Taste Modification in the Biotech Era
Advances in understanding the science of taste are allowing taste modifiers such as bitter blockers and sweetness potentiators.
The food and beverage industry is facing a critical time in its evolution. On the one hand, scientific developments are identifying increasing numbers of active components in foods and beverages which provide health and functional benefits such as impeding cancer cell growth, improving immune response, and protecting against bacteria and viruses. On the other hand, it is becoming increasingly clear that the Western diet is leading to serious health problems such as obesity, diabetes, and hypertension.
Concurrent with the increased visibility of these issues, advances in the understanding of the science of taste are allowing new approaches to address them. These new approaches involve the application of biotechnology to characterize taste and identify novel taste modifiers (Fig. 1 on page 26).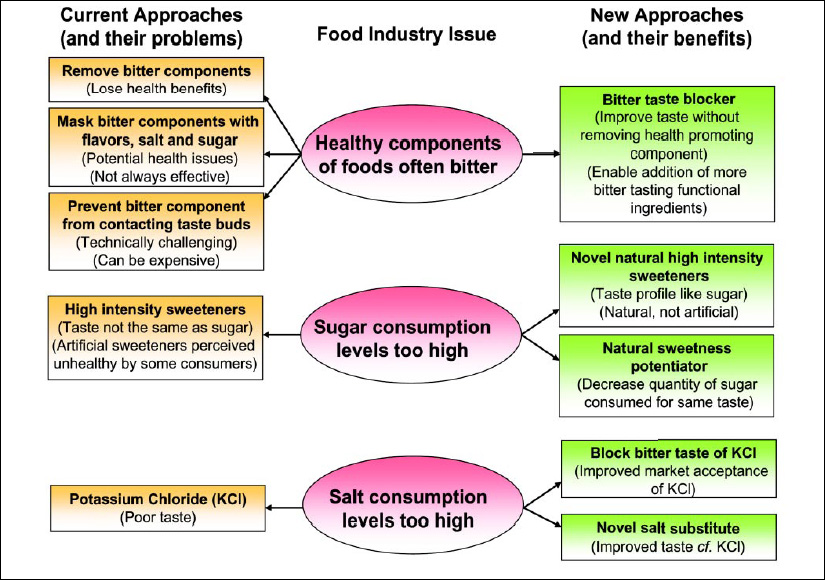
This article outlines the advances being made in understanding the science of taste and describes how this understanding is being applied to help overcome the issues facing the food, beverage, and nutritional industries.
The Science of Taste
The molecular era of taste science was initiated by the discovery in the early 1990s of gustducin, the protein inside the taste cell that interacts with taste receptors, leading to taste cell activation (McLaughlin et al., 1992). The identification of this protein, and subsequently other taste cell components, at the molecular level has led to a step change in the way taste can be studied and taste problems addressed. Now it is possible to use the powerful techniques of biotechnology to search for novel taste modifiers that would quite possibly never be discovered using traditional approaches.
Understanding how taste works at the molecular level allows for the development of tests, or assays, that monitor taste effectively in a test tube. Using the same techniques as the biopharmaceutical industry, this process can be automated to enable hundreds to thousands of samples to be screened per day to identify compounds that modulate taste.
There are currently five recognized taste modalities: sweet, salty, sour, bitter, and umami (savory) (Smith and Margolskee, 2001; le Coutre, 2003). It is recognition of these various tastes that enables humans and animals to discern important information about the quality of food. Sweet, salty, and umami tastes typically are associated with foods that contain nutrients important for well-being. Sweet-tasting foods are typically high in carbohydrates, salty food contains important minerals, and umami taste is coupled to the presence of amino acids. Sour and bitter taste perception is characteristically a protective mechanism against ingesting substances that may be deleterious to the body, such as spoiled food or poisons.
Taste perception occurs in specialized structures called taste buds. These are groups of elongated taste receptor cells that respond to the presence of a tastant in the mouth by generating nerve responses that relay this information to the brain. The function of taste receptor cells is to transduce the physical binding of tastants at the apical surface of the cell (exposed to the buccal cavity) into release of neurotransmitter at the baso-lateral surface of the cell (exposed to the peripheral nervous system), resulting in a nerve impulse.
