia and the Pacific region (WHO, 1995) underscores the need for increased awareness of this class of human pathogens. Particularly with the consumption of raw or undercooked seafood and the advent of a global market place, the need for precautions in processing methods and consumption habits is essential to prevent the spread of foodborne parasitic infections. This awareness also applies to food-related illnesses caused by gastrointestinal protozoa, although statistics comparable to those for worms are not available.
In the U.S., the latest survey of foodborne illnesses by the Centers for Disease Control and Prevention estimates that there are 2.5 million cases annually due to food- and beverage-borne parasites (Mead et al., 1999). This is approximately 7% of the annual food- and beverage-borne disease incidence caused by known infectious agents—fewer than the 13% caused by bacteria and the 80% caused by viruses. However, the parasite Toxoplasma gondii, a coccidian protozoa, is responsible for 20.7% of foodborne deaths due to known infectious agents. The flagellated protozoa, Giardia (lamblia) intestinalis, causes the greatest number of parasite-related disease cases, with an estimated 2,000,000 illnesses annually, equaling 1.4% of the food- and beverage-borne total for known pathogenic agents. Cryptosporidium parvum is reported to cause 30,000 cases (0.2%) and the recently recognized Cyclospora cayetanensis caused 14,638 cases (0.1%), due primarily to imported fresh produce. Although it is difficult to distinguish foodborne from waterborne illnesses attributed to these species, their impact on food safety and public health both nationally and internationally appears to be significant (Käferstein, 2000).
--- PAGE BREAK ---
The relationship between enteric parasitic protozoa, the environment, contamination of food, and human illness is extremely complex. Environmental factors play a significant role in the transmission of most foodborne parasitic diseases. This impact is particularly apparent with protozoa, which are readily transported to food by contaminated water (Slifko et al., 2000a). Fecal contamination of water sources used in crop irrigation, food processing and meal preparation are important sources of human infection. In this regard, contamination of fresh fruits and vegetables is causing the greatest concern. These commodities are intimately influenced by the environment and agricultural practices, and often receive no processing that is lethal to protozoa.
The deployment of more efficient and rapid means of transporting perishable goods worldwide enables fresh produce to be available in the U.S. nearly year-round. Together, these factors have influenced the emergence and recognition of some parasites as new human pathogens.
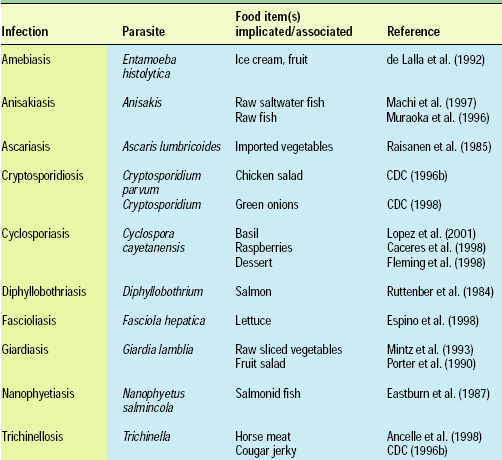 Cryptosporidium parvum and Cyclospora cayetanensis, for example, rapidly became important human pathogens in the U.S. during the 1980s and 1990s, infecting immunocompromised and immunocompetent individuals alike. While not generally associated with human disease earlier in the 20th century, C. parvum and C. cayetanensis are now frequently identified as causative agents in human illness. Additionally, each is linked epidemiologically to the consumption of fresh produce in both sporadic and clustered outbreaks (Table 2). Although both these protozoa can be foodborne pathogens, it is highly likely that they are more often waterborne organisms and transmitted to humans by environmental factors and agricultural practices. The microsporidia, another rapidly emerging group of human pathogens, may also belong in this category.
Cryptosporidium parvum and Cyclospora cayetanensis, for example, rapidly became important human pathogens in the U.S. during the 1980s and 1990s, infecting immunocompromised and immunocompetent individuals alike. While not generally associated with human disease earlier in the 20th century, C. parvum and C. cayetanensis are now frequently identified as causative agents in human illness. Additionally, each is linked epidemiologically to the consumption of fresh produce in both sporadic and clustered outbreaks (Table 2). Although both these protozoa can be foodborne pathogens, it is highly likely that they are more often waterborne organisms and transmitted to humans by environmental factors and agricultural practices. The microsporidia, another rapidly emerging group of human pathogens, may also belong in this category.
Although the contribution of these protozoan parasites to overall foodborne illness, as reflected in recent statistics (Mead et al., 1999), appears to be small, it is highly likely that the numbers are underestimates.
Analytical Methods
Methods for the detection of food- and water-borne parasites have expanded from traditional microscopic techniques to include such molecular tools as the polymerase chain reaction (PCR) and species-specific immunologically-based assays. Whereas morphological identification of worms (helminths) and protozoan pathogens remains a vital aspect of analysis, molecular and sometimes immunologic diagnoses are rapidly being incorporated as more sensitive standard practices.
Detection and identification of tissue-encysted or encapsulated helminths rely heavily on morphological characterization by visual inspection and use methods ranging from direct tissue examination to mechanical and enzymatic tissue disruption. Candling is still the standard practice for detecting and recovering anisakid nematodes in fish flesh (Bier et al., 1995). This method can be applied to fresh or frozen white-fleshed fish that are processed as fillets, steaks, or minced fish. Using a “cool white” light source, the appearance of parasitic worms may vary from reddish to a chalky white. A similar procedure using reflected long-wave ultraviolet light (366 nm) in which the worm’s larval stages fluoresce blue or green may be used for fish with dark flesh (Brattey, 1988). Compression candling is applied to such translucent foods as shellfish. Alternatively, tissue disruption is highly effective in liberating parasites for easier identification and establishing accurate counts. The simple technique of homogenizing fish flesh in a food processor and inspecting the diluted debris under shortwave UV light is an efficient means of detecting larval anisakid nematodes of the genera Anisakis and Phocanema (Brattey, 1988). Alone or in combination with saline elution or pepsin-HCl (enzyme acid) digestion of the homogenate, this approach offers the investigator a greater degree of parasite recovery. Using these types of disruptive methods, the whitish plerocercoids of Dyphyllobothrium spp. (larval tapeworms) can also be more easily recovered and identified (Arambulo, 1982). Metacercaria and mesocercaria (larval forms of trematodes) are also more easily detected and identified with the aid of these methods.
--- PAGE BREAK ---
Visual examination is the predominant method of inspection for the presence of the cysticerci (larval forms) of Taenia solium (pork tapeworm) in pig carcasses and Taenia saginata (beef tapeworm) in bovine carcasses. Post-mortem macroscopic inspection of selective muscle tissue is the primary means of detecting these parasites, although enzyme-acid digestion of tissue similar to that used to examine fish is also employed (Arambulo, 1982). Ante-mortem detection methods using serological tests are currently available. Although hemagluttination tests show some promise, these and other procedures have demonstrated variable specificity and sensitivity and may not be practical due to high cost. Additionally, a host’s weak immunological response to light infections may complicate definitive diagnoses.
In the past, detection and identification of pathogenic protozoa from water sources and foods were equally dependent on traditional microscopy. However, success with microscopy has proven to be much more difficult for protozoa than for the helminths. Definitive microscopic identification of coccidian parasites such as C. cayetanensis and C. parvum is linked to such criteria as oocyst size, shape, and sporulation characteristics (Goodgame, 1996). Staining and autofluorescent properties (such as those of C. cayetanensis) further distinguish these parasites, although, these means of identification are complicated by variability in staining patterns (Negm, 1998; Visvesvera et al.,1997). Inefficient isolation and concentration techniques—used to counteract small size, low numbers, and the large sample sizes required—create additional problems for analyses that already are laborious and time-consuming. Additionally, as in all microscopic diagnoses, identification is highly dependent on the skill, experience and expertise of the microscopist (Goodgame, 1996).
While microscopy remains an important diagnostic component for definitive identification of protozoan parasites, newer techniques are more sensitive, specific, and time-efficient. PCR protocols for Cyclospora and Cryptosporidium detection have been used successfully over the last several years for clinical, environmental and, more recently, food surveys (Rose and Slifko, 1999). These protocols include methods to differentiate among species or closely related genera. To distinguish between C. cayetanensis and Eimeria spp., PCR products also must be examined either by restriction fragment length polymorphism analysis (RFLP) (Jinneman et al., 1998) or an oligonucleotide ligation assay (OLA) (Jinneman et al., 1999).
With the recent description of several Cyclospora-like species isolated from non-human primates (Eberhard et al., 1999; Lopez et al., 1999; Smith et al., 1996), a new nested PCR protocol, capable of differentiating C. cayetanenesis from related non-human parasites (Orlandi, 2001) is currently under development at the U.S. Food and Drug Administration (FDA).
Molecular techniques provide considerable advances in detection sensitivity, specificity and ease of analysis. These methods, however, still depend on our ability to isolate and concentrate the parasite from the sample matrix and prepare a suitable DNA template free of matrix-derived substances that may inhibit PCR. An extraction-free, filter-based method of preparing DNA templates now exists for the PCR detection of such protozoa as C. cayetanensis, C. parvum, and the various microsporidia genera and species in a va riety of complex matrices (foods, environmental samples, and clinical specimens) (Orlandi and Lampel, 2000). This protocol increased the sensitivity of PCR detection and alleviated the need for time-consuming purification schemes that can contribute to significant sample losses and affect detection sensitivity. The protocol was successfully used to identify C. cayetanensis as a contaminant in a chicken-basil-pasta salad implicated in a 1999 outbreak of cyclosporiasis in Missouri (Lopez et al., 2001).