Sweet, bitter, and umami tastants bind to specialized receptor proteins in the cell membrane. These receptors traverse the membrane and interact within the cell with specialized transduction proteins called G proteins. Hence, the receptor proteins are known as G protein coupled receptors (GPCRs). Binding of tastant to receptors leads to activation of the G protein, which in turn activates enzymes within the cell known as effector enzymes. It is the job of these effector enzymes to modulate the internal concentration of molecules known as second messengers. Changes in the level of second messengers within a taste cell leads to opening of various ion channels both within the cell and on the cell surface, which results in a depolarization of the cell as ion gradients maintained by the cell in its resting state are depleted. This depolarization of the cell results in release of neurotransmitter into the synaptic cleft between the taste receptor cell and its neighboring neuron. These neurotransmitters depolarize the neuron and begin the electrical signal that is transmitted to the higher levels of the central nervous system to be interpreted as taste.
--- PAGE BREAK ---
Sweet taste is transduced by two receptors, known as T1R2 and T1R3, that work together as a sweet detector (Kitagawa et al., 2001; Max et al., 2001; Montmayeur et al., 2001; Sainz et al., 2001; Nelson et al., 2001; Bachmanov et al., 2001; Li et al., 2002). Recent evidence using transgenic knockout mice lacking one of these receptors (Damak et al., 2003) shows that with nutritive sweeteners this is not the whole story, which may account for the differences in taste between nutritive sweeteners and their nonnutritive brethren.
The etiological importance of the detection of poisons has led to the detection of bitter-taste stimuli being transduced by at least 25 GPCRs known as the T2Rs (Adler et al., 2000; Matsunami et al., 2000). Having many proteins involved in this process allows for the structural diversity necessary to interact with the many and varied types of bitter tastants and for protection against catastrophic mutations which if few receptors were involved would most likely lead to death of the organism from poisoning, if one of the receptors was nonfunctional.
There is some controversy over the identity of the umami receptor. A GPCR called mGluR4 has been hypothesized as an umami receptor (Chaudari et al., 2000). What seems to be certain is that the T1R3 receptor involved in sweet taste and a related protein, T1R1, act together to detect a number of umami stimuli (Li et al., 2002).
Salty and sour tastes are perceived through receptor proteins, as are other tastes, except that the proteins that respond to these tastes are ion channels instead of GPCRs. There is uncertainty over the identity of the salt receptor, with the relative contributions of different ion channels being a matter of conjecture (Doolin and Gilbertson, 1996; Formaker and Hill, 1988; Elliott and Simon, 1990; Stewart et al., 1997; Miyamoto et al., 2001); however, the effect of sodium ion entry is taste cell depolarization and neurotransmitter release. Sourtaste detection appears to be even more complex, with a number of pathways possibly involved. Hydrogen ions in acids enter taste cells through proton channels and also interact with a variety of channels, such as potassium, sodium, calcium, and chloride channels (DeSimone, 2001). The overall effect is a depolarization of the taste cell and the generation of a nerve impulse.
A variety of assay techniques familiar to the world of biotechnology are used in this new approach to taste modification. Recombinant DNA technology allows for the building of taste sensors—in-vitro biochemical assays and in-vivo behavioral assays—that allow quantitative monitoring of tastes. Calcium imaging, radionucleotide binding, and fluorescence polarization are all used in high-throughput screening programs that can process hundreds to thousands of unknown compounds a day, looking for new taste modifiers.
The further characterization of the receptors involved in taste will allow for additional advances in taste technology. With the structural elucidation of receptors, it will be possible in the coming years to screen for taste modifiers using computers that model the binding of receptors and tastants entirely using algorithms. This in-silico approach will lead to designer taste modifiers that can be physically produced once their functionality has been determined by computer.