In addition to PCR, several immunologically-based assays were developed for C. parvum. Monoclonal antibodies to oocyst surface antigens are currently available for use in immunofluorescence microscopy or as a component in commercial (enzyme-linked-immune-sorbent assay) kits. Immunomagnetic separation techniques are also being refined and applied as a means of isolating, concentrating, and purifying C. parvum oocysts from complex matrices. No commercially available immunological reagents currently exist for C. cayetanensis.
Whereas molecular methods have provided a means for rapid and sensitive detection of parasites in foods, the question of whether those organisms detected in complex matrices are viable and therefore infective still remains. Animal infectivity studies, where possible (i.e., C. parvum, G. intestinalis), provide the most definitive answer to the question of viability in comparison to sporulation, excystation, and tissue culture models (in vitro studies). However, animal infectivity studies are laborious, time-consuming, and expensive (Neumann et al., 2000).
--- PAGE BREAK ---
Deng et al. (1997) proposed an alternative method using immunomagnetic capture PCR to distinguish viable and dead C. parvum. In addition, viability stain assessment and fluorescent in situ hybridization studies have also proved useful (Deere et al., 1998; Neumann et al., 2000; Vesey et al., 1998). For other parasitic protozoa such as Cyclospora and the microsporidia, where genetic and surface antigen information is lacking, assessment of viability remains difficult. Sporulation, excystation (e.g., Cyclospora) and spore germination in conjunction with tissue culture infectivity (e.g., microsporidia) are currently the only method for gauging viability (Ortega et al., 1998; Wittner and Weiss, 1999).
Control Measures
Contamination of food products by parasites may occur at several points along the path from growing and harvesting food at the farm or fishing grounds to consumption by the consumer. Possible contamination sources include the use of parasite contaminated irrigation or spraying water, contamination of surfaces during harvesting or processing, and contamination of food products during final preparation and packaging. Furthermore, certain genotypes of C. parvum, T. gondii and G. intestinalis have reservoir hosts that increase their frequency in the environment and thereby their threat to public health.
A number of control measures are used to protect food products from parasites. Foremost among the traditional measures are the cleaning and cooking of food items prior to consumption. Most parasites’ heat resistance is not impressive, and temperatures as low as 56–60°C for several minutes will, in many instances, eliminate the infectivity of helminths (Fayer et al., 2001). Heating to > 72°C for 1 min or 45°C for 10–20 min inactivates C. parvum oocysts (Steiner et al., 1997). However, the heat must uniformly penetrate the entire food matrix because parasites such as Trichinella or Anisakis may be encysted deep inside the tissues.
Other processes, used primarily for the preparation and preservation of fish are effective in inactivating helminths. These include hot smoking, fermentation in brine, and drying (WHO, 1995); cold smoking and salting, however, may not be effective against fishborne anisakid nematodes. Cleaning methods include such actions as peeling, washing or scrubbing fresh produce that is usually contaminated at or near the surface. Mixtures of chemicals such as the insitu generation of mixed oxidants, may be more effective than a single chemical, such as chlorine, which does not inactivate protozoan cysts and oocysts (Venczel et al., 1997).
Consumer preferences for the consumption of fresh fruits and vegetables preclude the use of heat as a control measure against parasitic contamination. Application of cold temperatures may serve as a useful alternative: freezing C. parvum to –20°C and –70°C rendered oocysts noninfectious and nonviable after 24 and 1 hr, respectively (Fayer and Nerad, 1996). FDA’s “Fish and Fisheries Products Hazards and Controls Guide” recommends that raw fish served for consumption should be frozen at either (1) –35°C (or below) until solid and stored at –35°C or below for 15 hr, (2) –35°C (or below) until solid and stored at –20°C (or below) for at least 24 hr, or (3) –20°C or below for 7 days (total time) prior to being sold (FDA, 2001). Proper freezing of fish products destroys helminths capable of causing disease after consumption of such raw fish dishes as sushi, sashimi, or ceviche. Freezing, particularly in the short term, however is unpredictable—freezing can inactivate parasites but under certain conditions also may preserve them.
Water serves as an important vehicle for transmission of foodborne parasites. In developed nations, treatment of public water sources with halogenated compounds, predominantly chlorine, significantly reduces the public health threat of bacterial pathogens such as Vibrio cholerae. Protozoa such as G. intestinalis and the coccidia that are resistant to antibacterial levels of chlorine are now among the dominant public health concerns in several nations and can cause disease outbreaks that encompass entire communities.
Furthermore, the Environmental Protection Agency standard for water quality is based on a total bacterial coliform count (EPA, 1990). This criterion does not appear to be a reliable indicator for parasite contamination of water. This was apparent during the 1993 C. parvum outbreak in Milwaukee, in which the implicated water source met all federal quality standards (Mac Kenzie et al., 1994). Filtration of water also has improved water quality, although the small size of some parasites may allow their passage through certain types of filters. An absolute 1-μ filter is required to exclude Cryptosporidium.
--- PAGE BREAK ---
A number of parasite control measures are being considered and evaluated for food processing (Rose and Slifko, 1999). Ozone is a powerful oxidizing agent and has been suggested as a possible disinfectant for some parasites. Treatment of Cryptosporidium parvum in ozone-demand-free buffered H2O with 1 ppm ozone for 5 min decreased both oocyst excystation and infectivity to mice by greater than 90% (Korich et al., 1990).
Ozone inactivation, however, is dependent on a number of parameters including temperature, medium pH, and the amount of extracellular organic matter residing around the parasite; therefore, penetration of ozone into food crevices where parasites may reside may not occur (Kim et al., 1999).
Irradiation serves as another possible measure for parasite control. UV irradiation, as a method for inactivating Cryptosporidium in apple cider, is currently being investigated. Preliminary results demonstrate a 5-log10 reduction in oocyst viability (Hanes, 2001). Whereas UV irradiation has the potential to serve as an efficient method for inactivation of Cryptosprodium for even small producers, its economic feasibility has yet to be determined.
Ionizing irradiation is effective in controlling helminths such as Opisthorchis viverrini, Anisakis simplex, Clonorchis sinensis, and Paragonimus westermani (Venugopal et al., 1999). In the control of Trichinella spiralis-infected pork, FDA approved the use of irradiation at an absorbed dose of 0.3 kGy-1.0 kGy (FDA, 1985). While ionizing irradiation is useful for inactivation of a number of parasites, it does not uniformly inactivate all parasites to the same degree; a considerable range in the log reduction is reported (Enigk et al., 1975).
Also, inactivation of parasites may require a range of doses because the effectiveness of the process is dependent on the parasite, the stage of the parasite that has contaminated the food matrix, and the types and characteristics of the food matrix itself (Farkas, 1998). Hence, many parameters must be examined before irradiation will become practical for a particular product. Another factor is some consumers’ concern about ingesting irradiated food. This has mitigated against the use of irradiation on food products.
Hydrostatic pressure is a recently proposed means by which we may be able to decontaminate foods. Studies by Slifko et al. (2000a) demonstrated that more than 99% of C. parvum oocysts in apple and orange juice were inactivated following > 60 sec high hydrostatic pressure treatment. The use of hydrostatic pressure on fish for inactivation of helminths is currently being investigated. While some of the control measures discussed exhibit efficacy against certain parasites, none of these procedures has been demonstrated to be as uniformly reliable as heat.
Control measures used to decontaminate food products once contaminated by parasites are only one intervention to prevent human illness caused by the ingestion of foods harboring parasitic pathogens. Surveillance programs are another means to control and limit the impact of foodborne parasites on public health. Such programs can serve as effective indicators of potential contamination problems through periodic testing. Though somewhat costly, surveillance of those foods, such as fresh produce, previously identified as “at-risk” for parasitic contamination acts to protect both the producer and the consumer and minimize the risk of large foodborne outbreaks. A step towards such a surveillance program is the development and implementation of policies, similar to seafood HACCP, designed to limit, control, and monitor sources of parasite contact with food.
In an effort to preserve the raspberry industry in Guatemala in 1999, the Model Plan of Excellence (MPE) was developed through the cooperative efforts of the U.S. Food and Drug Administration, the Guatemalan government, and the Guatemalan Berry Commission to guard berries intended for export against contamination with C. cayetanensis. The MPE is a strict version of Good Agricultural Practices and is intended to decrease the risk of contaminating crops with Cyclospora. As a system designed to monitor many food safety aspects of farming, it provides for oversight and surveillance of sanitation practices, water source development, irrigation methods, food handling, and employee hygiene. Though originally designed and implemented to stem the growing problem of cyclosporiasis attributed to contaminated berries epidemiologically linked to the spring crop of Guatemalan raspberries, the MPE has the potential to serve as a model policy document for other produce-trading partners to limit the risk of contaminating crops with other pathogens (Calvin et al., 2000).
--- PAGE BREAK ---
Conclusions
In the past, in the U.S., consideration of parasitic animals as foodborne pathogens waned with the incorporation of better food handling and sanitation practices, and inspection procedures. However, events during the latter part of the 20th century forced us to focus again on the potential health risks posed by parasitic protozoa and helminths. Globalization of food trade, preferences for raw and undercooked dishes, ease of international travel, and increasing numbers of immunocompromised individuals are factors that have contributed to the increase in foodborne parasitic infections. This is further complicated by the emergence of parasites not previously associated with pathogenesis in humans.
Educational outreach and research programs developed through the U.S. government’s Food Safety Initiative have been relatively successful in increasing public awareness of food safety issues and potential health risks (FSI, 2001). Nevertheless, it is probable that the incidence of illness attributed to parasitic contamination of food and water is underreported. The reasons for the under-reporting are: the lack of recognition of potential effects of parasite contamination on public health and the absence of routine screening protocols for these pathogens.