Overcoming Bitterness
As people look to consume healthier and more-functional foods and beverages, the industry is faced with a significant problem. Many of the compounds in foods that provide health benefits, such as polyphenols in soy and chocolate and phytonutrients in nutritional products and functional foods, are bitter. The same is true for functional ingredients such as hydrolyzed proteins used as stabilizers and texturants in nutritional products. One way of overcoming the bitterness of foods containing these bitter compounds is to remove the offensive-tasting compound from the final product. However, this approach nullifies the benefit provided by the compound.
--- PAGE BREAK ---
Another approach is to mask the offending taste. There have traditionally been two methods of masking bitter taste. One method is to physically prevent the bitter tastant from interacting with the taste-receptor cells of the tongue. In the pharmaceutical industry, this is done by encapsulating or microencapsulating bitter-tasting drugs in a pill; this approach is also used in the food industry. The other method of bitter-taste masking is to confound the taste centers of the brain by stimulating competing tastes such as sweet and salty. However, overpowering the higher brain functions that decipher the nerve signals coming from the taste buds with vast amounts of competing taste signals—the “spoonful of sugar helps the medicine go down” approach—results in large quantities of salt and sugar being used in food and beverage products, which is perceived to be exacerbating the problems of hypertension, obesity, and diabetes.
In the new molecular era of taste, there is a third option. By identifying compounds that interfere with the transduction mechanism of bitter taste in taste-receptor cells, it is now possible to prevent the taste cells from ever being activated and generating a nerve impulse. In this way, the brain never even knows that a bitter compound is present in the mouth. Using this approach, the food industry can have the health-promoting and functional compounds present in food at normal or even increased levels and prevent the consumer from sensing the off-taste produced without the addition of too much salt or sugar.
The Solae Co., a joint venture of DuPont and Bunge Ltd., is addressing this issue in soy protein by working with the biotech company Linguagen Corp. Linguagen’s proprietary technology allows for the identification of taste-modifying compounds by using the biotechnology industry’s approach of screening large libraries of compounds. Linguagen’s focus is on natural-compound libraries, particularly food botanicals which are either Generally Recognized as Safe (GRAS), or have a high likelihood of GRAS status. This approach allows the regulatory pathway for taste modifiers to be quicker and less expensive.
Excess sodium is a major contributor to hypertension and other health problems in the United States. More than 60 million Americans have been found to have elevated blood pressure, and most are under age 65. Cardiovascular disease alone costs the U.S. nearly $300 billion per year. Despite these terrifying statistics, alternatives to sodium chloride have never gained widespread acceptance in the marketplace. One of the main reasons for this is taste. Potassium chloride is an excellent sodium chloride substitute which has many properties that make it an ideal replacement. However, it has one major drawback—it is bitter tasting to a substantial portion of the population.
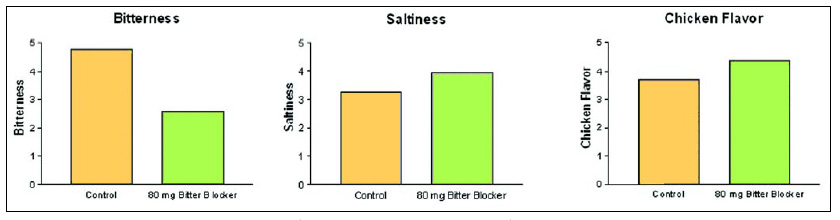
Biotechnology is being used to address the issue of high sodium levels in foods and beverages by identifying sodium chloride substitutes or by improving the taste of the current alternatives. In 2003, Linguagen received patent protection and regulatory approval for a bitter blocker, adenosine 5'-monophosphate (AMP), identified using biochemical assays (Ming et al., 1999; Margolskee and Ming, 2003). AMP works by blocking the activation of gustducin in taste receptor cells and thereby preventing taste nerve stimulation. AMP is being assessed by a number of food and beverage companies and has been shown to decrease bitter taste in a number of applications, including grapefruit juice, beer, and diet carbonated soft drinks.
This bitter blocker improves the taste of potassium chloride in food applications where it is used to replace sodium chloride (Fig. 2). In so doing, Linguagen became the first company to successfully bring to market a taste modifier discovered using an in-depth understanding of the underlying molecular mechanisms of taste. This bitter blocker is only the first of what will in the coming years become a stream of products which owe their existence to the convergence of food technology and biotechnology.