With the emphasis on food safety continuing and food security being a major concern since September 11, 2001, the development of new technologies for the prevention, detection and inactivation of foodborne parasites is being accelerated. In conjunction with an increased awareness of the health risks associated with parasitic contamination and illness, the control of foodborne parasites is expected to become more effective.
INSTITUTE OF FOOD TECHNOLOGISTS
The Society for Food Science and Technology
World Headquarters: 525 W. Van Buren Street, Suite 1000, Chicago, IL 60607 Voice: 312–782–8424 • Fax: 312–782–8348 e-mail: [email protected] • www.ift.org
Washington, D.C.: 1025 Connecticut Ave., NW, Suite 503, Washington, DC 20036 Voice: 202–466–5980 • Fax: 202–466–5988 e-mail: [email protected] • www.ift.org
Scientific Status Summaries are published in Food Technology by the Institute of Food Technologists’ Expert Panel on Food Safety and Nutrition. IFT’s Expert Panel on Food Safety and Nutrition, which studies significant food-related issues and oversees timely production of Scientific Status Summaries, comprises academicians representing expertise in one or more areas of food science/technology and nutrition. Scientific Status Summaries, which are not necessarily written by the Expert Panel, are rigorously peer-reviewed by the Expert Panel as well as by individuals outside the panel who have specific expertise in the subject.
The Scientific Status Summaries may be reprinted or photocopied without permission, provided that suitable credit is given.
--- PAGE BREAK ---
Discover the value potential of the scientific insights contained within the Journal of Food Science. Wherever your interest lies in product or process development or improvement, you.ll find important content in JFS. Peer-reviewed reports of original research and critical reviews of all basic and applied aspects of food science include:
Concise Reviews and Hypotheses in Food Science Food Chemistry and Toxicology Food Engineering and Physical Properties Food Microbiology and Safety Sensory and Nutritive Qualities of Food
Subscribe to JFS and get 9 issues per year.more than ever before.plus supplements on hot topics. Subscribers receive website access for online searches of the latest five years of JFS issues to aid their own research.
Order your subscription to JFS by calling 800-IFT-FOOD (800-438-3663) or 312-782-8424. Or visit www.ift.org to view rates and download a faxable order form.
"There is a message that science has to offer . . . the style of thinking, the integrity of argument; you honor your adversaries, and you put all the evidence on the table. You have an absolute obligation to feed your critics with all the data."
—Joshua Lederberg, 1958 Nobel Prize in Physiology and Medicine
The Nobel Prize Centennial 1901.2001 Information at www.nobel.se
The Journal of Food Science is a publication of the Institute of Food Technologists, a not-for-profit society for food science and technology.
Remember to visit IFT’s Web site— www.ift.org —for information about IFT’s scientific publications and the association’s resources.
Palmer A. Orlandi, Dan-My T. Chu, Jeffrey W. Bier and George J. Jackson
All authors are or were with the Center for Food Safety and Applied Nutrition, Food and Drug Administration, College Park, MD 20740-3835. Palmer Orlandi is a biochemical parasitologist in the Office of Applied Research and Safety Assessment. Dan-My Chu is a parasitologist in the Office of Plant, Dairy Foods and Beverages. Jeff Bier is a parasitologist and consultant, formerly with the Office of Seafood. George Jackson is a parasitologist in the Office of Science and the Dean of the Center for Food Safety and Applied Nutrition’s Staff College.
References
Ancelle, T., Dupouy-Camet, J., Desenclos, J.C., Maillot, R., Savage-Houze, S., Charlet, F., Drucker, J., and Moren, A. 1998. A multifocal outbreak of trichinellosis linked to horse meat imported from North America to France in 1993. Am. J. Trop. Med. Hyg. 59: 615-619.
Arambulo, P. 1982. The cestode zoonoses. Part 2, Section C. In b�0;0;0;0;0;0;0;0;0;0;0;0;1C;CRC Handbook Series in Zoonoses,b�0;0;0;0;0;0;0;0;0;0;0;0;1D; ed. J.H. Steele, pp. 209-348. CRC Press, Inc., Boca Raton, Fla.
Bier, J.W., Jackson, G.J., Adams, A.M., and Rude, R.A. 1995. Parasitic animals in foods. Chpt. 19 in b�0;0;0;0;0;0;0;0;0;0;0;0;1C;U.S. Food and Drug Administration: Bacteriological Analytical Manual,b�0;0;0;0;0;0;0;0;0;0;0;0;1D; 8th ed., pp. 19.01-19.18. AOAC Intl., Gaithersburg, Md.
Brattey, J. 1988. A simple technique for recovering larval ascaridoid nematodes from the flesh of marine fish. J. Parasitol. 74: 735-737.
Caceres, V.M., Ball, R.T., Somerfeldt, S.A., Mackey, R.L., Nichols, S.E., MacKenzie, W.R., and Herwaldt, B.L. 1998. A foodborne outbreak of cyclosporiasis caused by imported raspberries. J. Fam. Pract. 47(3): 231-234.
Calvin, L., Foster, W., Solorzano, L., Mooney, J.D., Flores, L., and Barrios, V. 2000. Response to a food safety problem in produce: A case study of a cyclosporiasis outbreak. Presented at International Agricultural Trade Research Consortium Conference on Global Food Trade and Demand for Quality, Montreal, Canada, June 26-27.
CDC. 1996a. Foodborne outbreak of diarrheal illness associated with Cryptosporidium parvumb�0;0;0;0;0;0;0;0;0;0;0;0;13;Minnesota, 1995. Morb. Mort. Wkly. Rept. 45(36): 783-784. U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, Ga.
CDC.1996b. Outbreak of trichinellosis associated with eating cougar jerky-Idaho, 1995. Atlanta, Ga. Morb. Mort. Wkly. Rept. 45(10): 205-206. U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, Ga.
CDC. 1998. Foodborne outbreak of cryptosporidiosis-Spokane, Washington, 1997. Morb. Mort. Wkly. Rept. 47: 565-567. U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, Ga.
Deere, D., Vesey, G., Milner, M., Williams, K., Ashbolt, N., and Veal, D. 1998. Rapid method for fluorescent in situ ribosomal RNA labelling of Cryptosporidium parvum. J. Appl. Microbiol. 85: 807-818.
de Lalla, F., Rinaldi, E., Santoro, D., Nicolin, R., and Tramarin, A. 1992. Outbreak of Entamoeba histolytica and Giardia lamblia infections in travellers returning from the tropics. Infection. 20(2): 78-82.
Deng, M.Q., Cliver, D.O., and Mariam, T.W. 1997. Immunomagnetic capture PCR to detect viable Cryptosporidium parvum oocysts from environmental samples. Appl. Environ. Microbiol. 63: 3134-3138.
Dobell, C. 1920. The discovery of the intestinal protozoa of man. Proc. Royal Soc. Med. 13: 1-15.
Eastburn, R.L., Fritsche, T.R., and Terhune, C.A. Jr. 1987. Human intestinal infection with Nanophyetus salmincola from salmonid fishes. Am. J. Trop. Med. Hyg. 36: 586-591.
Eberhard, M.L., da Silva, A.J., Lilley, B.G., and Pieniazek, N.J. 1999. Morphologic and molecular characterization of new Cyclospora species from Ethiopian monkeys: C. cercopitheci sp.n., C. colobi sp.n., and C. papionis sp.n. Emerg. Infect. Dis. 5: 651-658.
Enigk, K., Holl, P., and Dey-Hazra, A. 1975. The destruction of parasitic resistant stages in sludge by irradiation with low accelerating voltage electrons. Zentralbl. Bakteriol. 161(1): 61-71.
EPA. 1990. Drinking water: National primary drinking water regulations; total coliforms; corrections and technical amendments; final rule. Environmental Protection Agency, Fed. Reg. 55: 25064-25065.
Espino, A.M., Diaz, A., Perez, A., and Finlay, C.M.. 1998. Dynamics of antigenemia and coproantigens during a human Fasciola hepatica outbreak. J. Clin. Microbiol. 36: 2723-2726.
Farkas, J. 1998. Irradiation as a method for decontaminating food. A review. Intl. J. Food. Microbiol. 44(3): 189-204.
Fayer, R., Gamble, H.R., Lichtenfels, J.R., and Bier, J.W. 2001. Waterborne and foodborne parasites. Chpt. 42 In b�0;0;0;0;0;0;0;0;0;0;0;0;1C;Compendium of Methods for the Microbiological Examination of Foods,b�0;0;0;0;0;0;0;0;0;0;0;0;1D; 4th ed. ed. F.P. Downes and K. Ito, pp. 429-438. Am. Publ. Health Assn., Washington, D.C.
Fayer, R. and Nerad, T. 1996. Effects of low temperatures on viability of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 62: 1431-1433.
FDA. 1985. Irradiation in the production, processing, and handling of food. Food and Drug Admin., Fed. Reg. 50: 29658-29659.
FDA. 2001. Parasites. Chpt. 5 in b�0;0;0;0;0;0;0;0;0;0;0;0;1C;Fish and Fisheries Products Hazards and Controls Guide,b�0;0;0;0;0;0;0;0;0;0;0;0;1D; 3rd ed. Food and Drug Admin., www.cfsan.fda.gov/~comm/haccpsea.html.
FSI. 2001. Food Safety Initiative. www.cfsan.fda.gov/~dms/fs-toc.html.
Fleming, C.A., Caron, D., Gunn, J.E., and Barry, M.A. 1998. A foodborne outbreak of Cyclospora cayetanensis at a wedding: Clinical features and risk factors for illness. Arch. Intern. Med.158: 1121-1125.