--- PAGE BREAK ---
Potentiating Sweetness
The Centers for Disease Control and Prevention recently reported that obesity now rivals smoking as the leading cause of avoidable death in the U.S. A sedentary lifestyle and poor diet are considered to be the leading contributors to this trend, which is expected to continue. Daily calorie requirements in the Western world have decreased markedly in recent decades. Today, few jobs require manual labor, and our leisure time is dominated by sedentary activities such as television, DVDs, and computers. The predominant form of transportation between these various activities is the car.
Caloric intake has increased considerably over the same time period. Sugar consumption in the U.S. has increased by almost one-third in the last two decades, and one in three American children born in 2000 are now predicted to suffer from diabetes during their lifetime if these trends are not addressed.
Historically, almost all the major noncaloric sweeteners have been discovered by serendipity. The folklore surrounding the discoveries of saccharin, aspartame, and sucralose by scientists accidentally tasting these sweeteners, as a result of either spillage or misunderstanding, is well known. What has not been possible before is the systematic screening (and effective tasting) of tens of thousands of compounds to identify new sweeteners. By using biotechnological methods, combined with a deeper understanding of how taste works at the molecular level, noncaloric sweeteners will finally be identified that have properties indistinguishable from nutritive sweeteners such as sucrose and fructose.
Even more intriguing is the idea of identifying sweetness potentiators. These are compounds that, while being bland-tasting themselves, enhance the sweetness of nutritive sweeteners. Using a sweetness potentiator would, for example, allow a carbonated soft drink manufacturer to use 2% high-fructose corn syrup (HFCS) in its product and achieve the same satisfying sweet taste as using 10% HFCS.
Potentiation of receptors similar to those responsible for sweet taste is well known. Sweet-taste receptors belong to the GPCR class of receptors, which also includes muscarinic and nicotinic receptors that have been shown to have allosteric activators. These are compounds that don’t interact with the receptor’s ligand-binding site directly but bind to another site on the receptor protein, leading to a change in affinity of the receptor for its ligand (Christopoulos and Kenakin, 2002; Conigrave and Franks, 2003). More pertinently, umami taste, which is signaled by GPCRs very similar to the sweet-taste receptors, undergoes potentiation of glutamate taste in the presence of inositol 5'-monophosphate and guanosine 5'-monophosphate (Yamaguchi, 1991). Thus, it is expected that biochemical screening of compound libraries will identify sweetness potentiators.
An Increasingly Prominent Role
In the coming years, biotechnology will play an increasingly prominent role in the development of new flavor ingredients. The new technologies now being developed will allow the food and beverage industry to address major health concerns that face first- and second-world nations and to improve the health of the global population with the development of better-tasting, healthier products.
Richard McGregor
The author is Director, Technology Assessment, Linguagen Corp., 2005 Eastpark Blvd., Cranbury, NJ 08512-3515
([email protected]).
References
Adler, E., Hoon, M.A., Mueller, K.L., Chandrashekar, J., Ryba, N.J.P., and Zuker, C.S. 2000. A novel family of mammalian taste receptors. Cell 100: 693-702.
Bachmanov, A.A., Li, X., Reed, D.R., Ohmen, J.D., Li, S., Chen, Z., Tordoff, M.G., de Jong, P.J., Wu, C., West, D.B., Chatterjee, A., Ross, D.A., and Beauchamp, G.K. 2001. Positional cloning of the mouse saccharin preference (Sac) locus. Chem. Senses 26: 925-933.
Boughter, J.D. Jr, Pumplin, D.W., Yu, C., Christy, R.C., and Smith, D.V. 1997. Differential expression of alpha-gustducin in taste bud populations of the rat and hamster. J. Neurosci 17: 2852-2858.
Chaudari, N., Landin, S.D., and Roper, S.D. 2000. A metabotropic glutamate receptor variant functions as a taste receptor. Nature Neurosci. 3: 113.