Goodgame, R.W. 1996. Understanding intestinal sporeforming protozoa: Cryptosporidia, Microsporida, Isospora and Cyclospora. Ann. Intern. Med. 124: 429-441.
Hanes, D. 2001. Personal communication. Food and Drug Admin., Beltsville, Md.
Jackson, G.J. 1990. Parasitic protozoa and worms relevant to the U.S. Food Technol. 44(5): 106-112.
Jackson, G.J., Bier, J.W., Payne, W.L., and McClure, F.D. 1981. Recovery of parasitic nematodes from fish by digestion or elution. Appl. Environ. Microbiol. 41: 912-914.
Jinneman, K.C., Wetherington, J.H., Hill, W.E., Adams, A.M., Johnson, J.M., Tenge, B.J., Dang, N., Manger, R.L. and Wekell, M.M. 1998. Template preparation of PCR and RFLP of amplification products for the detection and identification of Cyclospora sp. and Eimeria spp. oocysts directly from raspberries. J. Food Protect. 61: 1497-1503.
Jinneman, K.C., Wetherington, J.H., Hill, W.E., Omiescinski, C.J., Adams, A.M., Johnson, J.M., Tenge, B.J., Dang, N., and Wekell, M. 1999. An oligonucleotide-ligation assay for the differentiation between Cyclospora and Eimeria spp. Polymerase chain reaction amplification products. J. Food Protect. 62: 682-685.
KC$ferstein, F.K. 2000. Diseases caused by foodborne parasites: The scope of the problem. Acta Parasitologica 45(3): 146.
Kim, J.G., Yousef, A.E., and Dave, S. 1999. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Food Protect. 62: 1071-1087.
Korich, D.G., Mead, J.R., Madore, M.S., Sinclair, N.A., and Sterling, C.R. 1990. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 56: 1423-1428.
Lopez, F.A., Manglicmot, J., Schmidt, T.M., Yeh, C., Smith, H.V., and Relman, D.A. 1999. Molecular characterization of Cyclospora-like organisms from baboons. J. Infect. Dis. 179: 670-676.
Lopez, A.S., Dodson, D.R., Arrowood, M.J., Orlandi Jr, P.A., da Silva, A.J., Bier, J.W., Hanauer, S.D., Kuster, R.L., Oltman, S., Baldwin, M.S., Won, K.Y., Nace, E.M., Eberhard, M.L., and Herwaldt, B.L. 2001. Outbreak of cyclosporiasis associated with basil in missouri in 1999. Clin. Infect. Dis. 32: 1010-1017.
Machi, T., Okino, S., Saito, Y., Horita, Y., Taguchi, T., Nakazawa, T., Nakamura, Y., Hirai, H., Miyamori, H., and Kitagawa, S. 1997. Severe chest pain due to gastric anisakiasis. Intern. Med. 36(1): 28-30.
MacKenzie, W.R., Hoxie, N.J., Proctor, M.E., Gradus, M.S., Blair, K.A., Peterson, D.E., Kazmierczak, J.J., Addiss, D.G., Fox, K.R., Rose, J.B., and Davies, J.P. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. New Engl. J. Med. 331(3): 161-167.
Mead, P.S., Slutsker, L., Dietz, V., McCraig, L.F., Bresee, J.S., Shapiro, C., Griffin, P.M., and Tauxe, R.V. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5: 607-625.
Michael, E., Bundy, D.A.P., Hall, A., Savioli, L., and Montresor, A. 1997. This wormy world: Fifty years on. The challenge of controlling common helminthiases of humans today. Parasit. Today. 13(11): 407-408.
Mintz, E.D., Hudson-Wragg, M., Mshar, P., Cartter, M.L., and Hadler, J. L. 1993. Foodborne giardiasis in a corporate office setting. J. Infect. Dis. 167(1): 250-253.
Muraoka, A., Suehiro, I., Fujii, M., Nagata, K., Kusunoki, H., Kumon, Y., Shirasaka, D., Hosooka, T., and Murakami, K. 1996. Acute gastric anisakiasis: 28 cases during the last 10 years. Dig. Dis. Sci. 41: 2362-2365.
Negm, A. 1998. Identification of Cyclospora cayetanensis in stool using different stains. J. Egypt. Soc. Parasitol. 28(2): 429-436.
Neuman, N.F., Gyurek, L.L., Gammie, L., Rinch, G.R., and Belosevic, M. 2000. Comparison of animal infectivity and nucleic acid staining for assessment of Cryptosporidium parvum viability in water. Appl. Envrion. Microbiol. 66: 406-412.
Orlandi, P.A. 2001. Personal communication. Food and Drug Admin.,Washington, D.C.
Orlandi, P.A. and Lampel, K.A. 2000. Extraction-free, filter-based template preparation for rapid and sensitive PCR detection of pathogenic parasitic protozoa. J. Clin. Microbiol. 38: 2271-2277.