Christopoulos, A. and Kenakin, T. 2002. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 54: 323-374.
Conigrave, A.D. and Franks, A.H. 2003. Allosteric activation of plasma membrane receptors- Physiological implications and structural origins. Prog. Biophys. Molec. Biol. 81: 219-240.
Damak S., Rong, M., Yasumatsu, K., Kokrashvili, Z., Varadarajan, V., Zou, S., Jiang, P., Ninomiya, Y., and Margolskee, R.F. 2003. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850-8533.
DeSimone, J.A., Lyall, V., Heck, G.L., and Feldman, G.M. 2001. Acid detection by taste receptor cells. Respir. Physiol. 129: 231 45.
Doolin, R.E. and Gilbertson, T.A. 1996. Distribution and characterization of functional amiloridesensitive sodium channels in rat tongue. J. Gen. Physiol. 107: 545-554.
Elliott, E.J. and Simon, S.A 1990. The anion in salt taste: A possible role for paracellular pathways. Brain Res. 535: 9-17.
Formaker, B.K. and Hill, D.L. 1988. An analysis of residual NaCl taste response after amiloride. Am. J. Physiol. Regulatory Integrative Comp. Physiol. 255: R1002-R1007.
Hoon, M.A., Adler, E., Lindemeier, J., Battey, J.F., Ryba, N.J., and Zuker, C.S. 1999. Putative mammalian taste receptors: A class of taste-specific GPCRs with distinct topographic selectivity. Cell 96: 541-551.
Kitagawa, M., Kusakabe, Y., Miura, H., Ninomiya, Y., and Hino, A. 2001. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem. Biophys. Res. Commun. 283: 236-242.
le Coutre, J. 2003. Taste: The metabolic sense. Food Technol. 57(8): 34-37.
Li, X., Staszewski, L., Xu, H., Durick, K., Zoller, M., and Adler, E. 2002. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 99: 4692-4696.
Margolskee, R.F. and Ming, D. 2003. Inhibitors of the bitter taste response. U.S. patent 6,540,978.
Matsunami, H., Montmayeur, J.-P., and Buck, L.B. 2000. A family of candidate taste receptors in human and mouse. Nature 404: 601-604.
Max, M., Shanker, Y.G., Huang, L., Rong, M., Liu, Z., Campagne, F., Weinstein, H., Damak, S., and Margolskee, R.F. 2001. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat. Genet. 28: 58-63.
McLaughlin, S.K., McKinnon, P.J., and Margolskee, R.F. 1992. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature 357: 563-569.
Ming, D., Ninomiya, Y., and Margolskee, R.F. 1999. Blocking taste receptor activation of gustducin inhibits gustatory responses to bitter compounds. Proc. Natl. Acad. Sci. USA 96: 9903-9908.
Miyamoto T., Miyazaki T., Fujiyama R., Okaday Y., and Sato T. 2001. Differential transduction mechanisms underlying NaCl- and KCl-induced responses in mouse taste cells. Chem. Senses 26: 67-77.
Montmayeur, J.P., Liberles, S.D., Matsunami, H., and Buck, L.B. 2001. A candidate taste receptor gene near a sweet taste locus. Nat. Neurosci. 4: 492-498.
Nelson, G., Hoon, M.A., Chandrashekar, J., Zhang, Y., Ryba, N.J.P., and Zuker, C.S. 2001. Mammalian sweet taste receptors. Cell 106: 381-390.
Sainz, E., Korley, J.N., Battey, J.F., and Sullivan, S.L. 2001. Identification of a novel member of the T1R family of putative taste receptors. J. Neurochem. 77: 896-903.
Smith, D.V. and Margolskee, R.F. 2001. Making sense of taste. Sci. Am. 284: 32-39.
Stewart, R.E., Hill, D.L., and DeSimone, J.A. 1997. New perspectives in gustatory physiology: Transduction, development and plasticity. Am. J. Physiol. Cell Physiol. 272: C1-C26.
Yamaguchi, S. 1991. Basic properties of umami and effects on humans. Physiol. Behav. 49: 833-841.