Ortega, Y.R., Sterling. C.R., and Gilman, R.H. 1998. Cyclospora cayetanensis. Adv. Parasitol. 40: 399-418.
Porter, J.D., Gaffney, C., Heymann, D., Parkin, W. 1990. Food-borne outbreak of Giardia lamblia. Am. J. Publ. Health 80: 259-1260.
Raisanen, S., Ruuskanen, L., and Nyman, S. 1985. Epidemic ascariasisb�0;0;0;0;0;0;0;0;0;0;0;0;14;Evidence of transmission by imported vegetables. Scand. J. Prim. Health Care. 3(3): 189-191.
Rose, J. B., and Slifko, T.R. 1999. Giardia, Cryptosporidium, and Cyclospora, and their impact on foods: A review. J. Food Protect. 62: 1059-1070.
Ruttenber, A.J., Weniger, B.G., Sorvillo, F., Murray, R.A., and Ford, S.L. 1984. Diphyllobothriasis associated with salmon consumption in Pacific Coast states. Am. J. Trop. Med. Hyg. 33(3): 455-459
Slifko, T.R., Smith, H.V., and Rose, J.B. 2000a. Emerging parasite zoonoses associated with water and food. Intl. J. Parasitol. 12-13: 1379-1393.
Slifko, T.R., Raghubeer, E., and Rose, J.B. 2000b. Effect of high hydrostatic pressure on Cryptosporidium parvum infectivity. J. Food Protect. 63: 1262-1267.
Smith, H.V., Paton, C.A., Girdwood, R.W., and Mtambo, M.M. 1996. Cyclospora in non-human primates in Gombe, Tanzania. Vet. Record. 138(21): 528
Smyth, J.D. 1990. b�0;0;0;0;0;0;0;0;0;0;0;0;1C;In Vitro Cultivation of Parasitic Helminths.b�0;0;0;0;0;0;0;0;0;0;0;0;1D; CRC Press Inc., Boca Raton, Fla.
Steiner, T.S., Thielman, N.M., and Guerrant, R.L. 1997. Protozoal agents: What are the dangers for the public water supply? Ann. Rev. Med. 48: 329-340.
Stoll, N.R. 1947. This wormy world. J. Parasitol. 33: 1-18.
Taylor, A. E. and Baker, J.R. 1978. b�0;0;0;0;0;0;0;0;0;0;0;0;1C;Methods of Cultivating Parasites in vitro.b�0;0;0;0;0;0;0;0;0;0;0;0;1D; Academic Press, N.Y.
USDC. 2000a. U.S. Trade in Goods, 1972-2000, Census Basis. Dept. of Commerce, Bureau of the Census. http://www.ita.doc.gov/td/industry/otea/usfth/aggregate/HL00T03.txt.
USDC. 2000b. International travelers to and from the U.S.b�0;0;0;0;0;0;0;0;0;0;0;0;13;International visitors (inbound) and U.S. residents (outbound) (1989b�0;0;0;0;0;0;0;0;0;0;0;0;13;1998). U.S. Dept. of Commerce, Office of Tourism Industries. http://tinet.ita.doc.gov/view/f-1998-06-001/index.html?ti_cart_cookie=20010604.140603.06592 and http://tinet.ita.doc.gov/view/f-2000-99-001/trend2003.html.
Venczel, L.V., Arrowood, M., Hurd, M., and Sobsey, M.D. 1997. Inactivation of Cryptosporidium parvum oocysts and Clostridium perfringens spores by a mixed-oxidant disinfectant and by free chlorine. App. Environ. Microbiol. 63: 1598-1601.
Venugopal, V., Doke, S.N., and Thomas, P. 1999. Radiation processing to improve the quality of fishery products. Crit. Rev. Food Sci. Nutr. 39(5): 391-440.
Vesey, G., Ashbolt, N., Fricker, E.J., Deere, D., Williams, K.L., Veal, D.A., and Dorsch, M. 1998. The use of a ribosomal RNA targeted oligonucleotide probe for fluorescent labeling of viable Cryptosporidium parvum oocysts. J. Appl. Microbiol. 85: 429-440.
Visvesvara, G., Moura, H., Kovacs, Nace, E., Wallace, S. and Eberhard, M.L. 1997. Uniform staining of Cyclospora oocysts in fecal smears by a modified safranin technique with microwave heating. J. Clin. Microbiol. 35: 730-733.
Wittner, M., Weiss.and L.M. 1999. b�0;0;0;0;0;0;0;0;0;0;0;0;1C;The Microsporidia and Microsporidiosis.b�0;0;0;0;0;0;0;0;0;0;0;0;1D; ASM Press, Washington, D.C.
WHO. 1995. Control of foodborne trematode infections. WHO Tech. Rept. Series. No. 849, pp. 1-157 World Health Organization, Rome.
